Microbiological and Clinical Aspects of Cervicofacial Actinomyces Infections: An Overview
Abstract
1. Introduction, Taxonomy
2. Epidemiology, Clinical Presentation
3. Pathogenesis of the Infection
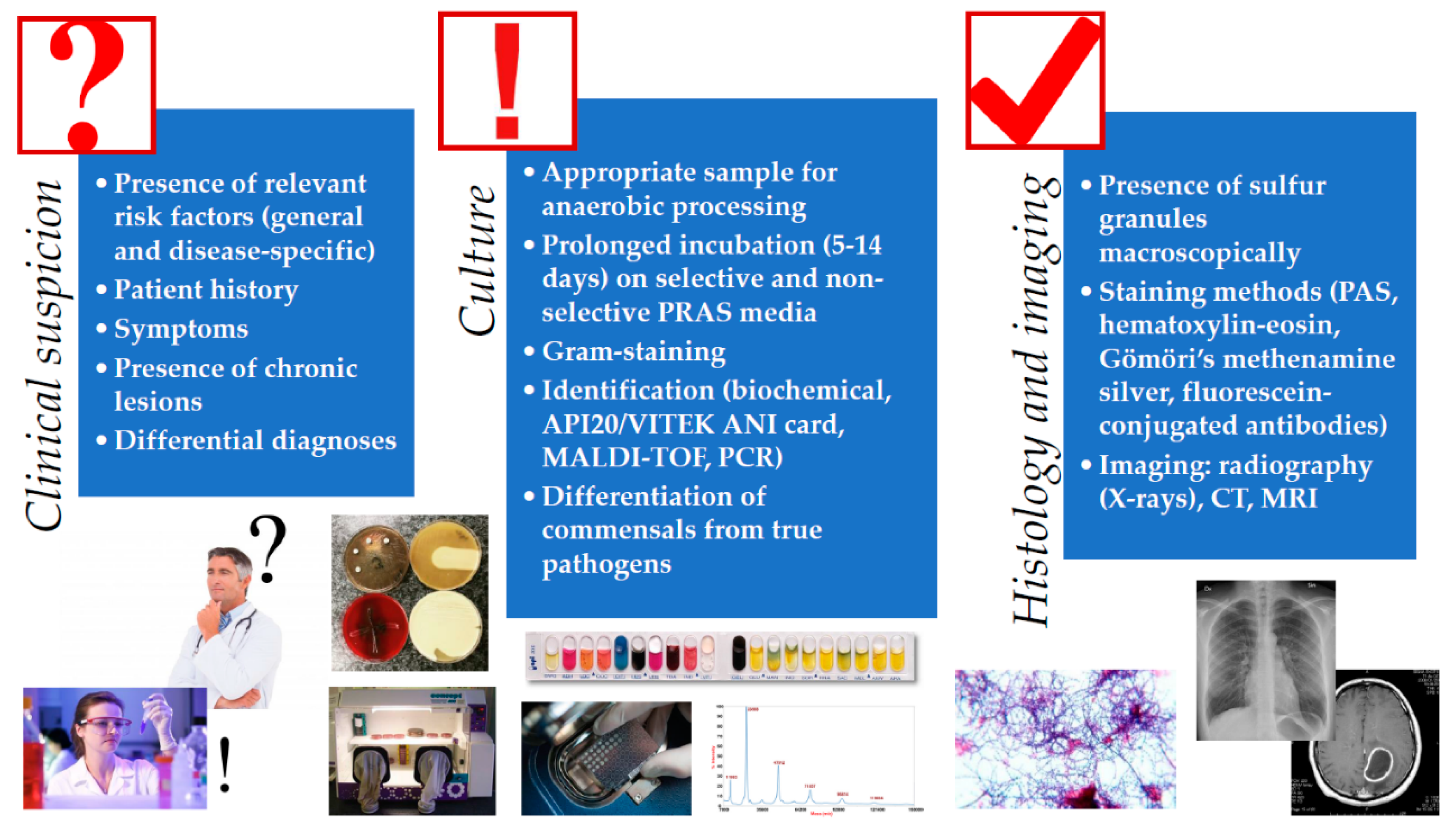
4. Isolation and Identification of Actinomyces spp. from Clinical Specimen
5. Therapy of Actinomycoses
6. Conclusions
7. Literature Search
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AIDS | acquired immunodeficiency syndrome |
| CNS | central nervous system |
| CRP | C-reactive protein |
| CT | computer tomography |
| DNA | deoxyribonucleic acid |
| ESR | erythrocyte sedimentation rate |
| G+C | guanine-cytosine content |
| GPAC | Gram-positive anaerobic cocci |
| HIV | human immunodeficiency virus |
| HMP | Human Microbiome Project |
| IUD | intrauterine device |
| MALDI-TOF MS | matrix-assisted laser desorption/ionization time-of flight mass spectrometry |
| MRI | magnetic resonance imaging |
| NSAID | non-steroidal anti-inflammatory drugs |
| NGS | next-generation sequencing |
| NIH | National Institutes of Health |
| PCR | polymerase chain reaction |
| PMN | polymorphonuclear neutrophils |
| PRAS | pre-reduced anaerobically sterilized |
| WHO | World Health Organization |
References
- Könönen, E.; Wade, W.G. Actinomyces and related organisms in human infections. Clin. Microbiol. Rev. 2015, 28, 419–442. [Google Scholar] [CrossRef] [PubMed]
- Finegold, S.M. Anaerobic infections: General concepts. In Principles and Practice of Infectious Diseases; Mandell, G.L., Bennett, J.E., Dolin, R., Eds.; Churchill Livingstone: London, UK, 2000; Volume 2. [Google Scholar]
- Zhao, K.; Li, W.; Kang, C.; Du, L.; Huang, T.; Zhang, X.; Wu, M.; Yue, B. Phylogenomics and evolutionary dynamics of the family Actinomycetaceae. Genome Biol. Evol. 2014, 6, 2625–2633. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, K.; Nord, C.E. Numerical taxonomy and laboratory identification of Actinomyces and Arachnia and some related bacteria. J. Gen. Microbiol. 1975, 91, 17–44. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boyanova, L.; Kolarov, R.; Mateva, L.; Markovska, R.; Mitov, I. Actinomycosis: A frequently forgotten disease. Future Microbiol. 2015, 10, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.R.; Baron, E.J.; Jorgensen, J.H.; Landry, M.L.; Pfaller, M.A. (Eds.) Manual of Clinical Microbiology, 9th ed.; American Society for Microbiology: Washington, DC, USA, 2007; Volume 1, ISBN 978-1-55581-371-0. [Google Scholar]
- Sullivan, D.C.; Chapman, S.W. Bacteria that masquerade as fungi: Actinomycosis/nocardia. Proc. Am. Thorac. Soc. 2010, 7, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Thukral, R.; Shrivastav, K.; Mathur, V.; Barodiya, A.; Shrivastav, S. Actinomyces: A deceptive infection of oral cavity. J. Korean Assoc. Oral Maxillofac. Surg. 2017, 43, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Bowden, G.H.W. Actinomyces, Propionibacterium propionicus, and Streptomyces. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; ISBN 978-0-9631172-1-2. [Google Scholar]
- Nagy, E.; Boyanova, L.; Justesen, U.S. How to isolate, identify and determine antimicrobial susceptibility of anaerobic bacteria in routine laboratories. Clin. Microbiol. Infect. 2018, 24, 1139–1148. [Google Scholar] [CrossRef]
- Pulverer, G.; Schütt-Gerowitt, H.; Schaal, K.P. Human cervicofacial actinomycoses: Microbiological data for 1997 cases. Clin. Infect. Dis. 2003, 37, 490–497. [Google Scholar] [CrossRef]
- Evaldson, G.; Heimdahl, A.; Kager, L.; Nord, C.E. The normal human anaerobic microflora. Scand. J. Infect. Dis. Suppl. 1982, 35, 9–15. [Google Scholar]
- Gajdács, M.; Urbán, E. Epidemiology and species distribution of anaerobic Gram-negative cocci: A 10-year retrospective survey (2008–2017). Acta Pharm. Hung. 2019, 89, 84–87. [Google Scholar] [CrossRef]
- Hall, V. Actinomyces—Gathering evidence of human colonization and infection. Anaerobe 2008, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.; Knight, R.; Gordon, J.I. The human microbiome project: Exploring the microbial part of ourselves in a changing world. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Finegold, S.M. Anaerobic Bacteria in Human Disease; Academic Press: New York, NY, USA, 1977; ISBN 978-0-12-256750-6. [Google Scholar]
- Bitschar, K.; Sauer, B.; Focken, J.; Dehmer, H.; Moos, S.; Konnerth, M.; Schilling, N.A.; Grond, S.; Kalbacher, H.; Kurschus, F.C.; et al. Lugdunin amplifies innate immune responses in the skin in synergy with host- and microbiota-derived factors. Nat. Commun. 2019, 10, 2730. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M.; Spengler, G.; Urbán, E. Identification and Antimicrobial Susceptibility Testing of Anaerobic Bacteria: Rubik’s Cube of Clinical Microbiology? Antibiotics 2017, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Vaulor, F.; Sénéchal, A.; Dupieux, C.; Karsenty, J.; Lustig, S.; Breton, P.; Gleizal, A.; Boussel, L.; Laurent, F.; Braun, E.; et al. Actinomycosis: Etiology, clinical features, diagnosis, treatment, and management. Infect. Drug Res. 2014, 7, 183–197. [Google Scholar]
- Leber, A.L. Clinical Microbiology Procedures Handbook, 4th ed.; ASM Press: Washington, DC, USA, 2016; ISBN 978-1-55581-880-7. [Google Scholar]
- Oostman, O.; Smego, R.A. Cervicofacial Actinomycosis: Diagnosis and Management. Curr. Infect. Dis. Rep. 2005, 7, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Clérigo, V.; Fernandes, L.; Feliciano, A.; Carvalho, L. A rare case of Actinomyces meyeri empyema: Still a challenging entity to manage. Respir. Med. Case Rep. 2017, 22, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Crossman, T.; Herold, J. Actinomycosis of the maxilla—A case report of a rare oral infection presenting in general dental practice. Br. Dent. J. 2009, 206, 201–202. [Google Scholar] [CrossRef]
- Garner, J.P.; Macdonald, M.; Kumar, P.K. Abdominal actinomycosis. Int. J. Surg. 2007, 5, 441–448. [Google Scholar] [CrossRef]
- Grzywa-Celińska, A.; Emeryk-Maksymiuk, J.; Szmygin-Milanowska, K.; Czekajska-Chehab, E.; Milanowski, J. Pulmonary actinomycosis—The great imitator. Ann. Agric. Environ. Med. 2017, 25, 211–212. [Google Scholar] [CrossRef]
- Matsuda, K.; Nakajima, H.; Khan, K.N.; Tanigawa, T.; Hamaguchi, D.; Kitajima, M.; Hiraki, K.; Moriyama, S.; Masuzaki, H. Preoperative diagnosis of pelvic actinomycosis by clinical cytology. Int. J. Womens Health 2012, 4, 527–533. [Google Scholar] [PubMed]
- Kim, S.R.; Jung, L.Y.; Oh, I.-J.; Kim, Y.-C.; Shin, K.-C.; Lee, M.K.; Yang, S.-H.; Park, H.S.; Kim, M.-K.; Kwak, J.Y.; et al. Pulmonary actinomycosis during the first decade of 21st century: Cases of 94 patients. BMC Infect. Dis. 2013, 13, 216. [Google Scholar] [CrossRef] [PubMed]
- Palmitessa, V.; Cuppone, R.; Monno, R.; Fumarola, L.; Lippolis, A. A case report of esophageal actinomycosis in an immunocompetent patient and review of the literature. New Microbiol. 2019, 42, 55–60. [Google Scholar] [PubMed]
- Reichenbach, J.; Lopatin, U.; Mahlaoui, N.; Beovic, B.; Siler, U.; Zbinden, R.; Seger, R.A.; Galmiche, L.; Brousse, N.; Kayal, S.; et al. Actinomyces in Chronic Granulomatous Disease: An Emerging and Unanticipated Pathogen. Clin. Infect. Dis. 2009, 49, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Zhou, Y.; Wang, C.; Wu, B.; Wan, J. Actinomyces and Alimentary Tract Diseases: A Review of Its Biological Functions and Pathology. BioMed Res. Int. 2018, 2018, 3820215. [Google Scholar] [CrossRef] [PubMed]
- Slutzker, A.D.; Claypool, W.D. Pericardial actinomycosis with cardiac tamponade from a contiguous thoracic lesion. Thorax 1989, 44, 442–443. [Google Scholar] [CrossRef][Green Version]
- Gajdács, M.; Urbán, E. The relevance of anaerobic bacteria in brain abscesses: A ten-year retrospective analysis (2008–2017). Infect. Dis. 2019, 51, 1–3. [Google Scholar] [CrossRef]
- Clancy, U.; Ronayne, A.; Prentice, M.B.; Jackson, A. Actinomyces meyeri brain abscess following dental extraction. BMJ Case Rep. 2015, 2015, bcr2014207548. [Google Scholar] [CrossRef]
- Hwang, C.S.; Lee, H.; Hong, M.P.; Kim, J.H.; Kim, K.-S. Brain abscess caused by chronic invasive actinomycosis in the nasopharynx. Medicine 2018, 97, e0406. [Google Scholar] [CrossRef]
- Vazquez Guillamet, L.J.; Malinis, M.F.; Meyer, J.P. Emerging role of Actinomyces meyeri in brain abscesses: A case report and literature review. IDCases 2017, 10, 26–29. [Google Scholar] [CrossRef]
- Fan, G.; Gu, J.; He, S.; Cai, X. Comprehensive management of cervical epidural spinal abscess followed by brain abscesses: A life-threatening and tortuous case. Int. J. Clin. Exp. Med. 2016, 9, 16867–16872. [Google Scholar]
- Benítez-Páez, A.; Álvarez, M.; Belda-Ferre, P.; Rubido, S.; Mira, A.; Tomás, I. Detection of Transient Bacteraemia following Dental Extractions by 16S rDNA Pyrosequencing: A Pilot Study. PLoS ONE 2013, 8, e57782. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, P.B.; Brennan, M.T.; Thornhill, M.; Michalowicz, B.S.; Noll, J.; Bahrani-Mougeot, F.K.; Sasser, H.C. Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. J. Am. Dent. Assoc. 2009, 140, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, P.B.; Brennan, M.T.; Sasser, H.C.; Fox, P.C.; Paster, B.J.; Bahrani-Mougeot, F.K. Bacteremia Associated with Tooth Brushing and Dental Extraction. Circulation 2008, 117, 3118–3125. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.N.R.; Brundin, M.; Sundqvist, G.; Sjögren, U. Building biofilms in vital host tissues: A survival strategy of Actinomyces radicidentis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 106, 595–603. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wong, V.K.; Turmezei, T.D.; Weston, V.C. Actinomycosis. BMJ 2011, 343, d6099. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.M.; Fjeldsøe-Nielsen, H.; Sulim, S.; Kemp, M.; Christensen, J.J. Actinomyces species: A Danish Survey on Human Infections and Microbiological Characteristics. Open Microbiol. J. 2009, 3, 113–120. [Google Scholar] [CrossRef]
- Bonnefond, S.; Catroux, M.; Melenotte, C.; Karkowski, L.; Rolland, L.; Trouillier, S.; Raffray, L. Clinical features of actinomycosis. Medicine 2016, 95, e3923. [Google Scholar] [CrossRef]
- Bulut, G.; Bayram, Y.; Bulut, M.D.; Garça, M.F.; Bayram, İ. Mandibular Actinomyces Infection Mimicking a Malignancy: Case Report. Turk. Patol. Derg. 2017, 33, 256–258. [Google Scholar] [CrossRef]
- Ji, W.; Kwak, J.M.; Kim, J.; Kim, S.H. Actinomycosis of the rectum mimicking a malignant neoplasm. ANZ J. Surg. 2014, 84, 497. [Google Scholar] [CrossRef]
- Gliga, S.; Devaux, M.; Gosset-Woimant, M.; Mompoint, D.; Perrone, C.; Davido, B. Actinomyces graevenitzii pulmonary abscess mimicking tuberculosis in a healthy young man. Can. Resp. J. 2014, 21, e75–e77. [Google Scholar]
- Murphy, E.C.; Frick, I.M. Gram-positive anaerobic cocci—Commensals and opportunistic pathogens. FEMS Microbiol. Rev. 2013, 37, 520–553. [Google Scholar] [CrossRef] [PubMed]
- Tanaka-Bandoh, K.; Watanabe, K.; Kato, N.; Ueno, K. Susceptibilities of Actinomyces species and Propionibacterium propionicus to antimicrobial agents. Clin. Infect. Dis. 1997, 25 (Suppl. 2), S262–S263. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.; Kunkel, M.; Kirkpatrick, C.J.; Weber, A. Actinomyces in infected osteoradionecrosis—Underestimated? Hum. Pathol. 2006, 37, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.; Kunkel, M.; Springer, E.; Walter, C.; Weber, A.; Siegel, E.; Kirkpatrick, C.J. Actinomycosis of the jaws—Histopathological study of 45 patients shows significant involvement in bisphosphonate-associated osteonecrosis and infected osteoradionecrosis. Virchows Arch. 2007, 451, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Støre, G.; Eribe, E.R.K.; Olsen, I. DNA-DNA hybridization demonstrates multiple bacteria in osteoradionecrosis. Int. J. Oral Maxillofac Surg. 2005, 34, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Gallay, L.; Bodard, A.-G.; Chidiac, C.; Ferry, T. Bilateral bisphosphonate-related osteonecrosis of the jaw with left chronic infection in an 82-year-old woman. BMJ Case Rep. 2013, 2013, bcr2013008558. [Google Scholar] [CrossRef]
- Brook, I. The role of anaerobic bacteria in bacteremia. Anaerobe 2010, 16, 183–189. [Google Scholar] [CrossRef]
- Hecht, D.W. Anaerobes: Antibiotic resistance, clinical significance, and the role of susceptibility testing. Anaerobe 2006, 12, 115–121. [Google Scholar] [CrossRef]
- Blinkhorn, R.J.; Strimbu, V.; Effron, D.; Spagnuolo, P.J. “Punch” actinomycosis causing osteomyelitis of the hand. Arch. Intern. Med. 1988, 148, 2668–2670. [Google Scholar] [CrossRef]
- Nair, P.N.R.; Sundqvist, G.; Sjögren, U. Experimental evidence supports the abscess theory of development of radicular cysts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 106, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M. The Concept of an Ideal Antibiotic: Implications for Drug Design. Molecules 2019, 24, 892. [Google Scholar] [CrossRef] [PubMed]
- Sarkonen, N.; Könönen, E.; Eerola, E.; Könönen, M.; Jousimies-Somer, H.; Laine, P. Characterization of Actinomyces species isolated from failed dental implant fixtures. Anaerobe 2005, 11, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Rôças, I.N.; Siqueira, J.F. Antibiotic resistance genes in anaerobic bacteria isolated from primary dental root canal infections. Anaerobe 2012, 18, 576–580. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental plaque as a biofilm and a microbial community—Implications for health and disease. BMC Oral Health 2006, 6, S14. [Google Scholar] [CrossRef] [PubMed]
- Loesche, W.J. Microbiology of dental decay and periodontal disease. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; ISBN 978-0-9631172-1-2. [Google Scholar]
- Lovegrove, J.M. Dental plaque revisited: Bacteria associated with periodontal disease. J. N. Z. Soc. Periodontol. 2004, 87, 7–21. [Google Scholar]
- Pizzo, K.; Arnold, C.; Wispelwey, B. Actinomyces neuii Causing Vertebral Osteomyelitis. Am. J. Med. Sci. 2017, 353, 407–410. [Google Scholar] [CrossRef]
- Shen, J.Y.; Futran, N.D.; Sardesai, M.G. Craniofacial Actinomyces osteomyelitis evolving from sinusitis. Radiol. Case Rep. 2017, 13, 104–107. [Google Scholar] [CrossRef]
- Lewis, R.P.; Sutter, V.L.; Finegold, S.M. Bone infections involving anaerobic bacteria. Medicine 1978, 57, 279–305. [Google Scholar] [CrossRef]
- Sato, T.; Watanabe, K.; Kumada, H.; Toyama, T.; Tani-Ishii, N.; Hamada, N. Peptidoglycan of Actinomyces naeslundii induces inflammatory cytokine production and stimulates osteoclastogenesis in alveolar bone resorption. Arch. Oral Biol. 2012, 57, 1522–1528. [Google Scholar] [CrossRef]
- Jousimies-Somer, H.; Summanen, P.; Citron, D.M.; Baron, E.J.; Wexler, H.M.; Finegold, S.M. KTL Anaerobic Bacteriology Manual, 6th ed.; Jousimies-Somer, H., Summanen, P., Citron, D.M., Baron, E.J., Wexler, H.M., Finegold, S.M., Eds.; Star Publishing Company: Belmont, CA, USA, 2003. [Google Scholar]
- Garg, R.; Kaistha, N.; Gupta, V.; Chander, J. Isolation, Identification and Antimicrobial Susceptibility of Anaerobic Bacteria: A Study Re-emphasizing Its Role. J. Clin. Diag. Res. 2014, 8, DL01-2. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.L.; Schmidt, T.M. Shallow breathing: Bacterial life at low O2. Nat. Rev. Microbiol. 2013, 11, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M. Anaerobes and laboratory automation: Like oil and water? Anaerobe 2019, 59, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Zimbro, M.J.; Power, D.A.; Miller, S.M.; Wilson, G.E.; Johnson, J.A. (Eds.) Manual of Microbiological Culture Media, 2nd ed.; BD Diagnostics—Diagnostic Systems; Becton, Dickinson and Company: Sparks, MD, USA, 2009; ISBN 978-0-9727207-1-7. [Google Scholar]
- Gajdács, M.; Paulik, E.; Szabó, A. The opinions of community pharmacists related to antibiotic use and resistance. Acta Pharm. Hung. 2018, 88, 249–252. (In Hungarian) [Google Scholar]
- Kalfas, S.; Edwardsson, S. Identification procedures for oral Actinomyces species. Oral Microbiol. Immunol. 1990, 5, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Jamal, W.Y.; Shahin, M.; Rotimi, V.O. Comparison of two matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry methods and API 20AN for identification of clinically relevant anaerobic bacteria. J. Med. Microbiol. 2013, 62, 540–544. [Google Scholar] [CrossRef]
- Sondag, J.E.; Ali, M.; Murray, P.R. Rapid presumptive identification of anaerobes in blood cultures by gas-liquid chromatography. J. Clin. Microbiol. 1980, 11, 274–277. [Google Scholar]
- Schreckenberger, P.C.; Blazevic, D.J. Rapid methods for biochemical testing of anaerobic bacteria. Appl. Microbiol. 1974, 28, 759–762. [Google Scholar]
- Nagy, E.; Becker, S.; Kostrzewa, M.; Barta, N.; Urban, E. The value of MALDI-TOF MS for the identification of clinically relevant anaerobic bacteria in routine laboratories. J. Med. Microbiol. 2012, 61, 1393–1400. [Google Scholar] [CrossRef]
- Kuyama, K.; Fukui, K.; Ochiai, E.; Maruyama, S.; Iwadate, K.; Saku, T.; Yamamoto, H. Identification of the actinomycete 16S ribosomal RNA gene by polymerase chain reaction in oral inflammatory lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 485–491. [Google Scholar] [CrossRef]
- Nagy, E.; Maier, T.; Urban, E.; Terhes, G.; Kostrzewa, M.; ESCMID Study Group on Antimicrobial Resistance in Anaerobic Bacteria. Species identification of clinical isolates of Bacteroides by matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry. Clin. Microbiol. Infect. 2009, 15, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, T.; Ross, P.L.; Rajamani, U. Detection of pathogenic and non-pathogenic bacteria by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectr. 1996, 10, 883–888. [Google Scholar] [CrossRef]
- Croxatto, A.; Prod’hom, G.; Greub, G. Applications of MALDI-TOF mass spectrometry in clinical diagnostic microbiology. FEMS Microbiol. Rev. 2012, 36, 380–407. [Google Scholar] [CrossRef] [PubMed]
- Seng, P.; Drancourt, M.; Gouriet, F.; La Scola, B.; Fournier, P.-E.; Rolain, J.M.; Raoult, D. Ongoing revolution in bacteriology: Routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Infect. Dis. 2009, 49, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Patel, R. MALDI-TOF MS for the diagnosis of infectious diseases. Clin. Chem. 2015, 61, 100–111. [Google Scholar] [CrossRef]
- Shannon, S.; Kronemann, D.; Patel, R.; Schuetz, A.N. Routine use of MALDI-TOF MS for anaerobic bacterial identification in clinical microbiology. Anaerobe 2018, 54, 191–196. [Google Scholar] [CrossRef]
- Veloo, A.C.; Knoester, M.; Degener, J.E.; Kuijper, E.J. Comparison of two matrix-assisted laser desorption ionisation-time of flight mass spectrometry methods for the identification of clinically relevant anaerobic bacteria. Clin. Microbiol. Infect. 2011, 17, 1501–1506. [Google Scholar] [CrossRef]
- Lynch, T.; Gregson, D.; Church, D.L. Species-Level Identification of Actinomyces Isolates Causing Invasive Infections: Multiyear Comparison of Vitek MS (Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry) to Partial Sequencing of the 16S rRNA Gene. J. Clin. Microbiol. 2016, 54, 712–717. [Google Scholar] [CrossRef]
- Veloo, A.C.; Erhard, M.; Welker, M.; Welling, G.W.; Degener, J.E. Identification of Gram-positive anaerobic cocci by MALDI-TOF mass spectrometry. Syst. Appl. Microbiol. 2011, 34, 58–62. [Google Scholar] [CrossRef]
- Ng, L.S.Y.; Sim, J.H.C.; Eng, L.C.; Menon, S.; Tan, T.Y. Comparison of phenotypic methods and matrix-assisted laser desorption ionisation time-of-flight mass spectrometry for the identification of aero-tolerant Actinomyces spp. isolated from soft-tissue infections. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1749–1752. [Google Scholar] [CrossRef]
- Fong, P.; Francis, M.J.; Hamblin, J.F.; Korman, T.M.; Graham, M. Identification and diversity of Actinomyces species in a clinical microbiology laboratory in the MALDI-TOF MS era. Anaerobe 2018, 54, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.H.; Shin, S.S.; Kim, J.W.; Lim, H.S.; Seon, H.J.; Jung, S.-I.; Jeong, Y.Y.; Kang, H.K. Imaging of actinomycosis in various organs: A comprehensive review. Radiographics 2014, 34, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Lan, L.; Feng, Y.; Huang, Z.; Chen, Y. Pulmonary Actinomycosis Imitating Lung Cancer on (18)F-FDG PET/CT: A Case Report and Literature Review. Korean J. Radiol. 2015, 16, 1262–1265. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Kaneda, T.; Uyeda, J.W.; Okada, H.; Sekiya, K.; Suemitsu, M.; Sakai, O. Actinomycosis in the Mandible: CT and MR Findings. Am. J. Neuroradiol. 2014, 35, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Soneja, M.; Batra, A.; Vikram, N.K.; Ahuja, A.; Mohan, A.; Sood, R. Actinomycosis and nocardiosis co-infection in chronic granulomatous disease. J. Assoc. Physicians India 2012, 60, 66–68. [Google Scholar] [PubMed]
- Nagy, E. Anaerobic Infections Update on Treatment Considerations. Drugs 2010, 70, 841–858. [Google Scholar] [CrossRef] [PubMed]
- Shinn, D.L.S. Metronidazole in acute ulcerative gingivitis. Lancet 1962, 279, 1191. [Google Scholar] [CrossRef]
- Alauzet, C.; Lozniewski, A.; Marchandin, H. Metronidazole resistance and nim genes in anaerobes: A review. Anaerobe 2019, 55, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Bryan, L.E.; Kowand, S.K.; Van Den Elzen, H.M. Mechanism of aminoglycoside antibiotic resistance in anaerobic bacteria: Clostridium perfringens and Bacteroides fragilis. Antimicrob. Agents Chemother. 1979, 15, 7–13. [Google Scholar] [CrossRef]
- Stein, G.E.; Goldstein, E.J.C. Fluoroquinolones and anaerobes. Clin. Infect. Dis. 2006, 42, 1598–1607. [Google Scholar] [CrossRef]
- Nagy, E.; Urbán, E.; Nord, C.E.; ESCMID Study Group on Antimicrobial Resistance in Anaerobic Bacteria. Antimicrobial susceptibility of Bacteroides fragilis group isolates in Europe: 20 years of experience. Clin. Microbiol. Infect. 2011, 17, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M. Intravenous or oral antibiotic therapy: Sophie’s choice? Gen. Int. Med. Clin. Innov. 2019, 4, 1–2. [Google Scholar]
- Martin, M.V. Antibiotic treatment of cervicofacial actinomycosis for patients allergic to penicillin: A clinical and in vitro study. Br. J. Oral Maxillofac. Surg. 1985, 23, 428–434. [Google Scholar] [CrossRef]
- Moghimi, M.; Salentijn, E.; Debets-Ossenkop, Y.; Karagozoglu, K.H.; Forouzanfar, T. Treatment of cervicofacial actinomycosis: A report of 19 cases and review of literature. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e627–e632. [Google Scholar] [CrossRef] [PubMed]
- Kolditz, M.; Bickhardt, J.; Matthiessen, W.; Holotiuk, O.; Höffken, G.; Koschel, D. Medical management of pulmonary actinomycosis: Data from 49 consecutive cases. J. Antimicrob. Chemother. 2009, 63, 839–841. [Google Scholar] [CrossRef] [PubMed]
- Atad, J.; Hallak, M.; Sharon, A.; Kitzes, R.; Kelner, Y.; Abramovici, H. Pelvic actinomycosis: Is long term antibiotic therapy necessary? J. Reprod. Med. 1999, 44, 939–944. [Google Scholar] [PubMed]
- Sudhakar, S.S.; Ross, J.J. Short-term treatment of actinomycosis: Two cases and a review. Clin. Infect. Dis. 2004, 38, 444–447. [Google Scholar] [CrossRef]
- Shah, K.M.; Karagir, A.; Kanitkar, S.; Koppikar, R. An atypical form of cervicofacial actinomycosis treated with short but intensive antibiotic regimen. BMJ Case Rep. 2013, 2013, bcr2013008733. [Google Scholar] [CrossRef]
- Barberis, C.; Budia, M.; Palombarani, S.; Rodriguez, C.H.; Ramírez, M.S.; Arias, B.; Bonofiglio, L.; Famiglietti, A.; Mollerach, M.; Almuzara, M.; et al. Antimicrobial susceptibility of clinical isolates of Actinomyces and related genera reveals an unusual clindamycin resistance among Actinomyces urogenitalis strains. J. Glob. Antimicrob. Resist. 2017, 8, 115–120. [Google Scholar] [CrossRef]
- Smith, A.J.; Hall, V.; Thakker, B.; Gemmell, C.G. Antimicrobial susceptibility testing of Actinomyces species with 12 antimicrobial agents. J. Antimicrob. Chemother. 2005, 56, 407–409. [Google Scholar] [CrossRef]
- Wexler, H.M.; Molitoris, E.; Murray, P.R.; Washington, J.; Zabransky, R.J.; Edelstein, P.H.; Finegold, S.M. Comparison of spiral gradient endpoint and agar dilution methods for susceptibility testing of anaerobic bacteria: A multilaboratory collaborative evaluation. J. Clin. Microbiol. 1996, 34, 170–174. [Google Scholar] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Available online: https://clsi.org/standards/products/microbiology/ (accessed on 10 September 2017).
- Tietz, A.; Aldridge, K.E.; Figueroa, J.E. Disseminated Coinfection with Actinomyces graevenitzii and Mycobacterium tuberculosis: Case Report and Review of the Literature. J. Clin. Microbiol. 2005, 43, 3017–3022. [Google Scholar] [CrossRef] [PubMed]
| A. bovis | A. graevenitzii | A. nasicola | A. radingae |
| A. cardiffiensis | A. hominis | A. neuii | A. timonensis |
| A. dentalis | A. hongkongiensis | A. odontolyticus | A. turicensis |
| A. europaeus | A. israelii | A. oris | A. urogenitalis |
| A. funkei | A. massiliensis | A. oricola | A. viscous |
| A. georgiae | A. meyeri | A. pyogenes | several novel geno-species |
| A. gerencseriae | A. naeslundii | A. radicidentis |
| Cervicofacial (including Central Nervous System (CNS)) | 40–60% |
| Affected areas: | |
| Upper and lower mandibles | 50% |
| Cheeks | 10–15% |
| Chin | 10–15% |
| Submaxillary ramus and angle, mandibular joints | 5–10% |
| CNS (brain abscess, meningitis, meningoencephalitis, epidural abscess, subdural empyema) | 5–10% |
| Tongue, sinuses, middle ear, larynx, lachrymal pathways, and thyroid gland | 0–5% |
| Abdominal | 20–30% |
| Thoracic (incl. pulmonary) | 20–25% |
| Pelvic | 3–5% |
| Cutaneous | 3–5% |
| General | Disease-Specific |
|---|---|
| Human Immunodeficiency Virus (HIV) infection or manifest AIDS | Erupting secondary teeth |
| Hematological malignancies or solid tumors | Poor dental hygiene |
| Organ transplantation (especially in case of the kidneys and lungs) | Dental caries |
| Use of monoclonal antibodies (e.g., anti-tumor necrosis-α-inhibitors, infliximab, etanercept) | Gingivitis |
| Cancer chemotherapy | Mucositis |
| Corticosteroid use | Dental extraction |
| Malnutrition | Introduction of dental implants |
| Diabetes | Cervicofacial surgery |
| Alcoholism | Traumatic injury |
| Smoking and/or inhalation of particles | Bisphosphonate therapy |
| Low socio-economic status | Radiation therapy |
| Seizure disorders | |
| Crohn’s disease | |
| Hereditary diseases (e.g., hereditary hemorrhagic telangiectasia, chronic granulomatous disease) | |
| Use of non-steroid anti-inflammatory drugs (NSAIDs) |
| Antibiotic Therapy | |
| Should be Considered | Should NOT be Considered |
| Penicillin G | Metronidazole * |
| Penicillin V | Aminoglycosides * |
| Ampicillin | Other β-lactam-β-lactamase-inhibitor combinations * |
| Amoxicillin | Penicillinase-stable penicillins |
| Piperacillin/tazobactam | First generation cephalosporins (cephalexin) |
| Second generation cephalosporins with anti-anaerobic activity (cefoxitin) | Aztreonam * |
| Third generation cephalosporins (ceftriaxone) | Sulfamethoxazole/trimethoprim * |
| Carbapenems | Fluoroquinolones * |
| Doxycycline | |
| Tigecycline | |
| Clindamycin | |
| Macrolides | |
| Chloramphenicol | |
| Adjunctive Therapy | |
| Surgical debridement, drainage | |
| Photodynamic therapy | |
| Laser therapy | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajdács, M.; Urbán, E.; Terhes, G. Microbiological and Clinical Aspects of Cervicofacial Actinomyces Infections: An Overview. Dent. J. 2019, 7, 85. https://doi.org/10.3390/dj7030085
Gajdács M, Urbán E, Terhes G. Microbiological and Clinical Aspects of Cervicofacial Actinomyces Infections: An Overview. Dentistry Journal. 2019; 7(3):85. https://doi.org/10.3390/dj7030085
Chicago/Turabian StyleGajdács, Márió, Edit Urbán, and Gabriella Terhes. 2019. "Microbiological and Clinical Aspects of Cervicofacial Actinomyces Infections: An Overview" Dentistry Journal 7, no. 3: 85. https://doi.org/10.3390/dj7030085
APA StyleGajdács, M., Urbán, E., & Terhes, G. (2019). Microbiological and Clinical Aspects of Cervicofacial Actinomyces Infections: An Overview. Dentistry Journal, 7(3), 85. https://doi.org/10.3390/dj7030085






