Assessment of Screening for Nasal Obstruction among Sleep Dentistry Outpatients with Obstructive Sleep Apnea
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Sample Size Calculation
2.3. Data Collection
2.4. PNIF Assessment
2.5. NOSE Scale Scores
2.6. Mallampati Classification
2.7. Statistical Analysis
3. Results
3.1. Basic Clinical Data in the OSA Group
3.2. PNIF Results between OSA Group and Control Group
3.3. NOSE and Mallampati Classification between the OSA and the Control Groups
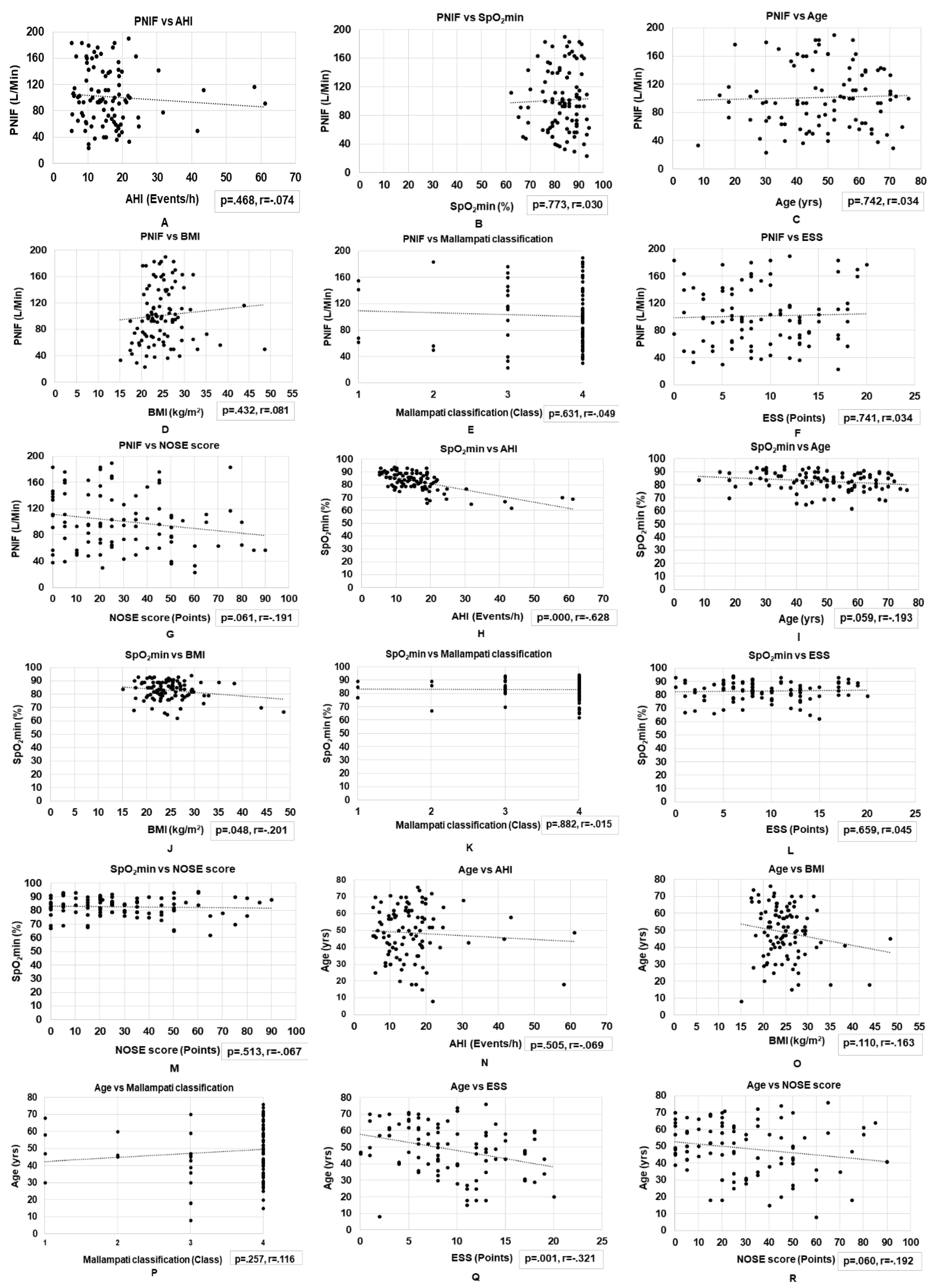
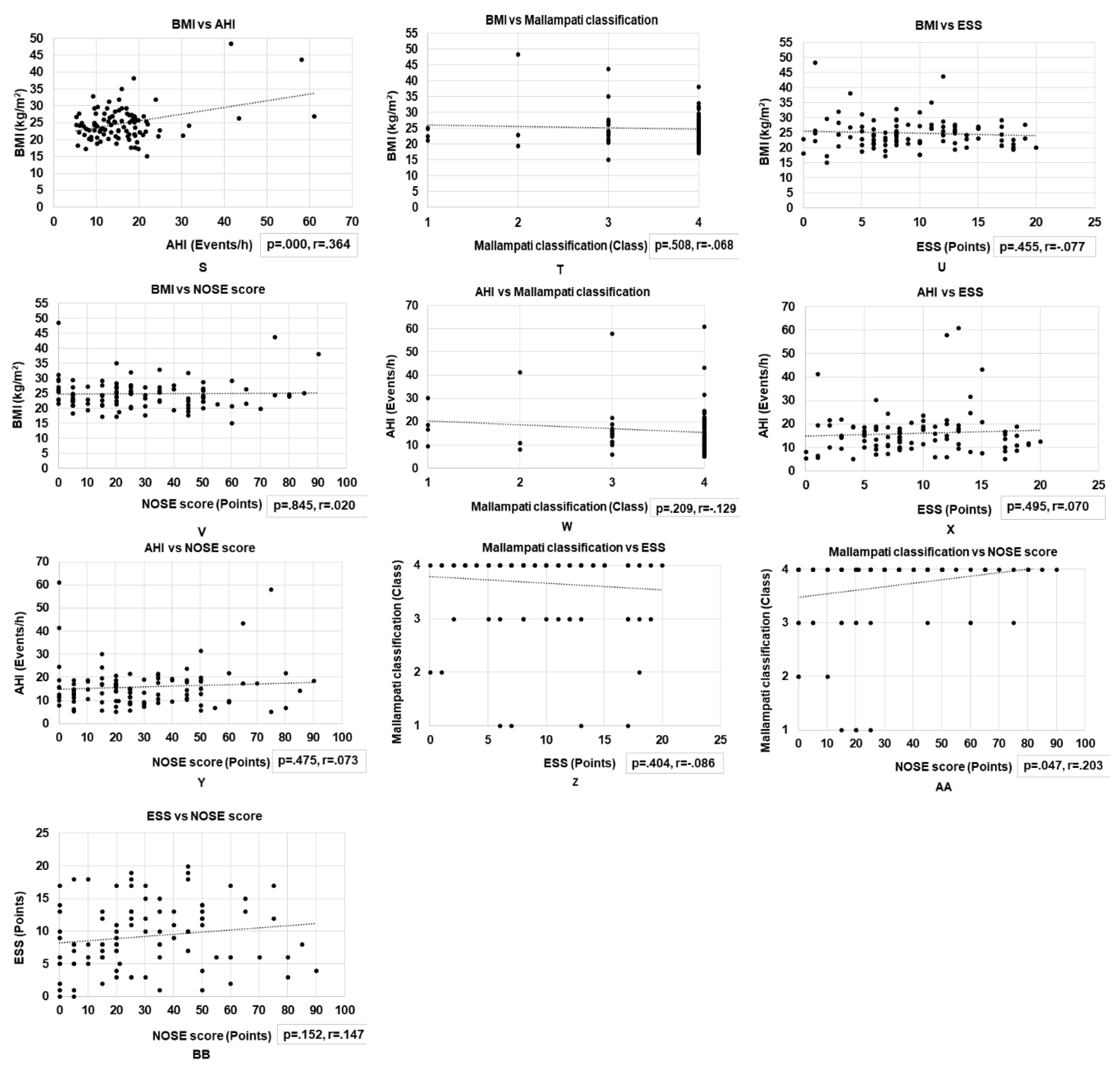
3.4. Correlations between Basic Clinical Variables and Nasal Obstruction Assessments in Patients with OSA
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The occurrence of sleep disordered breathing among middle aged adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.S.; McSharry, D.G.; Malhotra, A. Adult obstructive sleep apnoea. Lancet 2014, 383, 736–747. [Google Scholar] [CrossRef]
- Vicini, C.; Colabianchi, V.; Giorgio Marrano, G.; Barbanti, F.; Spedicato, G.A.; Lombardo, L.; Siciliani, G. Description of the relationship between NOHL classification in drug-induced sleep endoscopy and initial AHI in patients with moderate to severe OSAS, and evaluation of the results obtained with oral appliance therapy. Acta. Otorhinolaryngol. Ital. 2020, 40, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Kribbs, N.B.; Pack, A.I.; Kline, L.R.; Smith, P.L.; Schwartz, A.R.; Schubert, N.M.; Redline, S.; Henry, J.N.; Getsy, J.E.; Dinges, D.F. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am. Rev. Respir. Dis. 1993, 147, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Ramar, K.; Dort, L.C.; Katz, S.G.; Lettieri, C.J.; Harrod, C.G.; Thomas, S.M.; Chervin, R.D. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: An update for 2015. J. Clin. Sleep Med. 2015, 11, 773–827. [Google Scholar] [CrossRef]
- Lee, W.H.; Wee, J.H.; Lee, C.H.; Kim, M.S.; Rhee, C.S.; Yun, P.Y.; Yoon, I.Y.; Kim, J.W. Comparison between mono-bloc and bi-bloc mandibular advancement devices for obstructive sleep apnea. Eur. Arch. Otorhinolaryngol. 2013, 270, 2909–2913. [Google Scholar] [CrossRef]
- Sutherland, K.; Deane, S.A.; Chan, A.S.; Schwab, R.J.; Ng, A.T.; Darendeliler, M.A.; Cistulli, P.A. Comparative effects of two oral appliances on upper airway structure in obstructive sleep apnea. Sleep 2011, 34, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.L.; Launois, S.H.; Isono, S.; Feroah, T.R.; Whitelaw, W.A.; Remmers, J.E. Pharyngeal narrowing and closing pressures in patients with obstructive sleep apnea. Am. Rev. Respir. Dis 1993, 148, 606–611. [Google Scholar] [CrossRef]
- Suzuki, M.; Furukawa, T.; Sugimoto, A.; Katada, K.; Kotani, R.; Yoshizawa, T. Relationship between oral flow patterns, nasal obstruction, and respiratory events during sleep. J. Clin. Sleep Med. 2015, 11, 855–860. [Google Scholar] [CrossRef][Green Version]
- Leiberman, A.; Ohki, M.; Forte, V.; Fraschetti, J.; Cole, P. Nose/mouth distribution of respiratory airflow in ‘mouth breathing’ children. Acta. Otolaryngol. 1990, 109, 454–460. [Google Scholar] [CrossRef]
- Jones, A.S.; Willatt, D.J.; Durham, L.M. Nasal airflow: Resistance and sensation. J. Laryngol. Otol. 1989, 103, 909–911. [Google Scholar] [CrossRef]
- Zeng, B.; Ng, A.T.; Qian, J.; Petocz, P.; Darendeliler, M.A.; Cistulli, P.A. Influence of nasal resistance on oral appliance treatment outcome in obstructive sleep apnea. Sleep 2008, 31, 543–547. [Google Scholar] [CrossRef]
- Ottaviano, G.; Lund, V.J.; Nardello, E.; Scarpa, B.; Mylonakis, I.; Frasson, G.; Iacono, V.; Manzato, E.; Marioni, G.; Staffieri, A. Peak nasal inspiratory flow: A useful and handy tool for the diagnosis of nasal obstruction in the elderly. Eur. Arch. Otorhinolaryngol. 2014, 271, 2427–2431. [Google Scholar] [CrossRef]
- Moxness, M.H.S.; Bugten, V.; Thorstensen, W.M.; Nordgård, S.; Bruskeland, G. A comparison of minimal cross sectional areas, nasal volumes and peak nasal inspiratory flow between patients with obstructive sleep apnea and healthy controls. Rhinology 2016, 54, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Ottaviano, G.; Fokkens, W.J. Measurements of nasal airflow and patency: A critical review with emphasis on the use of peak nasal inspiratory flow in daily practice. Allergy 2016, 71, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.G.; Witsell, D.L.; Smith, T.L.; Weaver, E.M.; Yueh, B.; Hannley, M.T. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngol. Head Neck Surg. 2004, 130, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Lebret, M.; Arnol, N.; Martinot, J.B.; Tamisier, R.; Deschaux, C.; Pépin, J.L.; Borel, J.C. Nasal obstruction symptom evaluation score to guide mask selection in CPAP- treated obstructive sleep apnea. Otolaryngol. Head Neck Surg. 2018, 159, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Yoon, A.; Guilleminault, C.; Zaghi, S.; Liu, S.Y. Distraction osteogenesis maxillary expansion (DOME) for adult obstructive sleep apnea patients with narrow maxilla and nasal floor. Sleep Med. 2020, 65, 172–176. [Google Scholar] [CrossRef]
- Samsoon, G.L.; Young, J.R. Difficult tracheal intubation: A retrospective study. Anaesthesia 1987, 42, 487–490. [Google Scholar] [CrossRef]
- Epstein, L.J.; Kristo, D.; Strollo, P.J., Jr.; Friedman, N.; Malhotra, A.; Patil, S.P.; Ramar, K.; Rogers, R.; Schwab, R.J.; Weaver, E.M.; et al. Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep Med. 2009, 5, 263–276. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum: Hillsdale, NJ, USA, 1998. [Google Scholar]
- Okuno, K.; Furuhashi, A.; Nakamura, S.; Suzuki, H.; Arisaka, T.; Taga, H.; Tamura, M.; Katahira, H.; Furuhata, M.; Iida, C. Japanese cross-sectional multicenter survey (JAMS) of oral appliance therapy in the management of obstructive sleep apnea. Int. J. Env. Res. Public Health 2019, 16, 3228. [Google Scholar] [CrossRef] [PubMed]
- Ottaviano, G.; Scadding, G.K.; Coles, S.; Lund, V.J. Peak nasal inspiratory flow; normal range in adult population. Rhinology 2006, 44, 32–35. [Google Scholar] [PubMed]
- Dor-Wojnarowska, A.; Rabski, M.; Fal, A.M.; Liebhart, J.; Panaszek, B.; Samoliński, B. An attempt to estimate potentially useful parameters to evaluate the normal range of nasal peak inspiratory flow. Pneumonol. Alergol. Pol. 2011, 79, 320–325. [Google Scholar]
- Loftus, P.A.; Wise, S.K.; Nieto, D.; Panella, N.; Aiken, A.; DelGaudio, J.M. Intranasal volume increases with age: Computed tomography volumetric analysis in adults. Laryngoscope 2016, 26, 2212–2215. [Google Scholar] [CrossRef]
- Harms, C.A.; Rosenkranz, S. Sex differences in pulmonary function during exercise. Med. Sci. Sports Exerc. 2008, 40, 664–668. [Google Scholar] [CrossRef]
- Schumacher, M.J. Nasal dyspnea: The place of rhinomanometry in its objective assessment. Am. J. Rhinol. 2004, 18, 41–46. [Google Scholar] [CrossRef]
- Behrakis, P.K.; Baydur, A.; Jaeger, M.J.; Milic-Emili, J. Lung mechanics in sitting and horizontal body positions. Chest 1983, 83, 643–646. [Google Scholar] [CrossRef]
- Kim, J.H.; Koo, Y.C.; Cho, H.J.; Kang, J.W. Relationship between various anthropometric measures and apnea-hypopnea index in Korean men. Auris. Nasus. Larynx 2018, 45, 295–300. [Google Scholar] [CrossRef]
- Li, M.; Ge, Q.; Sheng, C.S.; Zhang, J.; Li, H.; Niu, W.; Tang, X.; Xu, J.; Gao, P.J.; Zhu, L. Clinical characteristics of snoring patients with primary aldosteronism and obstructive sleep apnea–hypopnea syndrome. J. Hum. Hypertens 2019, 33, 693–700. [Google Scholar] [CrossRef]
- Mohammadi, H.; Rezaei, M.; Sharafkhaneh, A.; Khazaie, H.; Ghadami, M.R. Serum testosterone/cortisol ratio in people with obstructive sleep apnea. J. Clin. Lab. Anal. 2020, 34, e23011. [Google Scholar] [CrossRef]
- Iannella, G.; Vicini, C.; Colizza, A.; Meccariello, G.; Polimeni, A.; Greco, A.; de Vincentiis, M.; de Vito, A.; Cammaroto, G.; Gobbi, R.; et al. Aging effect on sleepiness and apneas severity in patients with obstructive sleep apnea syndrome: A meta-analysis study. Eur. Arch. Otorhinolaryngol. 2019, 276, 3549–3556. [Google Scholar] [CrossRef]
- Kale, S.S.; Kakodkar, P.; Shetiya, S.H. Assessment of oral findings of dental patients who screen high and no risk for obstructive sleep apnea (OSA) reporting to a dental college-A cross sectional study. Sleep Sci. 2018, 11, 112–117. [Google Scholar] [CrossRef]
- Ishii, L.; Godoy, A.; Ishman, S.L.; Gourin, C.G.; Ishii, M. The nasal obstruction symptom evaluation survey as a screening tool for obstructive sleep apnea. Arch. Otolaryngol. Head Neck Surg. 2011, 137, 119–123. [Google Scholar] [CrossRef]
- Liistro, G.; Rombaux, P.; Belge, C.; Dury, M.; Aubert, G.; Rodenstein, D.O. High Mallampati score and nasal obstruction are associated risk factors for obstructive sleep apnoea. Eur. Respir. J. 2003, 21, 248–252. [Google Scholar] [CrossRef]
- Yagi, H.; Nakata, S.; Tsuge, H.; Yasuma, F.; Noda, A.; Morinaga, M.; Tagaya, M.; Nakashima, T. Morphological examination of upper airway in obstructive sleep apnea. Auris. Nasus. Larynx 2009, 36, 444–449. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
| Control | OSA | p Value | |
|---|---|---|---|
| N | 105 (100) | 97 (100) | |
| Males, n (%) | 68 (64.8) | 65 (67.0) | |
| Females, n (%) | 37 (35.2) | 32 (33.0) | |
| Age (years) | 35.0 ± 11.1 | 48.7 ± 15.3 | |
| BMI (kg/m2) | 22.6 ± 2.0 | 24.8 ± 5.1 | |
| AHI (/h) | N/A | 16.0 ± 9.2 | |
| Mild, n (%) | N/A | 49 (50.5) | |
| Moderate, n (%) | N/A | 42 (43.3) | |
| Severe, n (%) | N/A | 6 (6.2) | |
| SpO2min (%) | N/A | 82.8 ± 7.1 | |
| ESS (point) | N/A | 9.2 ± 5.0 | |
| PNIF (L/min) | 134.2 ± 31.5 | 101.3 ± 44.4 | <0.001 |
| Male (L/Min) | 143.4 ± 33.6 | 118.0 ±45.5 | <0.001 |
| Female (L/Min) | 117.4 ±17.7 | 81.1 ±34.3 | <0.001 |
| NOSE questionnaire(point×5) | 8.1 ± 5.5 | 29.1 ± 22.6 | <0.001 |
| Mallampati (Class) | 2.5 ± 0.6 | 3.7 ± 0.7 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawa, A.; Suzuki, H.; Niwa, H.; Oguchi, S.; Yagi, T.; Iwata, Y.; Makiyama, Y.; Chow, C.M.; Komiyama, O. Assessment of Screening for Nasal Obstruction among Sleep Dentistry Outpatients with Obstructive Sleep Apnea. Dent. J. 2020, 8, 119. https://doi.org/10.3390/dj8040119
Sawa A, Suzuki H, Niwa H, Oguchi S, Yagi T, Iwata Y, Makiyama Y, Chow CM, Komiyama O. Assessment of Screening for Nasal Obstruction among Sleep Dentistry Outpatients with Obstructive Sleep Apnea. Dentistry Journal. 2020; 8(4):119. https://doi.org/10.3390/dj8040119
Chicago/Turabian StyleSawa, Arisa, Hiroshi Suzuki, Hideo Niwa, Sumito Oguchi, Tatsuo Yagi, Yoshihiro Iwata, Yasuhide Makiyama, Chin Moi Chow, and Osamu Komiyama. 2020. "Assessment of Screening for Nasal Obstruction among Sleep Dentistry Outpatients with Obstructive Sleep Apnea" Dentistry Journal 8, no. 4: 119. https://doi.org/10.3390/dj8040119
APA StyleSawa, A., Suzuki, H., Niwa, H., Oguchi, S., Yagi, T., Iwata, Y., Makiyama, Y., Chow, C. M., & Komiyama, O. (2020). Assessment of Screening for Nasal Obstruction among Sleep Dentistry Outpatients with Obstructive Sleep Apnea. Dentistry Journal, 8(4), 119. https://doi.org/10.3390/dj8040119






