Human Gingival Crevicular Fluids (GCF) Proteomics: An Overview
Abstract
:1. Introduction
2. GCF as a Diagnostic Tool for Analysis of Oral Diseases
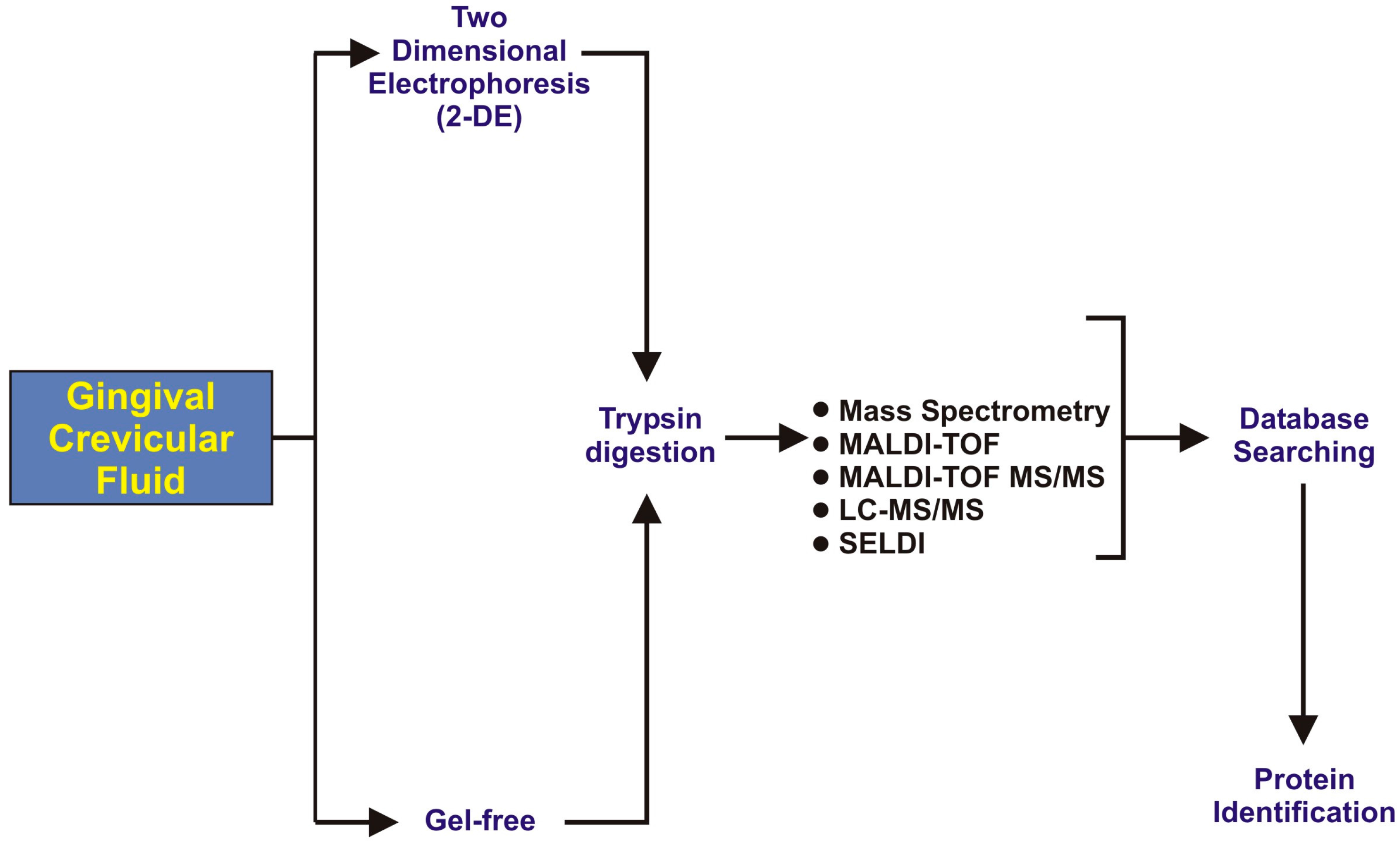
3. GCF Proteomic Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Duarte, T.; Spencer, C. Personalized Proteomics: The Future of Precision Medicine. Proteomes 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Naseem, M.; Sheikh, Z.; Najeeb, S.; Shahab, S.; Zafar, M.S. Oral antimicrobial peptides: Types and role in the oral cavity. Saudi Pharm. J. 2016, 24, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Brill, N.; Björn, H. Passage of Tissue Fluid Into Human Gingival Pockets. Acta Odontol. Scand. 1959, 17, 11–21. [Google Scholar] [CrossRef]
- Waerhaug, J. The Source of Mineral Salts in Subgingival Calculus. J. Dent. Res. 1955, 34, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Waerhaug, J.; Steen, E. The presence or absence of bacteria in gingival pockets and the reaction in healthy pockets to certain pure cultures; a bacteriological and histological investigation. Odontol. Tidskr. 1952, 60, 1–24. [Google Scholar] [PubMed]
- Alfano, M.C. The origin of gingival fluid. J. Theor. Biol. 1974, 47, 127–136. [Google Scholar] [CrossRef]
- Lin, P.H.; Yeh, S.K.; Huang, W.C.; Chen, H.Y.; Chen, C.H.; Sheu, J.R.; Lin, C.T.; Huang, Y.K. Research performance of biomarkers from biofluids in periodontal disease publications. J. Dent. Sci. 2015, 10, 61–67. [Google Scholar] [CrossRef]
- Lamster, I.B. Evaluation of components of gingival crevicular fluid as diagnostic tests. Ann. Periodontol. 1997, 2, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Barros, S.P.; Williams, R.; Offenbacher, S.; Morelli, T. Gingival crevicular fluid as a source of biomarkers for periodontitis. Periodontol. 2000 2016, 70, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Challacombe, S.J.; Russell, M.W.; Hawkes, J. Passage of intact IgG from plasma to the oral cavity via crevicular fluid. Clin. Exp. Immunol. 1978, 34, 417–422. [Google Scholar] [PubMed]
- Cimasoni, G. Crevicular fluid updated. Monogr. Oral Sci. 1983, 12, 1–152. [Google Scholar]
- Egelberg, J. The blood vessels of the dento-gingival junction. J. Periodontal Res. 1966, 1, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Egelberg, J. Permeability of the dento-gingival blood vessels. J. Periodontal Res. 1966, 1, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, S.; Satoh, M.; Umemura, H.; Sogawa, K.; Kawashima, Y.; Kado, S.; Sawai, S.; Nishimura, M.; Kodera, Y.; Matsushita, K.; et al. Proteomic analysis of gingival crevicular fluid for discovery of novel periodontal disease markers. Proteomics 2012, 12, 2190–2202. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Najeeb, S.; Mali, M.; Moin, S.F.; Raza, S.Q.; Zohaib, S.; Sefat, F.; Zafar, M.S. Histatin peptides: Pharmacological functions and its applications in dentistry. Saudi Pharm. J. 2017, 25, 25–31. [Google Scholar] [CrossRef]
- Khurshid, Z.; Zohaib, S.; Najeeb, S.Z.M. Proteomics Advancements in Dentistry. J. Dent. Health Oral. Disord. Ther. 2016, 4, 00104. [Google Scholar]
- Tsuchida, S.; Satoh, M.; Sogawa, K.; Kawashima, Y.; Kado, S.; Ishige, T.; Beppu, M.; Sawai, S.; Nishimura, M.; Kodera, Y.; et al. Application of proteomic technologies to discover and identify biomarkers for periodontal diseases in gingival crevicular fluid: A review. PROTEOMICS Clin. Appl. 2014, 8, 232–240. [Google Scholar] [CrossRef]
- Carneiro, L.G.; Venuleo, C.; Oppenheim, F.G.; Salih, E. Proteome data set of human gingival crevicular fluid from healthy periodontium sites by multidimensional protein separation and mass spectrometry. J. Periodontal Res. 2012, 47, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Silva-Boghossian, C.M.; Colombo, A.P.V.; Tanaka, M.; Rayo, C.; Xiao, Y.; Siqueira, W.L. Quantitative proteomic analysis of gingival crevicular fluid in different periodontal conditions. PLoS ONE 2013, 8, e75898. [Google Scholar] [CrossRef] [PubMed]
- Pisano, E.; Cabras, T.; Montaldo, C.; Piras, V.; Inzitari, R.; Olmi, C.; Castagnola, M.; Messana, I. Peptides of human gingival crevicular fluid determined by HPLC-ESI-MS. Eur. J. Oral Sci. 2005, 113, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.S.; Morelli, T.; Oh, M.; Braun, T.M.; Ramseier, C.A.; Sugai, J.V.; Giannobile, W.V. Crevicular fluid biomarkers and periodontal disease progression. J. Clin. Periodontol. 2014, 41, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Huynh, A.H.S.; Veith, P.D.; McGregor, N.R.; Adams, G.G.; Chen, D.; Reynolds, E.C.; Ngo, L.H.; Darby, I.B. Gingival crevicular fluid proteomes in health, gingivitis and chronic periodontitis. J. Periodontal Res. 2015, 50, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Rody, W.J., Jr.; Holliday, L.S.; McHugh, K.P.; Wallet, S.M.; Spicer, V.; Krokhin, O. Mass spectrometry analysis of gingival crevicular fluid in the presence of external root resorption. Am. J. Orthod. Dentofac. Orthop. 2014, 145, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Siddiqui, F.; Qasim, S.B.; Khurshid, Z.; Zohaib, S.; Zafar, M.S. Influence of uncontrolled diabetes mellitus on periodontal tissues during orthodontic tooth movement: A systematic review of animal studies. Prog. Orthod. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Serra, E.; Perinetti, G.; D’Attilio, M.; Cordella, C.; Paolantonio, M.; Festa, F.; Spoto, G. Lactate dehydrogenase activity in gingival crevicular fluid during orthodontic treatment. Am. J. Orthod. Dentofac. Orthop. 2003, 124, 206–211. [Google Scholar] [CrossRef]
- Perinetti, G.; Paolantonio, M.; Attilio, M.D. Alkaline phosphatase activity in gingival crevicular fluid during human orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 2002, 122, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, D.B.; Southard, K.A.; Qian, F.; Marshall, S.D.; Franciscus, R.G.; Southard, T.E. Cervical vertebrae maturation method: Poor reproducibility. Am. J. Orthod. Dentofac. Orthop. 2009, 136, 478.e1–478.e7. [Google Scholar] [CrossRef]
- Wen, X.; Gu, Y.; Chen, F. Gingival Crevicular Fluid as a Novel Potential Source of Biomarkers Distinguishes Pubertal from Post-Pubertal Subjects. Diagnostics 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Kunimatsu, K.; Yamamoto, K.; Ichimaru, E.; Kato, Y.; Kato, I. Cathepsins B, H and L activities in gingival crevicular fluid from Chronic adult periodontitis patients and exprimental gingivitis subjects. J. Periodontal Res. 1990, 25, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Symposium, C.F. Protein Degradation in Health and Disease; John Wiley & Sons: Amterdam, Netherland, 2009. [Google Scholar]
- Baliban, R.C.; Sakellari, D.; Li, Z.; DiMaggio, P.A.; Garcia, B.A.; Floudas, C.A. Novel protein identification methods for biomarker discovery via a proteomic analysis of periodontally healthy and diseased gingival crevicular fluid samples. J. Clin. Periodontol. 2012, 39, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Ngo, L.H.; Darby, I.B.; Veith, P.D.; Locke, A.G.; Reynolds, E.C. Mass spectrometric analysis of gingival crevicular fluid biomarkers can predict periodontal disease progression. J. Periodontal Res. 2013, 48, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, L.G.; Nouh, H.; Salih, E. Quantitative gingival crevicular fluid proteome in health and periodontal disease using stable isotope chemistries and mass spectrometry. J. Clin. Periodontol. 2014, 41, 733–747. [Google Scholar] [CrossRef] [PubMed]
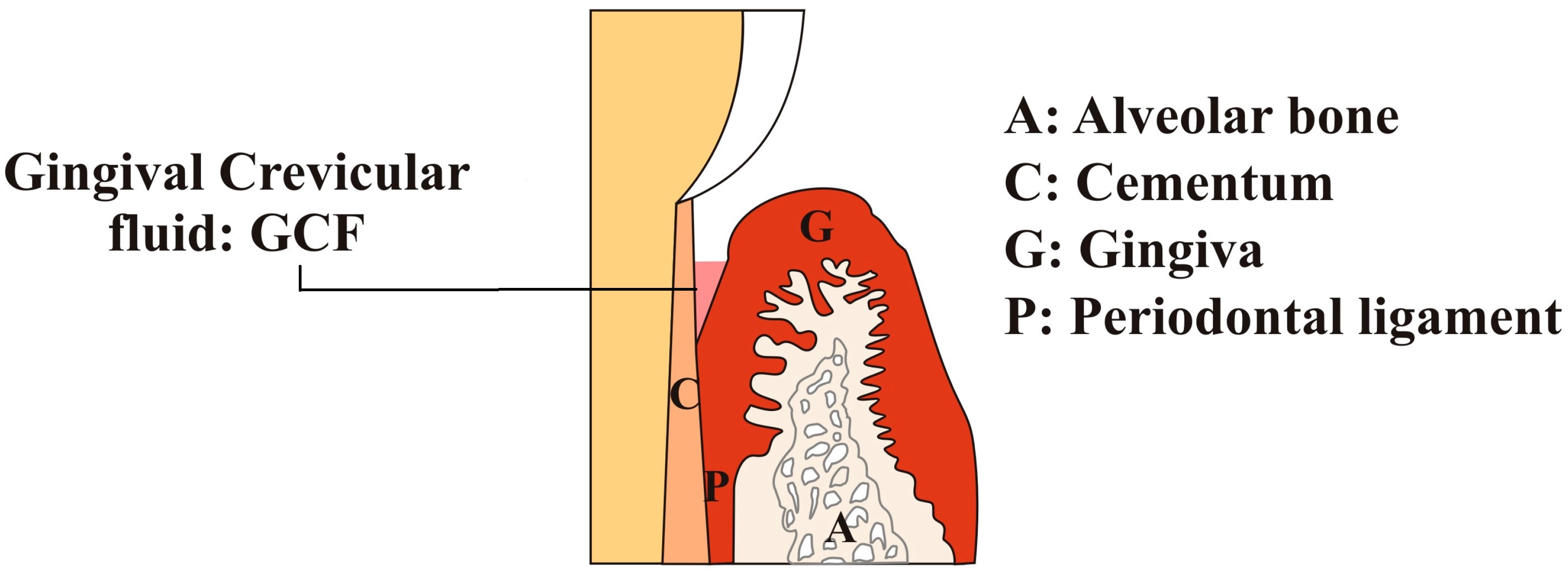
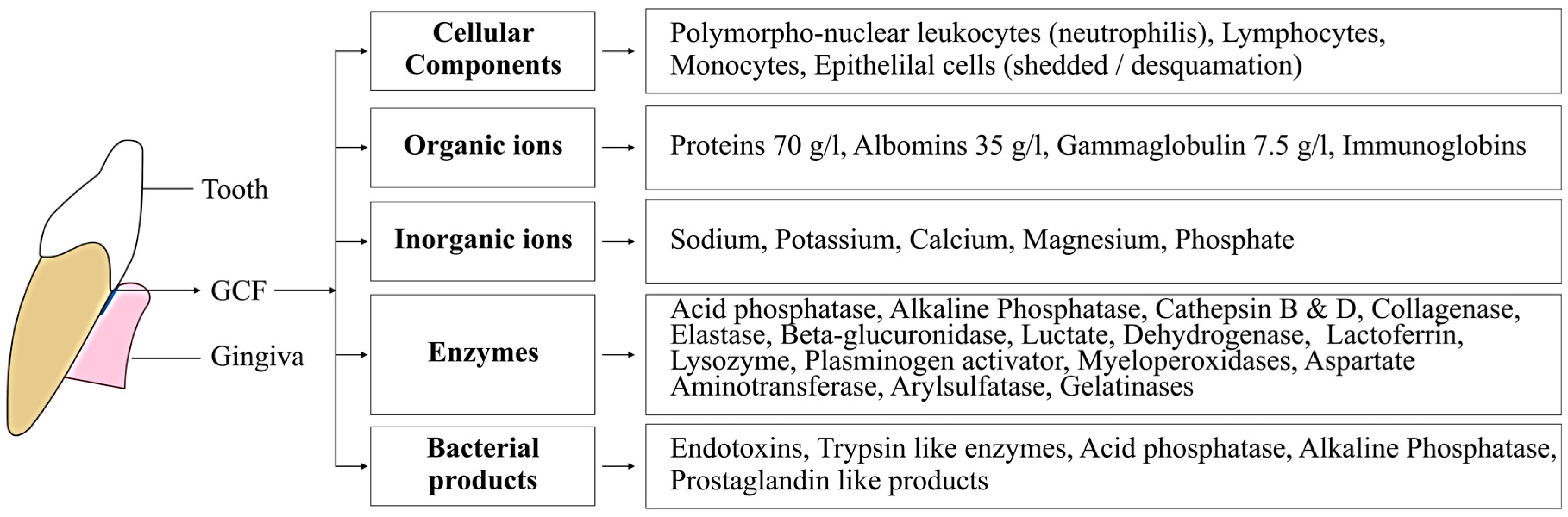
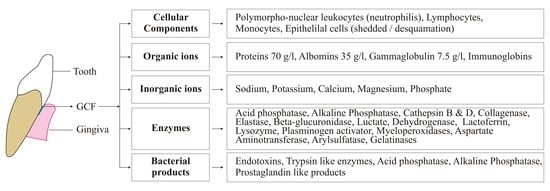
| Factors | Description |
|---|---|
| Mechanical | Chewing coarse foods, vigorous brushing and gingival massage are known to increase GCF production |
| Circadian periodicity | The amount of GCF increases gradually from 6 a.m. to 10 p.m. and it decreases after that |
| Periodontal surgeries | GCF production increases after periodontal surgeries, during the healing period |
| Smoking | Smoking increases GCF flow.
This increase in GCF due to smoking is immediate and transient |
| Author & Year | Sample Collection Sites | Collection Method | Proteomic Tool | Number of Identified Proteins | Outcome of Study | Ref. |
|---|---|---|---|---|---|---|
| Baliban et al., 2012 | Collected from pre-selected sites with probing depth >6 mm and <8 mm in periodontitis patients and for periodontal health from mesio-buccal sites of first molar | Filter strips (Periopapers®, Interstate Drug Exchange, Amityville, NY, USA) | Protein digest with trypsin, HPLC, fragmented analysis with tandem mass spectrometry (MS/MS) | 432 human proteins identified (120 new) | Studied identified novel biomarkers from GCF of periodontal healthy and chronic periodontitis patients | [31] |
| S.Tsuchida et al., 2012 | Labial side of maxillary incisors without crown and restoration | Absorbent paper points (ZIPPERER®, Munich, Germany) | 2DE, SDS-PAGE, Western Blot analysis, HPLC with LTQ-XL, HPLC with LTQ-Orbitrap XL, LC-MS/MS | 327 proteins identified | SOD1 and DCD were significantly ↑ in GCF of periodontal patients | [14] |
| Carneiro et al., 2012 | Healthy gingival sulcus of the second and third molar teeth | Periopapers®, USA | Trypsin digested followed by nano-flow liquid chromatography electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) analysis and enzyme-linked immunosorbent essay (ELISA) for human albumin analysis | 199 proteins identified | Provide protein analysis of healthy periodontium and explore GCF composition with new groups of proteins specific to GCF microenvironment | [18] |
| Ngo et al., 2013 | Five deepest sites and molar sites except mesial surface were excluded | Microcaps (glass micocapillary tubes); Drummed Scientific, Brookmall, PA, USA | Matrix-assisted laser desposition/ionization time-of-flight (MALDI-TOF) mass spectrometry | - | GCF mass spectra could be best for analyzing attachment loss and diagnosis of periodontal diseases | [32] |
| Carina M., et al. 2013 | Chronic Periodontitis patients sample were taken from different sites (5 deep sites, 5 shallow sites with gingivitis, and 4 without bleeding on probing sites) | Periopaper strip (ProFlow Inc. Amityville, NY, USA) | Reversed-phase LC-ESi-MS/MS and ELISA | 230 proteins identified | Concluded marked differences in GCF proteomics in different disease profiles | [33] |
| Carneiro et al., 2014 | The pre-selected specific sites with moderate and severe chronic periodontal disease were defined by pocket depth of 5–7 mm (24 patients) and >7 mm (16 patients) | Periopaper strips (Oraflow, Plainview, NY, USA) | SDS-PAGE, Isotope-Coded-Affinity-Tag (ICAT) labeling, mTRAQ labeling, Nano-LC-ESI-MS/MS, Human Albumin ELISA Kit, and S100-A9 protein quantification by ELISA | 199 proteins Identified | Innovative approach concluded the novel changes in host and microbial derived GCF proteome of periodontal patients | [19] |
| Rody, Jr. et al., 2014 | Collected from a deciduous second molar with radiographic evidence of root resorption on 1 quadrant (experimental site) and from the permanent first molar on the contralateral quadrant (control site) in the same jaw. | Periopaper strips (Oraflow, Plainview, NY, USA) | One dimensional LC-MS and Two dimensional LC-MS | 2789 proteins in control group and 2421 proteins in root resorption group | Mass spectrometry is a useful tool for analyzing external root resorption | [23] |
| Kinney et al., 2014 | Collection from the mesio-buccal aspect of each site (tooth) for up-to 28 teeth per patient. | Methylcellulose strip (Pro Flow, Inc., Amityville, NY, USA) | ELISA and Quantibody Human Cytokine Array | - | This method offers improved patient monitoring and disease control | [21] |
| Huynh et al., 2015 | Collection were chosen based on how well they represented the healthy, gingivitis, and chronic periodontitis inclusion criteria | Glass-microcapillary tube (Drummond Scientific, Brookmall, PA, USA) | One-dimensional Gel Electrophoresis and Nano-LC-ESI-MS | 121 proteins identified | Concluded various biomarkers which differentiate between healthy periodontium, gingivitis, and chronic periodontitis | [22] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khurshid, Z.; Mali, M.; Naseem, M.; Najeeb, S.; Zafar, M.S. Human Gingival Crevicular Fluids (GCF) Proteomics: An Overview. Dent. J. 2017, 5, 12. https://doi.org/10.3390/dj5010012
Khurshid Z, Mali M, Naseem M, Najeeb S, Zafar MS. Human Gingival Crevicular Fluids (GCF) Proteomics: An Overview. Dentistry Journal. 2017; 5(1):12. https://doi.org/10.3390/dj5010012
Chicago/Turabian StyleKhurshid, Zohaib, Maria Mali, Mustafa Naseem, Shariq Najeeb, and Muhammad Sohail Zafar. 2017. "Human Gingival Crevicular Fluids (GCF) Proteomics: An Overview" Dentistry Journal 5, no. 1: 12. https://doi.org/10.3390/dj5010012
APA StyleKhurshid, Z., Mali, M., Naseem, M., Najeeb, S., & Zafar, M. S. (2017). Human Gingival Crevicular Fluids (GCF) Proteomics: An Overview. Dentistry Journal, 5(1), 12. https://doi.org/10.3390/dj5010012