Q-Switch Nd:YAG Laser-Assisted Decontamination of Implant Surface
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental and Control Group
2.2. Irradiation and Treatment Protocol
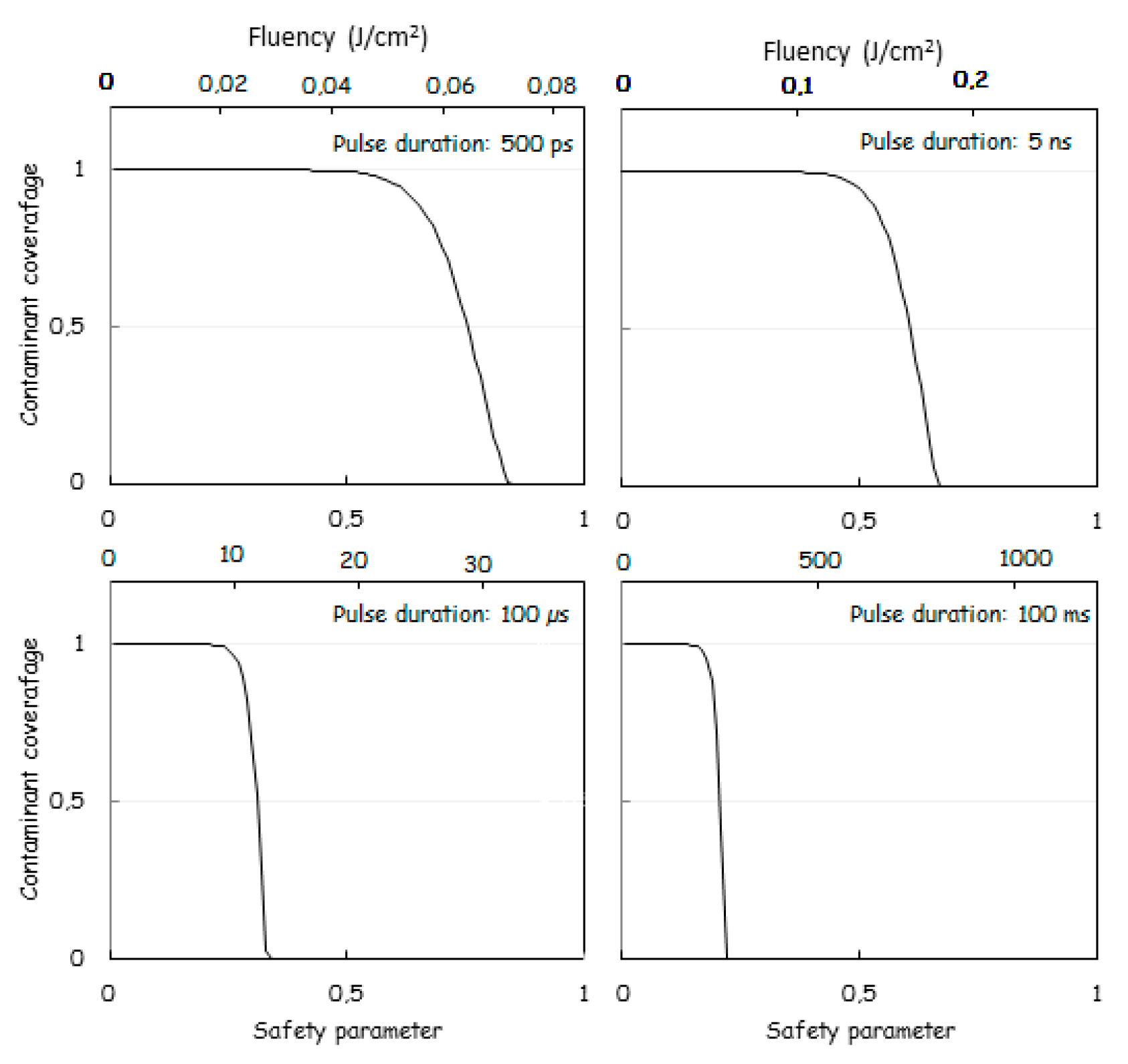
2.3. Theoretical Modelling and Calculation of the Laser Fluency
2.4. Scanning Electron Microscope (SEM) Measurements of the Three Groups
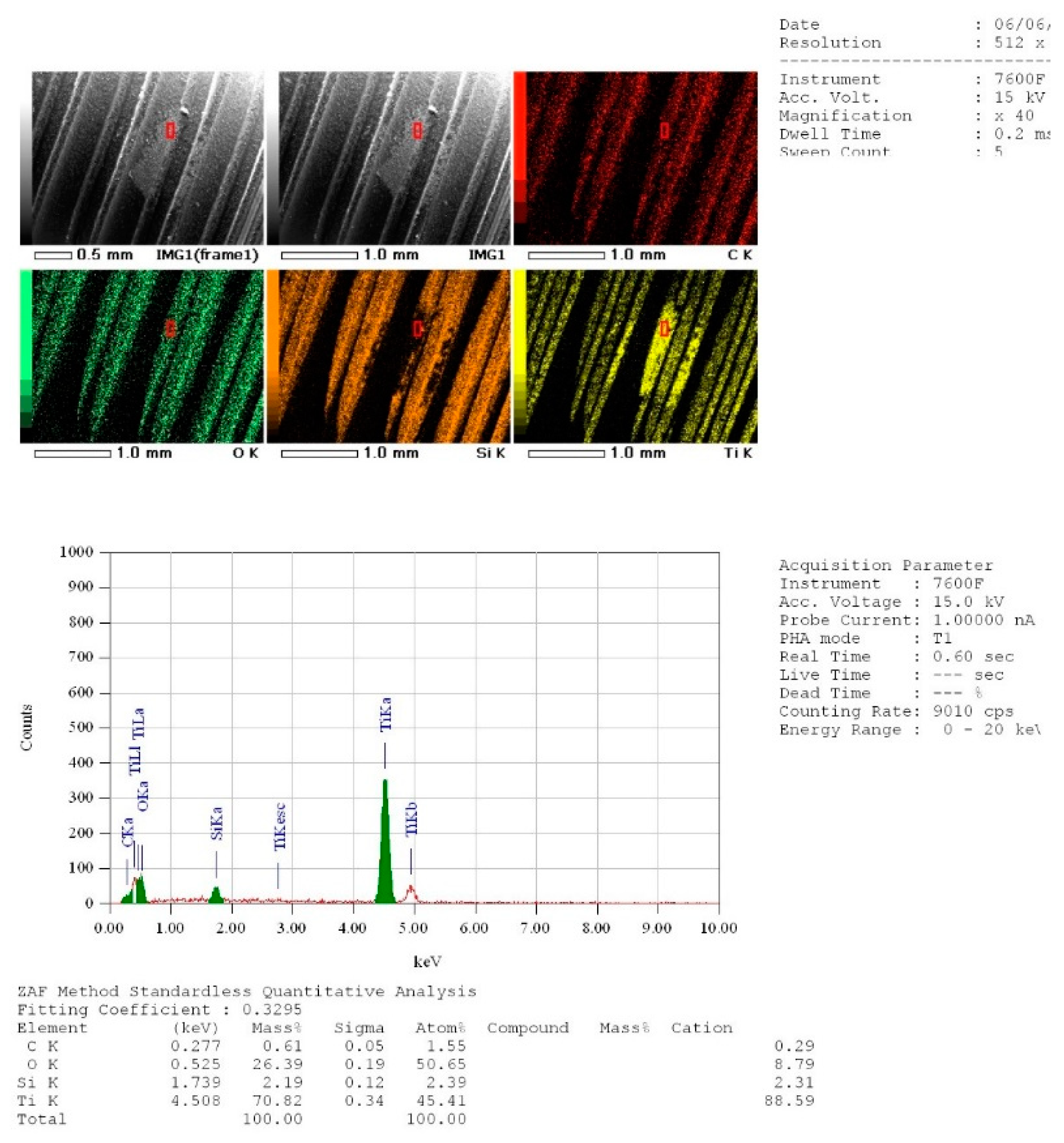
2.5. Energy Dispersive X-ray (EDX) Spectroscopy Measurements of the Three Groups
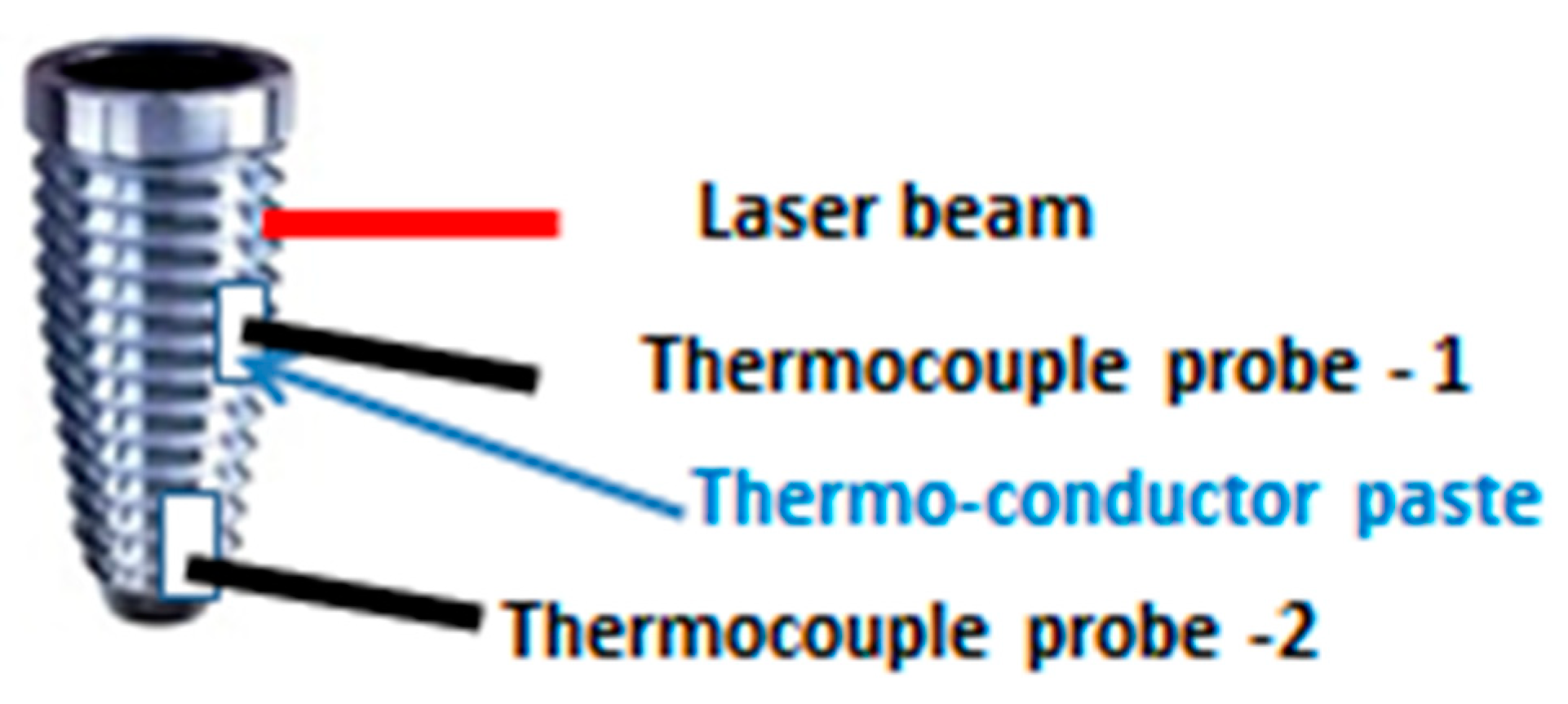
2.6. Temperature Increase Measurements
2.7. Statistical Analysis
3. Results
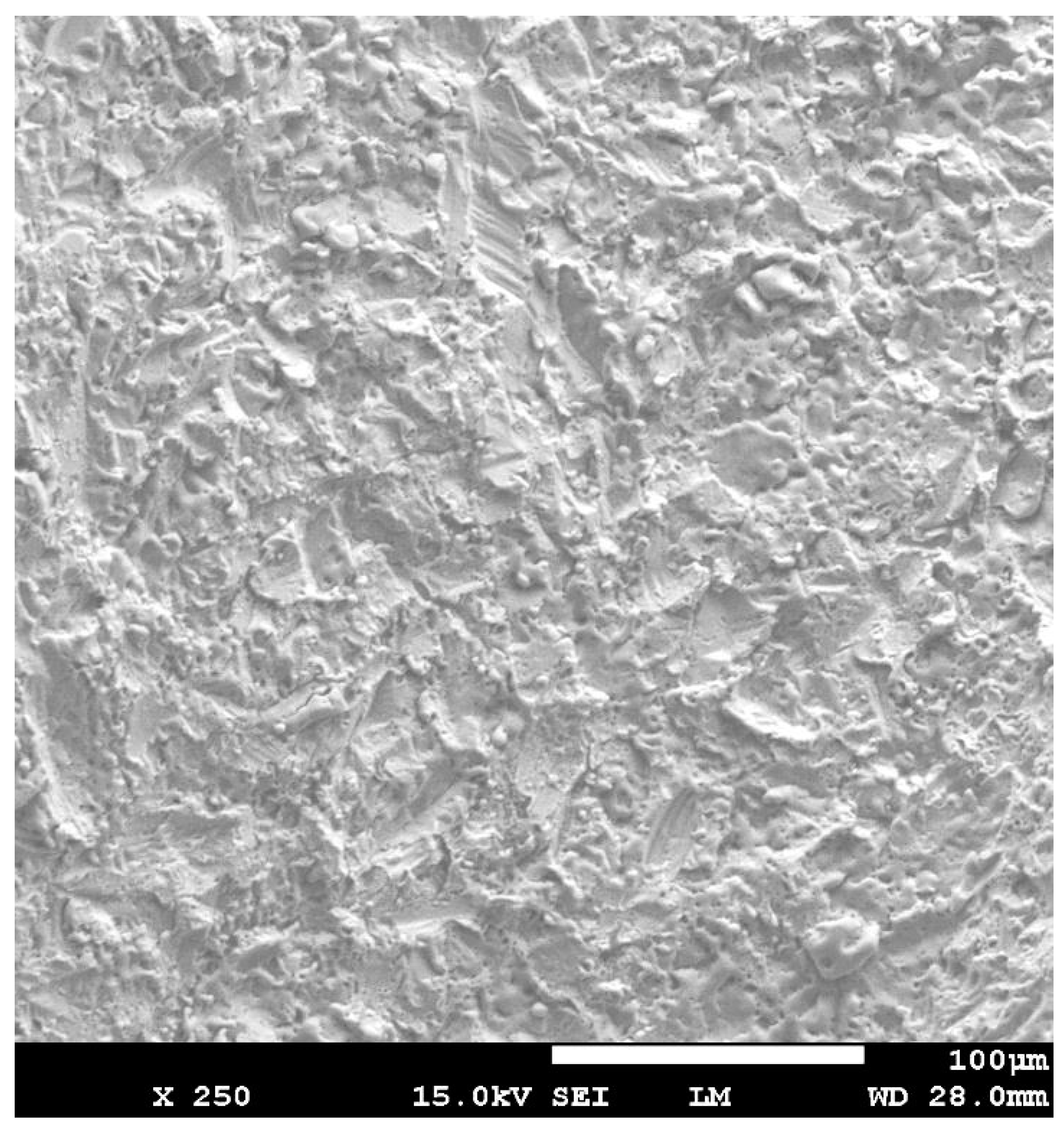
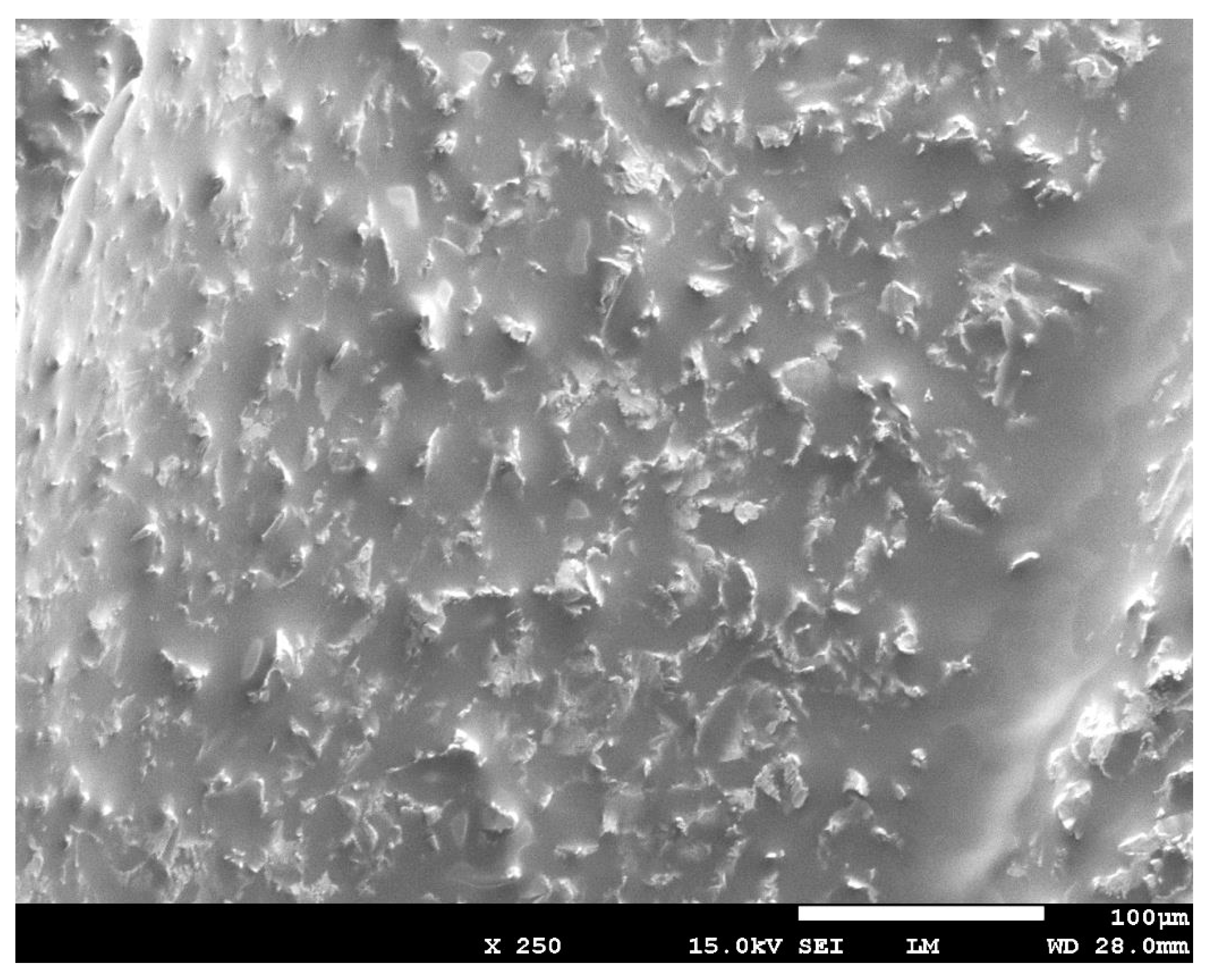
3.1. Scanning Electron Microscope (SEM) Analysis
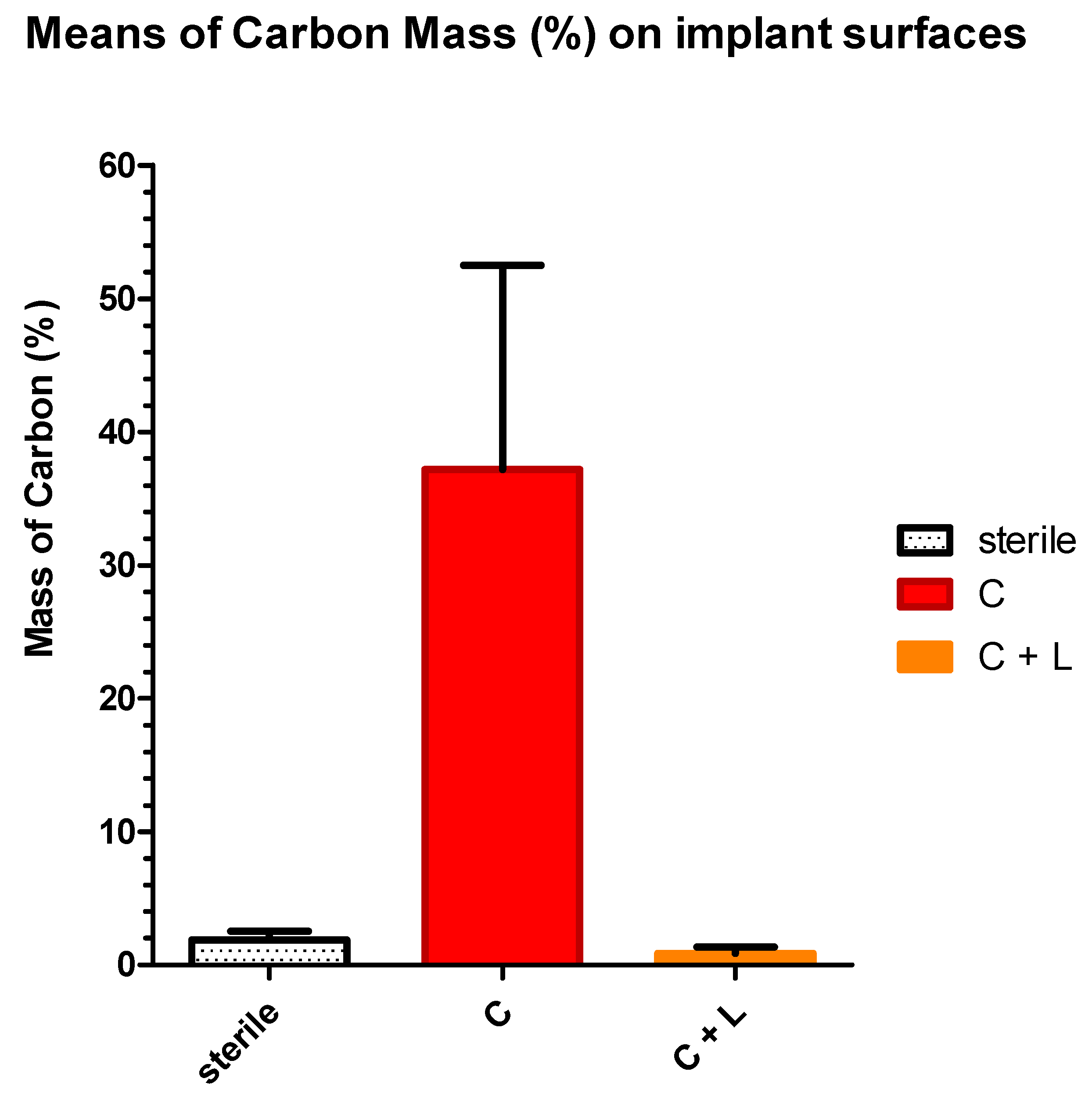
3.2. EDX Quantitative Measurements
3.3. Temperature Increase Measurements (°C)
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Atieh, M.A.; Alsabeeha, N.H.; Faggion, C.M.; Duncan, W.J. The frequency of peri-implant diseases: A systematic review and meta-analysis. J. Clin. Periodontol. 2013, 84, 1586–1598. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, N.U.; Berglundh, T. Definition and prevalence of peri-implant diseases. J. Clin. Periodontol. 2008, 35, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Rakic, M.; Galindo-Moreno, P.; Monje, A.; Radovanovic, S.; Wang, H.L.; Cochran, D.; Sculean, A.; Canullo, L. How frequent does peri-implantitis occur? A systematic review and meta-analysis. Clin. Oral Investig. 2018, 22, 1805–1816. [Google Scholar] [CrossRef] [PubMed]
- Montanaro, N.J.; Bekov, G.; Romanos, G.E. Optimization of thermal protocols during diode irradiation of dental implants. In Proceedings of the International Laser Safety Conference, Kissimmee, FL, USA, 18–21 March 2019; Volume 2019, p. P101. LIA. [Google Scholar]
- Hwey-Chin, Y.; Jang-Jih, L.; Shih-Cheng, C.; Mao-Cheng, G. Identification of microbiota in peri-implantitis pockets by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Sci. Rep. 2019, 9, 774. [Google Scholar]
- Renvert, S.; Loannis, N.P. Clinical approaches to treat peri-implant mucositis and peri-implantitis. Periodontol. 2000 2015, 68, 369–404. [Google Scholar] [CrossRef]
- Koyanagi, T.; Sakamoto, M.; Takeuchi, Y.; Ohkuma, M.; Izumi, Y. Analysis of microbiota associated with peri-implantitis using 16S rRNA gene clone library. J. Oral Microbiol. 2010, 2, 5104. [Google Scholar] [CrossRef]
- Świder, K.; Dominiak, M.; Grzech-Leśniak, K.; Matys, J. Effect of Different Laser Wavelengths on Periodontopathogens in Peri-Implantitis: A Review of In Vivo Studies. Microorganisms 2019, 7, 189. [Google Scholar] [CrossRef]
- Smeets, R.; Henningsen, A.; Jung, O.; Heiland, M. Definition, etiology, prevention and treatment of peri-implantitis—A review. Head Face Med. 2014, 10, 34. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A.; Salvi, G.E.; Botticelli, D.; Mombelli, A.; Faddy, M.; Lang, N.P. Anti-infective treatment of peri-implant mucositis: A randomisesd controlled clinical trial. Clin. Oral Implant. Res. 2011, 22, 237–241. [Google Scholar] [CrossRef]
- Hallstrom, H.; Persson, G.R.; Lindgren, S.; Olofsson, M.; Renvert, S. Systemic antibiotics and debridement of peri-implant mucositis. A randomized clinical trial. J. Clin. Periodontol. 2012, 39, 574–581. [Google Scholar] [CrossRef]
- Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Yukna, R.A.; Takasaki, A.A. Periodontal and peri-implant wound healing following laser therapy. Periodontol. 2000 2015, 68, 217–269. [Google Scholar] [CrossRef] [PubMed]
- Aoki, A.; Sasaki, K.; Watanabe, H.; Ishikawa, I. Lasers in non-surgical periodontal therapy. Periodontol. 2000 2004, 36, 59–97. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, I.; Aoki, A.; Takasaki, A. Potential applications of Er: YAG laser in periodontics. J. Periodontal Res. 2004, 39, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, I.; Aoki, A.; Takasaki, A.A.; Mizutani, K.; Sasaki, K.M.; Izumi, Y. Application of lasers in periodontics: True innovation or myth? Periodontol. 2000 2009, 50, 90–126. [Google Scholar] [CrossRef]
- Ghasemi, M.; Etemadi, A.; Nedaei, M.; Chiniforush, N. Antimicrobial efficacy of photodynamic therapy using two different light sources on the titanium-adherent biofilms of Aggregatibacter actinomycetemcomitans: An in vitro study. Photodiagnosis Photodyn. Ther. 2019, 26, 85–89. [Google Scholar] [CrossRef]
- Nejem Wakim, R.; Namour, M.; Nguyen, H.; Peremans, A.; Zeinoun, T.; Vanheusden, A.; Rompen, E.; Nammour, S. Decontamination of Dental Implant Surfaces by the Er: YAG Laser Beam: A Comparative in vitro Study of Various Protocols. Dent. J. 2018, 6, 66. [Google Scholar] [CrossRef]
- Romanos, G.E.; Motwani, S.V.; Montanaro, N.J.; Javed, F.; Delgado-Ruiz, R. Photothermal Effects of Defocused Initiated Versus Noninitiated Diode Implant Irradiation. Photomed. Laser Surg. 2019, 37, 356–361. [Google Scholar] [CrossRef]
- Peremans, A.; Dereux, A.; Maseri, F.; Darville, J.; Gilles, J.M.; Vigneron, J.P. Resonant ir laser stimulation of the desorption of methanol adsorbed on Cu (110). Phys. Rev. B Condens. Matter. 1992, 45, 8598. [Google Scholar] [CrossRef]
- Corkum, P.B.; Brunel, F.; Sherman, N.K.; Srinivasan-Rao, T. Thermal response of metals to ultrashort-pulse laser excitation. Phys. Rev. Lett. 1988, 61, 2886. [Google Scholar] [CrossRef]
- Bechtel, J.H. Heating of solid targets with laser pulses. J. Appl. Phys. 1975, 46, 1585–1593. [Google Scholar] [CrossRef]
- Roberts, I.; Mountford, R.; High, N.; Bitter-Suermann, D.; Jann, K.; Timmis, K.; Boulnois, G. Molecular cloning and analysis of genes for production of K5, K7, K12, and K92 capsular polysaccharides in Escherichia coli. J. Bacteriol. 1986, 168, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- King, D.A. Thermal desorption from metal surfaces: A review. Surf. Sci. 1975, 47, 384–402. [Google Scholar] [CrossRef]
- Aronsson, B.O.; Hjörvarsson, B.; Frauchiger, L.; Taborelli, M.; Vallotton, P.H.; Descouts, P. Hydrogen desorption from sand-blasted and acid-etched titanium surfaces after glow-discharge treatment. J. Biomed. Mater. Res. 2001, 54, 20–29. [Google Scholar] [CrossRef]
- Chichkov, B.N.; Momma, C.; Nolte, S.; Von Alvensleben, F.; Tünnermann, A. Femtosecond, picosecond and nanosecond laser ablation of solids. Appl. Phys. A 1996, 63, 109–115. [Google Scholar] [CrossRef]
- Umana, M.; Heysselaer, D.; Tielemans, M.; Compere, P.; Zeinoun, T.; Nammour, S. Dentinal tubules sealing by means of diode lasers (810 and 980 nm): A preliminary in vitro study. Photomed. Laser Surg. 2013, 31, 307–314. [Google Scholar] [CrossRef]
- Namour, A.; Nammour, S.; Peremans, A.; Heysselaer, D.; De-Moor, R.J. Treatment of dentinal hypersensitivity by means of Nd: YAP Laser: A preliminary in vitro study. Sci. World J. 2014. [Google Scholar] [CrossRef]
- El-Mobadder, M.; Namour, A.; Namour, A.; Dib, W.; El-Mobadder, W.; Maalouf, E. Dentinal Hypersensitivity Treatment Using Diode Laser 980 nm: In Vivo Study. Dent. J. 2019, 7, 5. [Google Scholar] [CrossRef]
- Mombelli, A.; Décaillet, F. The characteristics of biofilms in peri-implant disease. J. Clin. Periodontol. 2011, 38, 203–213. [Google Scholar] [CrossRef]
- Haas, R.; Haimböck, W.; Mailath, G.; Watzek, G. The relationship of smoking on peri-implant tissue: A retrospective study. J. Prosthet. Dent. 1996, 76, 592–596. [Google Scholar] [CrossRef]
- Tang, E.; Khan, I.; Andreana, S.; Arany, P. Laser-activated TGF-β1 induces Human β-Defensin 2: Implications for Laser Therapies for Periodontitis and Peri-implantitis. J. Periodontal Res. 2017, 52, 360. [Google Scholar] [CrossRef]
- Monteiro, L.; Delgado, M.L.; Garcês, F.; Machado, M.; Ferreira, F.; Martins, M.; Salazar, F.; Pacheco, J.J. A histological evaluation of the surgical margins from human oral fibrous-epithelial lesions excised with CO2 laser, Diode laser, Er: YAG laser, Nd: YAG laser, electrosurgical scalpel and cold scalpel. Med. Oral Patol. Oral Y Cir. Buccal 2019, 24, e271. [Google Scholar]
- Richter, L.J.; Buntin, S.A.; King, D.S.; Cavanagh, R.R. State-resolved studies of the laser-induced desorption of NO from Si (111) 7 × 7: Low coverage results. J. Chem. Phys. 1992, 96, 2324–2338. [Google Scholar] [CrossRef]
- Fukutani, K.; Peremans, A.; Mase, K.; Murata, Y. Photo-stimulated desorption of NO from a Pt (001) surface. Surf. Sci. 1993, 283, 158–168. [Google Scholar] [CrossRef]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Wismeijer, D. Decontamination of titanium implant surface and re-osseointegration to treat peri-implantitis: A literature review. Int. J. Oral Maxillofac. Implants 2012, 27, 1043–1054. [Google Scholar]
- Mailoa, J.; Lin, G.H.; Chan, H.L.; MacEachern, M.; Wang, H.L. Clinical outcomes of using lasers for peri-implantitis surface detoxification: A systematic review and meta-analysis. J. Periodontol. 2014, 85, 1194–1202. [Google Scholar] [CrossRef]
- Ferreira, C.F.; Babu, J.; Migliorati, E.K.; Stein, S.; Garcia-Godoy, F. Assessment of the Effect of CO2 Laser Irradiation on the Reduction of Bacteria Seeded on Commercially Available Sandblasted Acid-Etched Titanium Dental Implants: An In Vitro Study. Int. J. Oral. Maxillofac. Implants 2015, 30, 588–595. [Google Scholar] [CrossRef][Green Version]
- Mouhyi, J.; Sennerby, L.; Wennerberg, A.; Louette, P. Re-establishment of the atomic composition and the oxide structure of contaminated titanium surfaces by means of carbon dioxide laser and hydrogen peroxide: An in vitro study. Clin. Implant Dent. Relat. Res. 2000, 2, 190–202. [Google Scholar] [CrossRef]
- Romanos, G.; Ko, H.H.; Froum, S.; Tarnow, D. The use of CO2 laser in the treatment of peri-implantitis. Photomed. Laser Surg. 2009, 27, 381–386. [Google Scholar] [CrossRef]
- Romanos, G.E.; Nentwig, G.H. Regenerative Therapy of Deep Peri-implant Infrabony Defects after CO2 Laser Implant Surface Decontamination. Int. J. Periodontics Restor. Dent. 2008, 28, 245–255. [Google Scholar]
- Ley, J. Conventional versus CO2 Laser-assisted Treatment of Peri-implant Defects with the Concomitant Use of Pure-phase [beta]-tricalcium Phosphate: A 5-year Clinical Report. J. Oral Implant. 2007, 33, 240. [Google Scholar]
- Deppe, H.; Greim, H.; Brill, T.; Wagenpfeil, S. Titanium deposition after peri-implant care with the carbon dioxide laser. Int. J. Oral Maxillofac. Implants 2002, 17, 707–714. [Google Scholar] [PubMed]
- Bilgiç, E.; Arin, T.; Ercan, E.; Uysal, C.; Kara, L.; Çandırlı, C. The effect of Er, Cr: YSGG laser irradiation on titanium discs with microtextured surface morphology. Lasers Med. Sci. 2015, 30, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Rothamel, D.; Becker, J. Influence of an Er: YAG laser on the surface structure of titanium implants. Schweiz. Mon. Fur Zahnmed. Rev. Mens. Suisse D’odonto-Stomatol. Riv. Mens. Svizz. Di Odontol. E Stomatol. 2003, 113, 660–671. [Google Scholar]
- Gonçalves, F.; Zanetti, A.L.; Zanetti, R.V.; Martelli, F.S.; Avila-Campos, M.J.; Tomazinho, L.F.; Granjeiro, J.M. Effectiveness of 980-mm diode and 1064-nm extra-long-pulse neodymium-doped yttrium aluminum garnet lasers in implant disinfection. Photomed. Laser Surg. 2010, 28, 273–280. [Google Scholar] [CrossRef]
- Vassalli, M.; Giannelli, M. Effect of Nd: YAG laser on titanium dental implants studied by AFM. Ital. J. Anat. Embryol. Arch. Ital. Di Anat. Ed Embriol. 2003, 108, 195–203. [Google Scholar]
- Block, C.M.; Mayo, J.A.; Evans, G.H. Effects of the Nd: YAG dental laser on plasma-sprayed and hydroxyapatite-coated titanium dental implants: Surface alteration and attempted sterilization. Int. J. Oral Maxillofac. Implants 1992, 7, 22–35. [Google Scholar]
- Fickl, S.; Kebschull, M.; Calvo-Guirado, J.L.; Hürzeler, M.; Zuhr, O. Experimental Peri-Implantitis around Different Types of Implants—A Clinical and Radiographic Study in Dogs. Clin. Implant Dent. Relat. Res. 2015, 2, e661–e669. [Google Scholar] [CrossRef]


| Groups | Number of Samples | Description |
|---|---|---|
| Group S | 36 | Sterile implants with untreated surfaces used as a control group. |
| Group C and Group C + L | 36 | Partial surfaces of the contaminated implants were kept unlased (second control group) |
| Partial surfaces of contaminated implant of group C were treated with Q-switch Nd:YAG laser. | ||
| Group T | 6 | Group used for the temperature rise measurements |
| Wavelength (λ) | n | k | Reflectivity (Equation (1)) |
|---|---|---|---|
| 0.532 µm (KTP laser) | 2.2080 | 2.8980 | 53% |
| 1.064 µm (Nd:YAG laser) | 3.3731 | 3.9777 | 61% |
| 2.94 µm (Er:YAG laser) | 3.6872 | 5.2688 | 70% |
| 10 µm (CO2 laser) | 9.6000 | 18.600 | 92% |
| Laser pulse Duration | Pulse Fluency for Obtaining | Heat Diffusion Length |
|---|---|---|
| 100 µs | 22 J/cm2 | 25 µm |
| 6 ns | 0.16 J/cm2 | 196 nm |
| 500 ps | 0.05 J/cm2 | 62 nm |
| Laser Pulse Duration | Safety Parameter (Sa) | Heat Diffusion Length (HDL) | Fluency (F) | Pulse Energy | Implant Temperature Rise (ITR) for 2000 Shots |
|---|---|---|---|---|---|
| 100 ms | 0.2 | 800 µm | 145 J/cm2 | 0.73 J | 464 °C |
| 100 µs | 0.32 | 25 µm | 12 J/cm2 | 60 mJ | 38 °C |
| 6 ns | 0.14 | 196 nm | 0.18 J/cm2 | 850 µJ | 0.54 °C |
| 600 ps | 0.81 | 62 nm | 0.07 J/cm2 | 350 µJ | 0.22 °C |
| Groups | Sterile (Group S) | Contaminated (Group C) | Contaminated and Lased (Group C +L) |
|---|---|---|---|
| Number of values | 36 | 36 | 36 |
| Mean (SD) | 1.851 (0.672) a | 41.43 (10.41) b | 0.975 (1.007) a |
| Std. Error | 0.07609 | 1.480 | 0.1808 |
| Irradiation Conditions | 20 Pulses, 10 HZ (Impact) | 20 Pulses, 10 Hz (Apex) | 40 Pulses, 10 Hz (Impact) | 40 Pulses, 10 Hz (Apex) |
|---|---|---|---|---|
| Number of values | 36 | 36 | 36 | 36 |
| Mean (SD) | 0.29 (0.0493) a | 0.17 (0.0480) b | 0.98 (0.040) c | 0.56 (0.081) d |
| Std. Error | 0.01369 | 0.01332 | 0.01667 | 0.03333 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namour, M.; El Mobadder, M.; Magnin, D.; Peremans, A.; Verspecht, T.; Teughels, W.; Lamard, L.; Nammour, S.; Rompen, E. Q-Switch Nd:YAG Laser-Assisted Decontamination of Implant Surface. Dent. J. 2019, 7, 99. https://doi.org/10.3390/dj7040099
Namour M, El Mobadder M, Magnin D, Peremans A, Verspecht T, Teughels W, Lamard L, Nammour S, Rompen E. Q-Switch Nd:YAG Laser-Assisted Decontamination of Implant Surface. Dentistry Journal. 2019; 7(4):99. https://doi.org/10.3390/dj7040099
Chicago/Turabian StyleNamour, Melanie, Marwan El Mobadder, Delphine Magnin, André Peremans, Tim Verspecht, Wim Teughels, Laurent Lamard, Samir Nammour, and Eric Rompen. 2019. "Q-Switch Nd:YAG Laser-Assisted Decontamination of Implant Surface" Dentistry Journal 7, no. 4: 99. https://doi.org/10.3390/dj7040099
APA StyleNamour, M., El Mobadder, M., Magnin, D., Peremans, A., Verspecht, T., Teughels, W., Lamard, L., Nammour, S., & Rompen, E. (2019). Q-Switch Nd:YAG Laser-Assisted Decontamination of Implant Surface. Dentistry Journal, 7(4), 99. https://doi.org/10.3390/dj7040099







