Layered Double Hydroxide Fluoride Release in Dental Applications: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Research Question
2.2. Search Methodology
- Layered double hydroxide
- Hydrotalcite
- Release
- Discharge
- Fluoride
- Fluoride ion
- Fluoridation
- 1 OR 2
- 3 OR 4
- 5 OR 6 OR 7
- 8 AND 9 AND 10.
2.3. Study Selection
2.4. Study Quality Assessment
3. Results
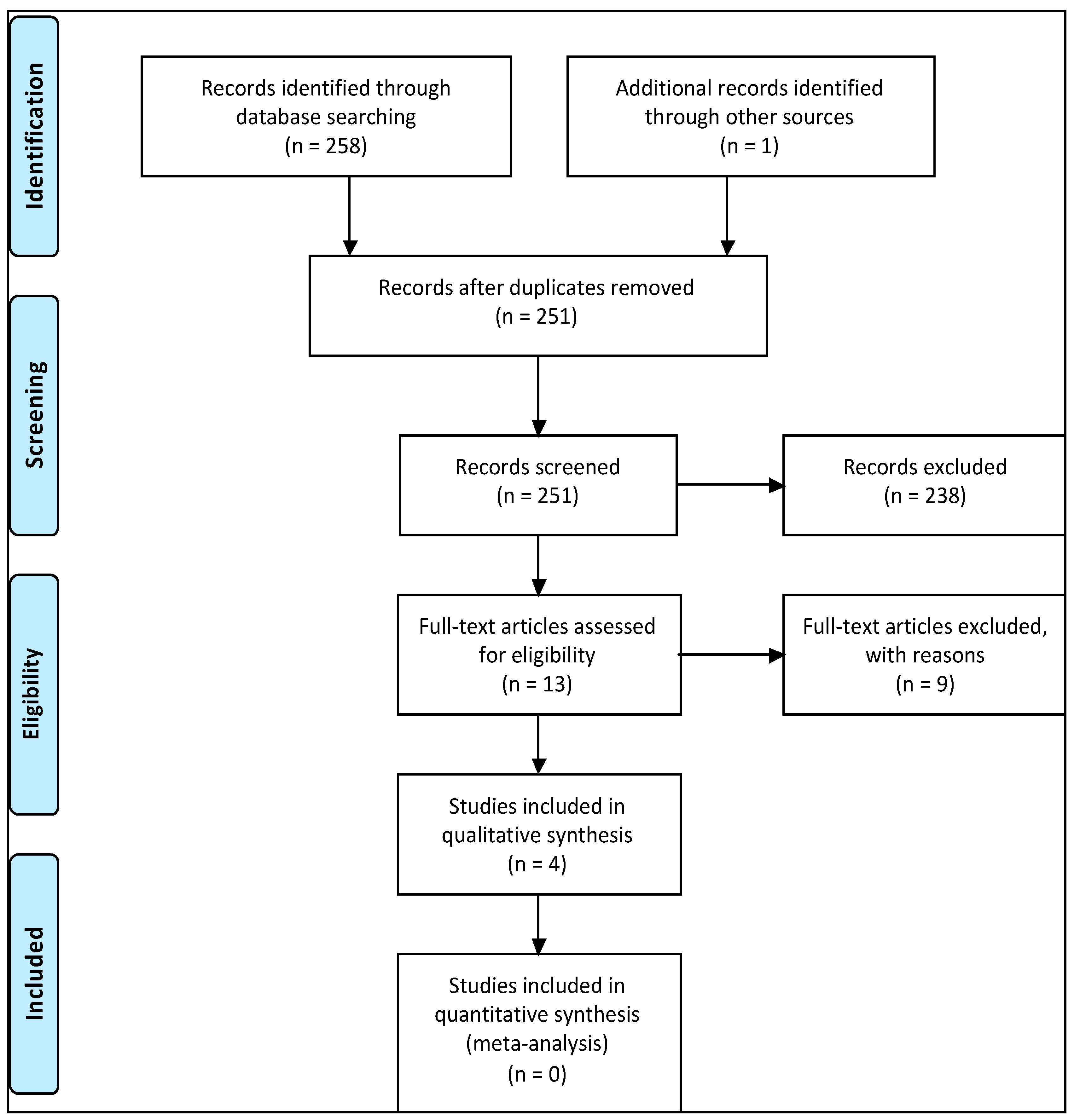
3.1. Study Selection
3.2. Study Quality Assessment
3.3. Study Characteristics
3.3.1. LDH—Polymer Composition and Study Characteristics
3.3.2. Characterisation Techniques
3.3.3. Fluoride Release Protocol and Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ten Cate, J.M.; Featherstone, J.D.B. Mechanistic Aspects of the Interactions between Fluoride and Dental Enamel. Crit. Rev. Oral Biol. Med. 1991, 2, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D.B. Prevention and reversal of dental caries: Role of low level fluoride. Community Dent. Oral Epidemiol. 1999, 27, 31–40. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.M.; Featherstone, J.D.B. Demineralization and remineralization around orthodontic appliances: An in vivo study. Am. J. Orthod. Dentofac. Orthop. 1987, 92, 33–40. [Google Scholar] [CrossRef]
- Friedl, K.-H.; Schmalz, G.; Miller, K.-A.; Shams, M. Resin-modified glass ionomer cements: Fluoride release and influence on Streptococcus mutans growth. Eur. J. Oral Sci. 1997, 105, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Furtos, G.; Cosma, V.; Prejmerean, C.; Moldovan, M.; Brie, M.; Colceriu, A.; Vezsenyi, L.; Silaghi-Dumitrescu, L.; Sirbu, C. Fluoride release from dental resin composites. Mater. Sci. Eng. C 2005, 25, 231–236. [Google Scholar] [CrossRef]
- Nakajo, K.; Imazato, S.; Takahashi, Y.; Kiba, W.; Ebisu, S.; Takahashi, N. Fluoride released from glass-ionomer cement is responsible to inhibit the acid production of caries-related oral streptococci. Dent. Mater. 2009, 25, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Grobler, S.R.; Rossouw, R.J.; Van Wyk Kotze, T.J. A comparison of fluoride release from various dental materials. J. Dent. 1998, 26, 259–265. [Google Scholar] [CrossRef]
- Creanor, S.L.; Carruthers, L.M.C.; Saunders, W.P.; Strang, R.; Foye, R.H. Fluoride Uptake and Release Characteristics of Glass lonomer Cements. Caries Res. 1994, 28, 322–328. [Google Scholar] [CrossRef]
- Aoba, T.; Fejerskov, O. Dental Fluorosis: Chemistry and Biology. Crit. Rev. Oral Biol. Med. 2002, 13, 155–170. [Google Scholar] [CrossRef]
- DenBesten, P.K. Biological mechanisms of dental fluorosis relevant to the use of fluoride supplements. Community Dent. Oral Epidemiol. 1999, 27, 41–47. [Google Scholar] [CrossRef]
- DenBesten, P.; Ko, H.S. Fluoride levels in whole saliva of preschool children after brushing with 0.25 g (pea-sized) as compared to 1.0 g (full-brush) of a fluoride dentifrice. Pediatr. Dent. 1996, 18, 277–280. [Google Scholar] [PubMed]
- Mohammed, N.R.; Kent, N.W.; Lynch, R.J.M.; Karpukhina, N.; Hill, R.; Anderson, P. Effects of Fluoride on in vitro Enamel Demineralization Analyzed by 19F MAS-NMR. Caries Res. 2013, 47, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Basu, D.; Das, A.; Stöckelhuber, K.W.; Wagenknecht, U.; Heinrich, G. Advances in layered double hydroxide (LDH)-based elastomer composites. Prog. Polym. Sci. 2014, 39, 594–626. [Google Scholar] [CrossRef]
- Mandal, S.; Mayadevi, S. Cellulose supported layered double hydroxides for the adsorption of fluoride from aqueous solution. Chemosphere 2008, 72, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Choy, J.-H.; Choi, S.-J.; Oh, J.-M.; Park, T. Clay minerals and layered double hydroxides for novel biological applications. Appl. Clay Sci. 2007, 36, 122–132. [Google Scholar] [CrossRef]
- Khan, A.I.; Lei, L.; Norquist, A.J.; O’Hare, D. Intercalation and controlled release of pharmaceutically active compounds from a layered double hydroxide. Chem. Commun. 2001, 22, 2342–2343. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, X.; Mi, Y.; Li, H.; Hou, W. Engineering of (10-hydroxycamptothecin intercalated layered double hydroxide)@ liposome nanocomposites with excellent water dispersity. J. Phys. Chem. Solids 2017, 108, 125–132. [Google Scholar] [CrossRef]
- Constantino, V.R.L.; Pinnavaia, T.J. Basic Properties of Mg2+1-xAl3+x Layered Double Hydroxides Intercalated by Carbonate, Hydroxide, Chloride, and Sulfate Anions. Inorg. Chem. 1995, 34, 883–892. [Google Scholar] [CrossRef]
- Watanabe, Y.; Ikoma, T.; Yamada, H.; Stevens, G.W.; Moriyoshi, Y.; Tanaka, J.; Komatsu, Y. Formation of Hydroxyapatite Nanocrystals on the Surface of Ca–Al-Layered Double Hydroxide. J. Am. Ceram. Soc. 2010, 93, 1195–1200. [Google Scholar] [CrossRef]
- Lynch, R.J.M. Zinc in the mouth, its interactions with dental enamel and possible effects on caries; a review of the literature. Int. Dent. J. 2011, 61, 46–54. [Google Scholar] [CrossRef]
- Lynch, E.; Brauer, D.S.; Karpukhina, N.; Gillam, D.G.; Hill, R.G. Multi-component bioactive glasses of varying fluoride content for treating dentin hypersensitivity. Dent. Mater. 2012, 28, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.G.; Chen, X.; Gillam, D.G. In vitro ability of a novel nanohydroxyapatite oral rinse to occlude dentine tubules. Int. J. Dent. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Mneimne, M.; Hill, R.G.; Bushby, A.J.; Brauer, D.S. High phosphate content significantly increases apatite formation of fluoride-containing bioactive glasses. Acta Biomater. 2011, 7, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Ray, S.; Acharya, R.; Chatterjee, T.K.; Chakraborty, J. Magnesium, zinc and calcium aluminium layered double hydroxide-drug nanohybrids: A comprehensive study. Appl. Clay Sci. 2017, 135 (Suppl. SC), 493–509. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernan, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of intervention. British Med. J. 2016, 355, 4919. [Google Scholar] [CrossRef] [PubMed]
- Calarco, A.; Di Salle, A.; Tammaro, L.; De Luca, I.; Mucerino, S.; Petillo, O.; Riccitiello, F.; Vittoria, V.; Peluso, G. Long-Term Fluoride Release from Dental Resins Affects STRO-1+ Cell Behavior. J. Dent. Res. 2015, 94, 1099–1105. [Google Scholar] [CrossRef]
- Tammaro, L.; Vittoria, V.; Calarco, A.; Petillo, O.; Riccitiello, F.; Peluso, G. Effect of layered double hydroxide intercalated with fluoride ions on the physical, biological and release properties of a dental composite resin. J. Dent. 2014, 42, 60–67. [Google Scholar] [CrossRef]
- Yokogawa, Y.; Namba, S.; Kinoshita, J.; Morita, Y.; Fujii, K.; Hotta, M. VSC Sorption onto Mg-Fe-F Layered Double Hydroxide and its Fluoride Release in Aqueous Solution. In Key Engineering Materials; Trans. Tech. Publications: Zürich, Switzerland, 2017; pp. 37–40. [Google Scholar]
- Perioli, L.; Nocchetti, M.; Giannelli, P.; Pagano, C.; Bastianini, M. Hydrotalcite composites for an effective fluoride buccal administration: A new technological approach. Int. J. Pharm. 2013, 454, 259–268. [Google Scholar] [CrossRef]
- Sarijo, S.H.; Ghazali, S.A.I.S.M.; Hussein, M.Z.; Ahmad, A.H. Intercalation, Physicochemical and Controlled Release Studies of Organic-inorganic -Herbicide (2, 4, 5 Tricholorphenoxy Butyric Acid) Nanohybrid into Hydrotalcite-like Compounds. Mater. Today Proc. 2015, 2, 345–354. [Google Scholar] [CrossRef]
- Kuthati, Y.; Kankala, R.K.; Lee, C.-H. Layered double hydroxide nanoparticles for biomedical applications: Current status and recent prospects. Appl. Clay Sci. 2015, 112–113 (Suppl. SC), 100–116. [Google Scholar] [CrossRef]
- Mandal, S.; Tripathy, S.; Padhi, T.; Sahu, M.K.; Patel, R.K. Removal efficiency of fluoride by novel Mg-Cr-Cl layered double hydroxide by batch process from water. J. Environ. Sci. 2013, 25, 993–1000. [Google Scholar] [CrossRef]
- Kameda, T.; Oba, J.; Yoshioka, T. Removal of boron and fluoride in wastewater using Mg-Al layered double hydroxide and Mg-Al oxide. J. Environ. Manag. 2017, 188 (Suppl. SC), 58–63. [Google Scholar] [CrossRef] [PubMed]
- Delorme, F.; Seron, A.; Gautier, A.; Crouzet, C. Comparison of the fluoride, arsenate and nitrate anions water depollution potential of a calcined quintinite, a layered double hydroxide compound. J. Mater. Sci. 2007, 42, 5799–5804. [Google Scholar] [CrossRef]
- Joshi, G.V.; Pawar, R.R.; Kevadiya, B.D.; Bajaj, H.C. Mesoporous synthetic hectorites: A versatile layered host with drug delivery application. Microporous Mesoporous Mater. 2011, 142, 542–548. [Google Scholar] [CrossRef]
- Louvain, N.; Peyroux, J.; Dubois, M.; Simond, W.; Leroux, F. Efficient Fluorinating Agent through Topochemical Fluorination of Co–Fe Layered Double Hydroxides. Inorg. Chem. 2013, 53, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Chen, Y.; Zhang, W.; Zhao, W. Performance and mechanism of Mg-Ca-Fe hydrotalcite-like compounds for fluoride removal from aqueous solution. J. Fluor. Chem. 2017, 200, 153–161. [Google Scholar] [CrossRef]
- Albini, A.; Iwamoto, Y.; Kleinman, H.K.; Martin, G.R.; Aaronson, S.A.; Kozlowski, J.M.; McEwan, R.N. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987, 47, 3239–3245. [Google Scholar] [PubMed]
- Lee, W.F.; Lee, S.C. Effect of hydrotalcite on the swelling and mechanical behaviors for the hybrid nanocomposite hydrogels based on gelatin and hydrotalcite. J. Appl. Polym. Sci. 2006, 100, 500–507. [Google Scholar] [CrossRef]
- Lv, L.; He, J.; Wei, M.; Evans, D.G.; Duan, X. Factors influencing the removal of fluoride from aqueous solution by calcined Mg–Al–CO3 layered double hydroxides. J. Hazard. Mater. 2006, 133, 119–128. [Google Scholar] [CrossRef]
- El Mallakh, B.F.; Sarkar, N.K. Fluoride release from glass-ionomer cements in de-ionized water and artificial saliva. Dent. Mater. 1990, 6, 118–122. [Google Scholar] [CrossRef]
- Miyata, S. Anion-exchange properties of hydrotalcite-like compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Mandal, S.; Mayadevi, S. Adsorption of fluoride ions by Zn–Al layered double hydroxides. Appl. Clay Sci. 2008, 40, 54–62. [Google Scholar] [CrossRef]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Oliveby, A.; Ekstrand, J.; Lagerlöf, F. Effect of Salivary Flow Rate on Salivary Fluoride Clearance after Use of a Fluoride-Containing Chewing Gum. Caries Res. 1987, 21, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Andersson, R.; Arvidsson, E.; Crossner, C.G.; Holm, A.K.; Mansson, B. The flow rate, pH and buffer effect of mixed saliva in children. J. Int. Assoc. Dent. Child. 1974, 5, 5. [Google Scholar] [PubMed]
- Edgar, W.M.; O’Mullane, D.M.; Dawes, C. Saliva and Oral Health; British Dental Association: London, UK, 2004. [Google Scholar]
- Hemamalathi, B.S.; Kandaswamy, D.; Gupta, T. A dynamic methodology for measuring fluoride release from restorative materials. J. Conserv. Dent. 2006, 9, 113–116. [Google Scholar] [CrossRef]
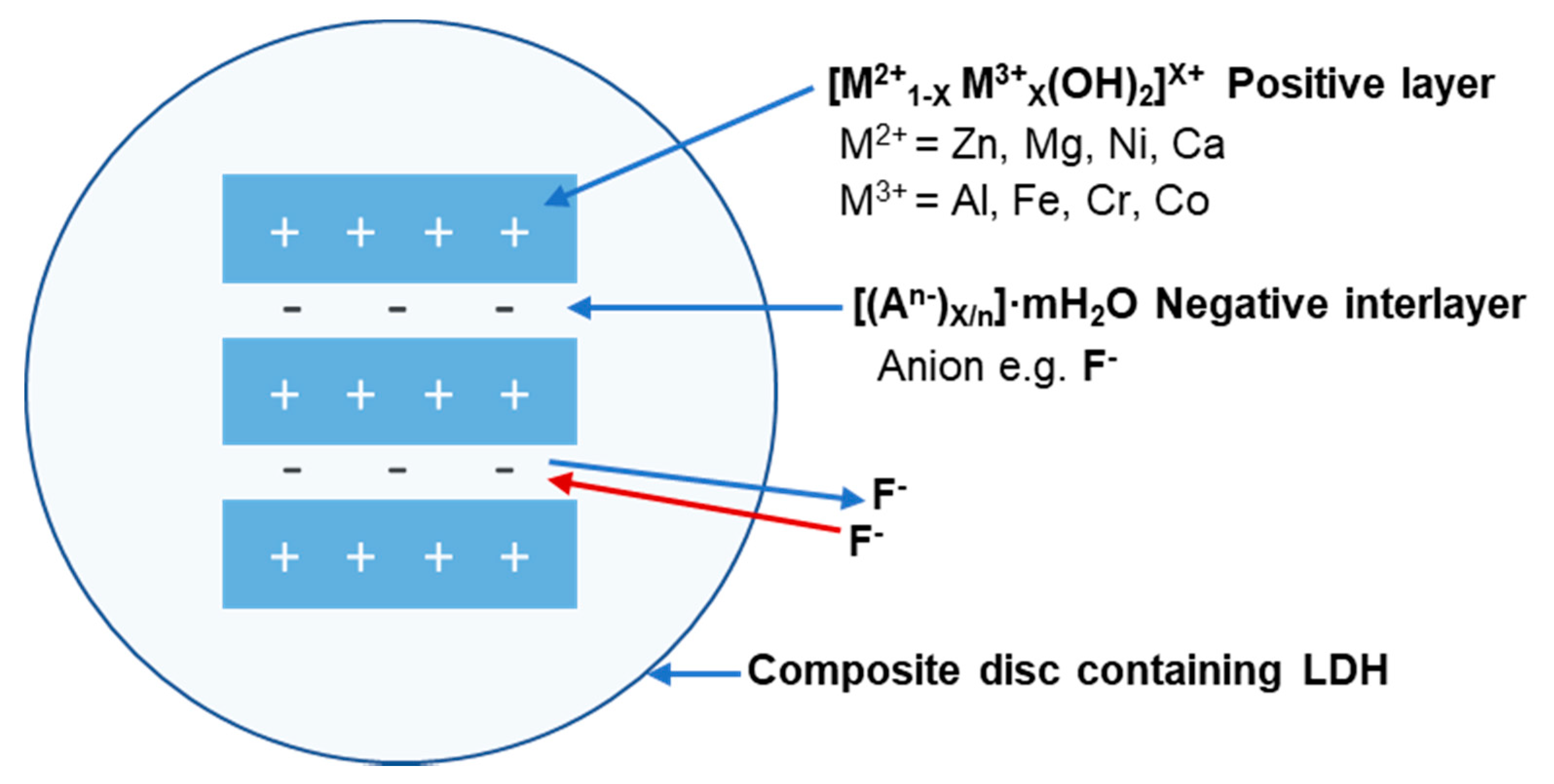
| Inclusion Criteria for Studies Included |
|
| Exclusion Criteria |
|
| Study | LDH Synthesis | LDH Characterization | F− Release | Cyto-Toxicity | Statistical Analysis | Incorporated into Dental Material | Control Group | Grade |
|---|---|---|---|---|---|---|---|---|
| Calarco et al. [27] | Yes | No | Yes | Yes | Yes | Yes | Yes | High |
| Tammaro et al. [28] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Yokogawa et al. [29] | Yes | Yes | Yes | No | No | No | No | Low |
| Perioli et al. [30] | Yes | Yes | Yes | No | Yes | Yes | Yes | High |
| Studies | Reason(s) for Exclusion |
|---|---|
| Sarijo et al. [31] | Ion release investigated for herbicide release, not release from dental materials. |
| Saha et al. [24] | Review on drug release; however, not dental related. |
| Kuthati et al. [32] | Review on drug release reporting only one dental study by Tammaro et al. [28], which is included in our review. |
| Mandal et al. [33] | Fluoride removal investigated. |
| Kameda et al. [34] | Fluoride removal investigated. |
| Delorme et al. [35] | Fluoride uptake and release investigated for water depollution. |
| Joshi et al. [36] | Layered double hydroxide not used in the study, mesoporous hectorites used. |
| Louvain et al. [37] | No fluoride release data published; the study was not dentally related. |
| Ma et al. [38] | Fluoride removal investigated. |
| Author(s) | LDH Type and Ratio M2+:M3+ | LDH Incorporated in: | Fluoride Release Protocol | Outcome/Analysis Technique | Summary of Results |
|---|---|---|---|---|---|
| Calarco et al. [27] | MgAl 2:1 | Composite: UDMA, Bis-GMA, TEGDMA, EBPADMA, glass filler |
|
| LDH-fluoride containing dental resins demonstrated:
|
| Tammaro et al. [28] | MgAl 2:1 | Composite: UDMA, Bis-GMA, TEGDMA, EBPADMA, glass filler |
|
| LDH-fluoride in dental resins (0.7, 5, 10, 20 wt.%):
|
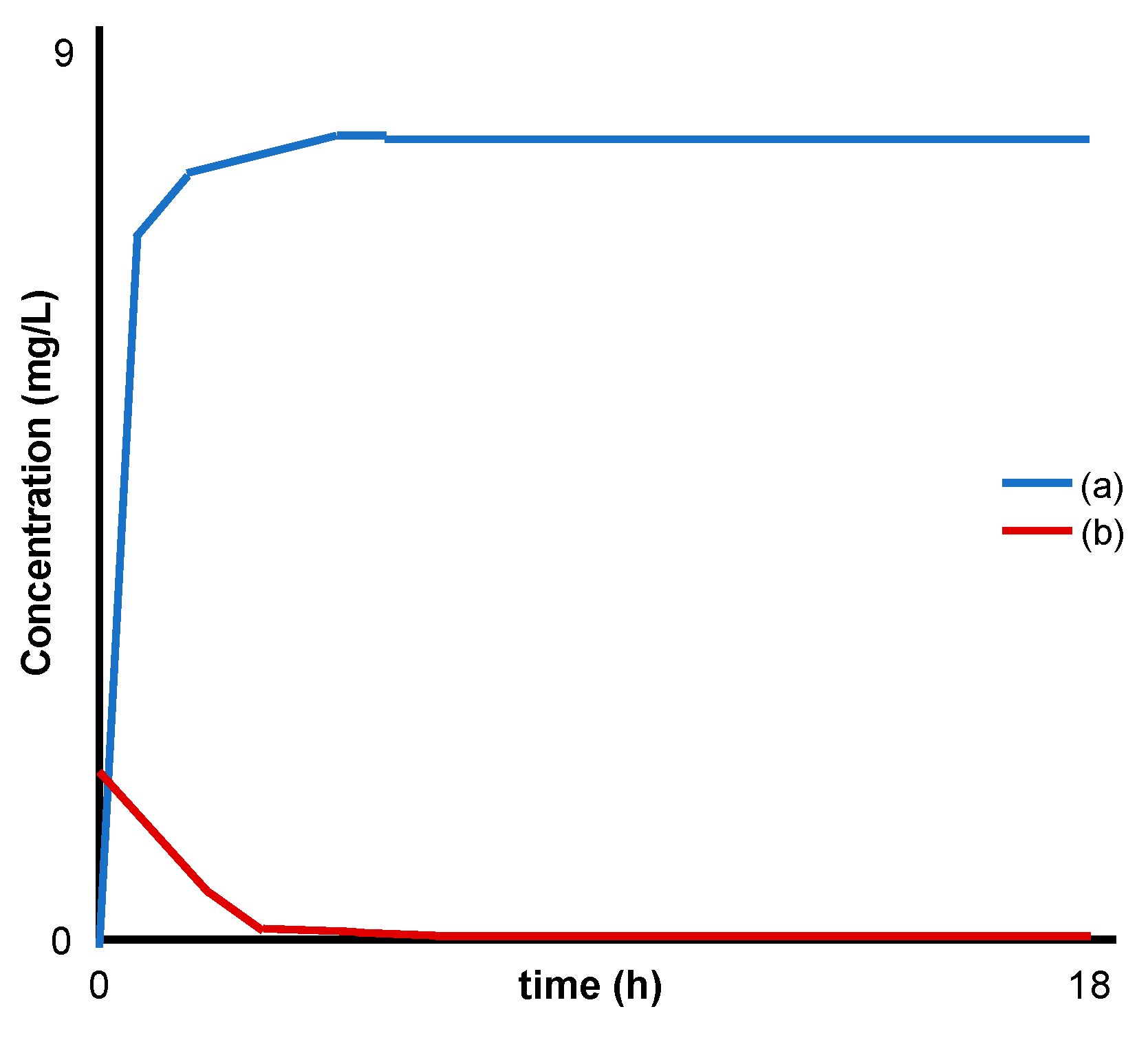
| Yokogawa el al [29] | MgFe 2.7:1 | Analysed LDH powder alone |
|
|
|
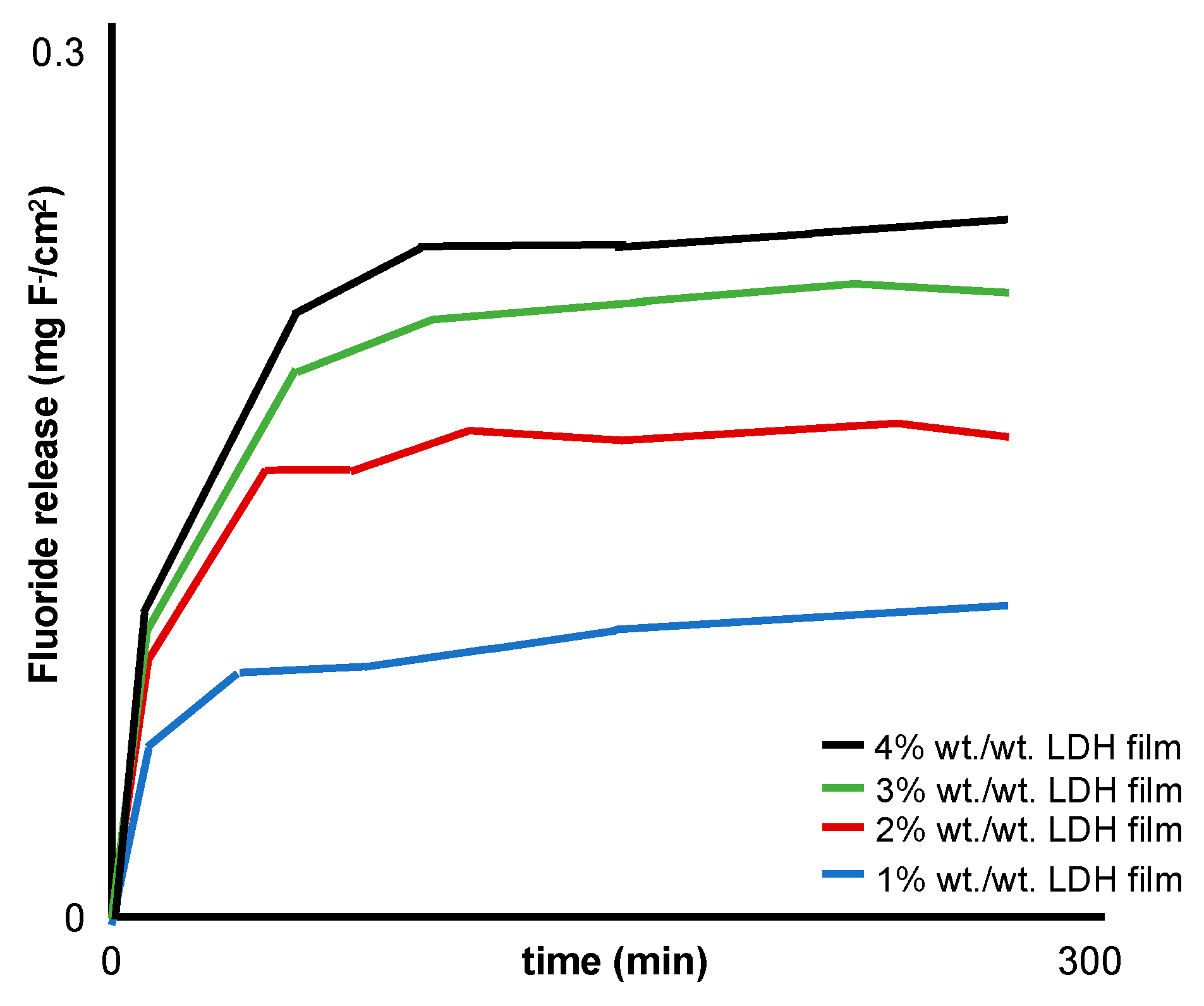
| Perioli et al. [30] | MgAl 2:1 | Muco-adhesive patches: sodium carboxy methyl cellulose, polycarbophil propylene glycol, de-ionised water | Circular films (diameter 25 mm) adhered to a Teflon cell with 100 mL of 1.2 mM NaHCO3 water (37 ± 0.1 °C) agitated at 60 rpm.
|
| LDH-fluoride (1–4% w/w) in a hydrophilic buccal mucoadhesive (2 cm2) attached to the gum of five healthy volunteers:
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoxha, A.; Gillam, D.G.; Bushby, A.J.; Agha, A.; Patel, M.P. Layered Double Hydroxide Fluoride Release in Dental Applications: A Systematic Review. Dent. J. 2019, 7, 87. https://doi.org/10.3390/dj7030087
Hoxha A, Gillam DG, Bushby AJ, Agha A, Patel MP. Layered Double Hydroxide Fluoride Release in Dental Applications: A Systematic Review. Dentistry Journal. 2019; 7(3):87. https://doi.org/10.3390/dj7030087
Chicago/Turabian StyleHoxha, Agron, David G. Gillam, Andy J. Bushby, Amani Agha, and Mangala P. Patel. 2019. "Layered Double Hydroxide Fluoride Release in Dental Applications: A Systematic Review" Dentistry Journal 7, no. 3: 87. https://doi.org/10.3390/dj7030087
APA StyleHoxha, A., Gillam, D. G., Bushby, A. J., Agha, A., & Patel, M. P. (2019). Layered Double Hydroxide Fluoride Release in Dental Applications: A Systematic Review. Dentistry Journal, 7(3), 87. https://doi.org/10.3390/dj7030087








