Dental Pulp Stem Cell Recruitment Signals within Injured Dental Pulp Tissue
Abstract
:1. Introduction
2. Migration of DPSC in Injured Pulp Microenvironment
2.1 Soluble Chemotactic Molecules
2.2. Both Chemotaxis and Haptotaxis are Required for DPSC Migration
2.3. Extracellular Matrix Remodeling and DPSC Migration
2.4. SDF-1/CXCR4 Axis in Injured Pulp
3. Influence of Inflammation
4. Influence of Pulp Capping Materials
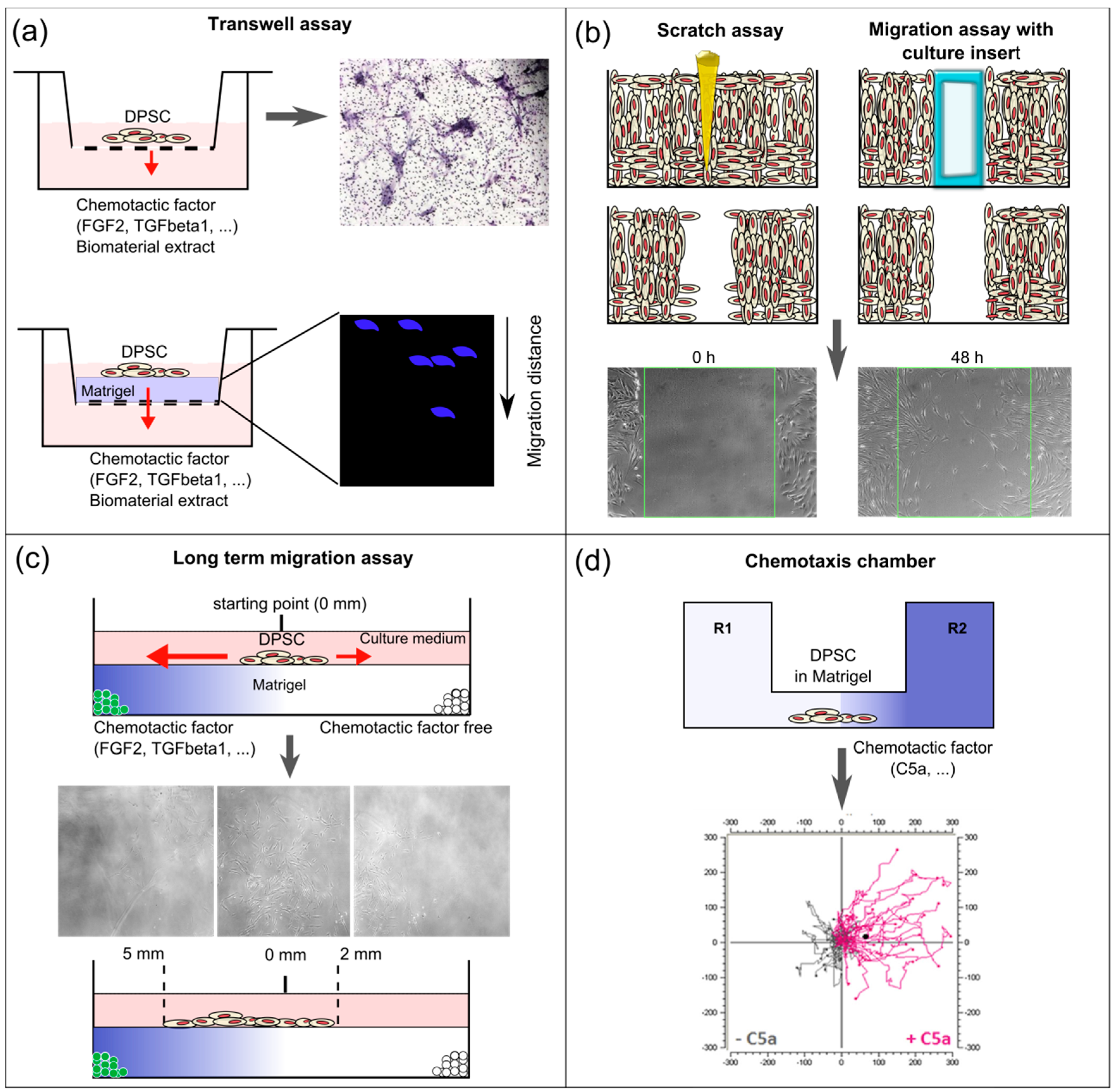
5. Methodologies Used to Study Dental Pulp Stem Cell Recruitment
5.1. In vitro Boyden Chamber (Transwell Assay)
5.2. In vitro Scratch Assay
5.3. Cell Migration Tracking in vivo
5.4. Ex vivo Entire Tooth Culture Reproduces in vivo Migration Conditions
6. Potential Use in Tissue Engineering
7. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| BMP | bone morphogenetic protein |
| BrdU | 5-bromo-20-deoxyuridine |
| BSP | bone sialoprotein |
| CAM | center of mass |
| CXCL14 | chemokine (C-X-C motif) ligand 14 |
| CXCR4 | C-X-C chemokine receptor type 4 |
| DMP-1 | dentin matrix protein-1 |
| DPSC | dental pulp stem cells |
| DAPI | 4’,6-diamidino-2-phenylindole |
| ECM | extracellular matrix |
| EDTA | ethylenediaminetetraacetic acid |
| FAK | focal adhesion kinase |
| FGF-2 | basic fibroblast growth factor |
| G-CSF | granulocyte-colony stimulating factor |
| HEMA | 2-hydroxyethyl methacrylate |
| HGF | hepatocyte growth factor |
| HMGB-1 | high mobility group box 1 |
| LPS | lipopolysaccharide |
| LTA | lipoteichoic acid |
| MAPK/ERK | mitogen-activated protein kinase/extracellular signal-regulated kinases |
| MCP-1 | monocyte chemoattractant protein 1 |
| MMPs | matrix metalloproteinases |
| MMP3 | matrix metalloproteinase-3 |
| MTA | mineral trioxide aggregate |
| OPN | osteopontin |
| PLGA | poly(lactic-co-glycolic acid) |
| RAGE | receptor for advanced glycation end products |
| S1P | sphingosine-1-phosphatase |
| SCAP | dental pulp stem cells of the apical papilla |
| SDF-1 | stromal cell-derived factor 1 |
| SIBLING | small integrin-binding ligand, N-linked glycoproteins |
| TEGDMA | triethylene-glycol- dimethacrylate |
| TGFβ-1 | transforming growth factor β 1 |
| TLR | Toll-like receptor |
| TLR4 | Toll-like receptor 4 |
References
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed]
- About, I.; Bottero, M.J.; de Denato, P.; Camps, J.; Franquin, J.C.; Mitsiadis, T.A. Human dentin production in vitro. Exp. Cell Res. 2000, 258, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Batouli, S.; Miura, M.; Brahim, J.; Tsutsui, T.W.; Fisher, L.W.; Gronthos, S.; Robey, P.G.; Shi, S. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J. Dent. Res. 2003, 82, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Kaukua, N.; Shahidi, M.K.; Konstantinidou, C.; Dyachuk, V.; Kaucka, M.; Furlan, A.; An, Z.; Wang, L.; Hultman, I.; Ahrlund-Richter, L.; et al. Glial origin of mesenchymal stem cells in a tooth model system. Nature 2014, 513, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Gronthos, S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J. Bone Miner. Res. 2003, 18, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Téclès, O.; Laurent, P.; Zygouritsas, S.; Burger, A.-S.; Camps, J.; Dejou, J.; About, I. Activation of human dental pulp progenitor/stem cells in response to odontoblast injury. Arch. Oral Biol. 2005, 50, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Andreas, K.; Sittinger, M.; Ringe, J. Toward in situ tissue engineering: chemokine-guided stem cell recruitment. Trends Biotechnol. 2014, 32, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Chmilewsky, F.; Jeanneau, C.; Laurent, P.; Kirschfink, M.; About, I. Pulp progenitor cell recruitment is selectively guided by a C5a gradient. J. Dent. Res. 2013, 92, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.; Murray, P.E.; Namerow, K.N. Dental pulp stem cell migration. J. Endod. 2010, 36, 1963–1966. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Murakami, M.; Kawamura, R.; Ishizaka, R.; Fukuta, O.; Nakashima, M. CXCL14 and MCP1 are potent trophic factors associated with cell migration and angiogenesis leading to higher regenerative potential of dental pulp side population cells. Stem Cell Res. Ther. 2015, 6, 111. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-W.; Zhang, Y.-F.; Wan, C.-Y.; Sun, Z.-Y.; Nie, S.; Jian, S.-J.; Zhang, L.; Song, G.-T.; Chen, Z. Autophagy in SDF-1α-mediated DPSC migration and pulp regeneration. Biomaterials 2015, 44, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Horibe, H.; Iohara, K.; Hayashi, Y.; Osako, Y.; Takei, Y.; Nakata, K.; Motoyama, N.; Kurita, K.; Nakashima, M. The use of granulocyte-colony stimulating factor induced mobilization for isolation of dental pulp stem cells with high regenerative potential. Biomaterials 2013, 34, 9036–9047. [Google Scholar] [CrossRef] [PubMed]
- Tomson, P.L.; Lumley, P.J.; Alexander, M.Y.; Smith, A.J.; Cooper, P.R. Hepatocyte growth factor is sequestered in dentine matrix and promotes regeneration-associated events in dental pulp cells. Cytokine 2013, 61, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, H.; Gong, Q.; Fan, C.; Huang, Y.; Ling, J. Expression of high mobility group box 1 in inflamed dental pulp and its chemotactic effect on dental pulp cells. Biochem. Biophys. Res. Commun. 2014, 450, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- Chmilewsky, F.; Jeanneau, C.; Dejou, J.; About, I. Sources of dentin-pulp regeneration signals and their modulation by the local microenvironment. J. Endod. 2014, 40, S19–S25. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, S.; Jeanneau, C.; Sheibat-Othman, N.; Kalaji, N.; Fessi, H.; About, I. Usefulness of controlled release of growth factors in investigating the early events of dentin-pulp regeneration. J. Endod. 2013, 39, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Lee, C.H.; Chen, M.; Zhao, W.; Fu, S.Y.; Qi, J.J.; Chotkowski, G.; Eisig, S.B.; Wong, A.; Mao, J.J. Induced Migration of Dental Pulp Stem Cells for in vivo Pulp Regeneration. J. Dent. Res. 2011, 90, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Laurent, P.; Camps, J.; About, I. Biodentine(TM) induces TGF-β1 release from human pulp cells and early dental pulp mineralization. Int. Endod. J. 2012, 45, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Sloan, A.J.; Couble, M.L.; Bleicher, F.; Magloire, H.; Smith, A.J.; Farges, J.C. Expression of TGF-beta receptors I and II in the human dental pulp by in situ hybridization. Adv. Dent. Res. 2001, 15, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Ko, I.K.; Lee, S.J.; Atala, A.; Yoo, J.J. In situ tissue regeneration through host stem cell recruitment. Exp. Mol. Med. 2013, 45, e57. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Chatterjee, M.; Schmid, H.; Beck, S.; Gawaz, M. CXCL14 as an emerging immune and inflammatory modulator. J. Inflamm. (Lond). 2016, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Iohara, K.; Murakami, M.; Takeuchi, N.; Osako, Y.; Ito, M.; Ishizaka, R.; Utunomiya, S.; Nakamura, H.; Matsushita, K.; Nakashima, M. A novel combinatorial therapy with pulp stem cells and granulocyte colony-stimulating factor for total pulp regeneration. Stem Cells Transl. Med. 2013, 2, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, N.; Hayashi, Y.; Murakami, M.; Alvarez, F.J.; Horibe, H.; Iohara, K.; Nakata, K.; Nakamura, H.; Nakashima, M. Similar in vitro effects and pulp regeneration in ectopic tooth transplantation by basic fibroblast growth factor and granulocyte-colony stimulating factor. Oral Dis. 2015, 21, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Obinata, H.; Hla, T. Sphingosine 1-phosphate in coagulation and inflammation. Semin. Immunopathol. 2012, 34, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.Y.; Yang, H.; Shao, M.Y.; Xu, J.; Zhang, P.; Cheng, R.; Hu, T. Sphingosine-1-phosphate mediates AKT/ERK maintenance of dental pulp homoeostasis. Int. Endod. J. 2015, 48, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhu, Y.-Q.; Du, R.; Gu, Y.-X.; Xia, L.; Qin, F.; Ritchie, H.H. The Expression and Role of Stromal Cell-derived Factor-1α-CXCR4 Axis in Human Dental Pulp. J. Endod. 2008, 34, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Peng, W.W.; Li, L.F.; Yang, Y.; Zhu, Y.Q. Proliferation and multilineage potential of CXCR4-positive human dental pulp cells in vitro. J. Endod. 2012, 38, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Chmilewsky, F.; Jeanneau, C.; Laurent, P.; About, I. Pulp fibroblasts synthesize functional complement proteins involved in initiating dentin-pulp regeneration. Am. J. Pathol. 2014, 184, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Chmilewsky, F.; Jeanneau, C.; Laurent, P.; About, I. LPS induces pulp progenitor cell recruitment via complement activation. J. Dent. Res. 2015, 94, 166–174. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, A.; Wang, J.; Redmond, H.P. HMGB1 as a key mediator of tissue response to injury: Roles in inflammation and tissue repair. Eur. Surg. Acta Chir. Austriaca 2006, 38, 283–292. [Google Scholar] [CrossRef]
- Haeger, A.; Wolf, K.; Zegers, M.M.; Friedl, P. Collective cell migration: guidance principles and hierarchies. Trends Cell Biol. 2015, 25, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Scheven, B.A.; Takahashi, Y.; Ferracane, J.L.; Shelton, R.M.; Cooper, P.R. Dentine as a bioactive extracellular matrix. Arch. Oral Biol. 2012, 57, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Tomson, P.L.; Grover, L.M.; Lumley, P.J.; Sloan, A.J.; Smith, A.J.; Cooper, P.R. Dissolution of bio-active dentine matrix components by mineral trioxide aggregate. J. Dent. 2007, 35, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Finkelman, R.D.; Mohan, S.; Jennings, J.C.; Taylor, A.K.; Jepsen, S.; Baylink, D.J. Quantitation of growth factors IGF-I, SGF/IGF-II, and TGF-β in human dentin. J. Bone Miner. Res. 1990, 5, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Roberts-Clark, D.; Smith, A. Angiogenic growth factors in human dentine matrix. Arch. Oral Biol. 2000, 45, 1013–1016. [Google Scholar] [CrossRef]
- Smith, J.G.; Smith, A.J.; Shelton, R.M.; Cooper, P.R. Recruitment of dental pulp cells by dentine and pulp extracellular matrix components. Exp. Cell Res. 2012, 318, 2397–2406. [Google Scholar] [CrossRef] [PubMed]
- Lymperi, S.; Taraslia, V.; Tsatsoulis, I.N.; Samara, A.; Velentzas, A.D.; Agrafioti, A.; Anastasiadou, E.; Kontakiotis, E. Dental Stem Cell Migration on Pulp Ceiling Cavities Filled with MTA, Dentin Chips, or Bio-Oss. Biomed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Palosaari, H.; Pennington, C.J.; Larmas, M.; Edwards, D.R.; Tjäderhane, L.; Salo, T. Expression profile of matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs in mature human odontoblasts and pulp tissue. Eur. J. Oral Sci. 2003, 111, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Bahuguna, R. Role of matrix metalloproteinases in dental caries, pulp and periapical inflammation: An overview. J. Oral Biol. Craniofacial Res. 2015, 5, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Chaussain-Miller, C.; Fioretti, F.; Goldberg, M.; Menashi, S. The Role of Matrix Metalloproteinases (MMPs) in Human Caries. J. Dent. Res. 2006, 85, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Amano, K.; Iohara, K.; Ito, M.; Imabayashi, K.; Into, T.; Matsushita, K.; Nakamura, H.; Nakashima, M. Matrix metalloproteinase-3 accelerates wound healing following dental pulp injury. Am. J. Pathol. 2009, 175, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.; Cambier, S.; Fjellbirkeland, L.; Baron, J.L.; Munger, J.S.; Kawakatsu, H.; Sheppard, D.; Broaddus, V.C.; Nishimura, S.L. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J. Cell Biol. 2002, 157, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Visse, R. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases: Structure, Function, and Biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.E.; Parks, W.C. Metalloproteinases and their inhibitors: regulators of wound healing. Int. J. Biochem. Cell Biol. 2008, 40, 1334–1347. [Google Scholar] [CrossRef] [PubMed]
- Ho, I.A.W.; Yulyana, Y.; Sia, K.C.; Newman, J.P.; Guo, C.M.; Hui, K.M.; Lam, P.Y.P. Matrix metalloproteinase-1-mediated mesenchymal stem cell tumor tropism is dependent on crosstalk with stromal derived growth factor 1/C-X-C chemokine receptor 4 axis. FASEB J. 2014, 28, 4359–4368. [Google Scholar] [CrossRef] [PubMed]
- Ries, C.; Egea, V.; Karow, M.; Kolb, H.; Jochum, M.; Neth, P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: Differential regulation by inflammatory cytokines. Blood 2007, 109, 4055–4063. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-Y.; Chen, X.; Yue, L.; Huang, G.T.-J.; Zou, X.-Y. CXC Chemokine Receptor 4 Is Expressed Paravascularly in Apical Papilla and Coordinates with Stromal Cell-derived Factor-1α during Transmigration of Stem Cells from Apical Papilla. J. Endod. 2015, 41, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-C.; Tsai, Y.-L.; Chang, H.-H.; Lee, S.-Y.; Lee, M.-S.; Chang, C.-W.; Chan, C.-P.; Yeh, C.-Y.; Cheng, R.-H.; Jeng, J.-H. IL-1β-induced MCP-1 expression and secretion of human dental pulp cells is related to TAK1, MEK/ERK, and PI3K/Akt signaling pathways. Arch. Oral Biol. 2016, 61, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Fu, L.; Zhang, Y.; Yu, Q.; Ma, F.; Wang, Z.; Luo, Z.; Zhou, Z.; Cooper, P.R.; He, W. The effects of LPS on adhesion and migration of human dental pulp stem cells in vitro. J. Dent. 2014, 42, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, Y.; Zhan, X.; Cui, L.; Xu, S.; Ma, D.; Yue, J.; Wu, B.; Gao, J. TLR4 Activation by Lipopolysaccharide and Streptococcus mutans Induces Differential Regulation of Proliferation and Migration in Human Dental Pulp Stem Cells. J. Endod. 2014, 40, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kwon, S.M.; Yoon, H.E.; Kim, S.A.; Ahn, S.G.; Yoon, J.H. Lipopolysaccharide promotes adhesion and migration of murine dental papilla-derived MDPC-23 cells via TLR4. Int. J. Mol. Med. 2011, 27, 277–281. [Google Scholar] [PubMed]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Schraufstatter, I.U.; Khaldoyanidi, S.K.; DiScipio, R.G. Complement activation in the context of stem cells and tissue repair. World J. Stem Cells 2015, 7, 1090–1108. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.C.; Cui, C.; Yan, Y.H.; Sun, G.H.; Zhu, S.R. Effects of high-mobility group box 1 on the proliferation and odontoblastic differentiation of human dental pulp cells. Int. Endod. J. 2013, 46, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, A.; Lipski, M.; Parafiniuk, M.; Sporniak-Tutak, K.; Lichota, D.; Kosierkiewicz, A.; Kaczmarek, W.; Buczkowska-Radlińska, J. Response of Human Dental Pulp Capped with Biodentine and Mineral Trioxide Aggregate. J. Endod. 2013, 39, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Hayashi, M.; Suzuki, Y.; Suzuki, N.; Maeno, M.; Ogiso, B. Calcium ions released from mineral trioxide aggregate convert the differentiation pathway of C2C12 cells into osteoblast lineage. J. Endod. 2013, 39, 68–75. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Gao, Y.; Ling, J.; Wei, X.; Xiao, Y. Calcium ions promote osteogenic differentiation and mineralization of human dental pulp cells: implications for pulp capping materials. J. Mater. Sci. Mater. Med. 2012, 23, 789–795. [Google Scholar] [CrossRef] [PubMed]
- AbdulQader, S.T.; Kannan, T.P.; Rahman, I.A.; Ismail, H.; Mahmood, Z. Effect of different calcium phosphate scaffold ratios on odontogenic differentiation of human dental pulp cells. Mater. Sci. Eng. C. Mater. Biol. Appl. 2015, 49, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.-C.; Kao, C.-T.; Huang, T.-H.; Hung, C.-J.; Shie, M.-Y.; Chung, H.-Y. Effect of verapamil, a calcium channel blocker, on the odontogenic activity of human dental pulp cells cultured with silicate-based materials. J. Endod. 2014, 40, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Hung, C.-J.; Huang, T.-H.; Lin, C.-C.; Kao, C.-T.; Shie, M.-Y. Odontogenic differentiation of human dental pulp cells by calcium silicate materials stimulating via FGFR/ERK signaling pathway. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 43, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.; Holland, G.R.; Chiego, D.; Hu, J.C.C.; Nör, J.E.; Botero, T.M. White mineral trioxide aggregate induces migration and proliferation of stem cells from the apical papilla. J. Endod. 2014, 40, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yang, J.; Zhang, J.; Peng, B. A comparative study of bioaggregate and ProRoot MTA on adhesion, migration, and attachment of human dental pulp cells. J. Endod. 2014, 40, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, L.X.; Cheng, X.; Lin, Y.; Yan, P.; Peng, B. Promotion of Dental Pulp Cell Migration and Pulp Repair by a Bioceramic Putty Involving FGFR-mediated Signaling Pathways. J. Dent. Res. 2015, 94, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Li, D.; Kohli, M.R.; Yu, Q.; Kim, S.; He, W.X. Effect of BiodentineTM on the proliferation, migration and adhesion of human dental pulp stem cells. J. Dent. 2014, 42, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.; González, A.; Planell, J.A.; Engel, E. Extracellular calcium modulates in vitro bone marrow-derived Flk-1+ CD34+ progenitor cell chemotaxis and differentiation through a calcium-sensing receptor. Biochem. Biophys. Res. Commun. 2010, 393, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.; Cooper, P.R.; Cassidy, N.; Nor, J.E.; Sloan, A.J.; Smith, A.J. The effect of calcium hydroxide on solubilisation of bio-active dentine matrix components. Biomaterials 2006, 27, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- Calarco, A.; Di Salle, A.; Tammaro, L.; De Luca, I.; Mucerino, S.; Petillo, O.; Riccitiello, F.; Vittoria, V.; Peluso, G. Long-Term Fluoride Release from Dental Resins Affects STRO-1+ Cell Behavior. J. Dent. Res. 2015, 94, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.W.; Wu, H.; Oh, J.-E.; Fakhar, C.; Kang, M.K.; Shin, K.-H.; Park, N.-H.; Kim, R.H. 2-Hydroxyethyl methacrylate inhibits migration of dental pulp stem cells. J. Endod. 2013, 39, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Tran-Hung, L.; Laurent, P.; Camps, J.; About, I. Quantification of angiogenic growth factors released by human dental cells after injury. Arch. Oral Biol. 2008, 53, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Paschalidis, T.; Bakopoulou, A.; Papa, P.; Leyhausen, G.; Geurtsen, W.; Koidis, P. Dental pulp stem cells’ secretome enhances pulp repair processes and compensates TEGDMA-induced cytotoxicity. Dent. Mater. 2014, 30, e405–e418. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-C. Boyden chamber assay. Methods Mol. Biol. 2005, 294, 15–22. [Google Scholar] [PubMed]
- Motasim, K.S.; Nystrom, M.L.; Thomas, G.J. Cell migration and invasion assays. Methods Mol. Biol. 2011, 731, 333–343. [Google Scholar]
- von Marschall, Z.; Fisher, L.W. Dentin matrix protein-1 isoforms promote differential cell attachment and migration. J. Biol. Chem. 2008, 283, 32730–32740. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S. Fibroblasts in three dimensional matrices: cell migration and matrix remodeling. Exp. Mol. Med. 2009, 41, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Kramer, N.; Walzl, A.; Unger, C.; Rosner, M.; Krupitza, G.; Hengstschläger, M.; Dolznig, H. In vitro cell migration and invasion assays. Mutat. Res. 2013, 752, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Smith, A.J. Cells and extracellular matrices of dentin and pulp: A biological basis for repair and tissue engineering. Crit. Rev. Oral Biol. Med. 2004, 15, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Zengel, P.; Nguyen-Hoang, A.; Schildhammer, C.; Zantl, R.; Kahl, V.; Horn, E. μ-Slide Chemotaxis: A new chamber for long-term chemotaxis studies. BMC Cell Biol. 2011, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Hirata, A.; Dimitrova-Nakov, S.; Djole, S.-X.; Ardila, H.; Baudry, A.; Kellermann, O.; Simon, S.; Goldberg, M. Plithotaxis, a collective cell migration, regulates the sliding of proliferating pulp cells located in the apical niche. Connect. Tissue Res. 2014, 55 Suppl 1, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Vanden Berg-Foels, W.S. In situ tissue regeneration: Chemoattractants for endogenous stem cell recruitment. Tissue Eng. Part B Rev. 2014, 20, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.-R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.-H.; Shin, U.S.; Kim, H.-W. Fibroblast growth factors: biology, function, and application for tissue regeneration. J. Tissue Eng. 2010, 2010, 218142. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.-M.; Wang, X.; Marin-Muller, C.; Wang, H.; Lin, P.H.; Yao, Q.; Chen, C. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev. Mol. Diagn. 2009, 9, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Ko, I.K.; Ju, Y.M.; Chen, T.; Atala, A.; Yoo, J.J.; Lee, S.J. Combined systemic and local delivery of stem cell inducing/recruiting factors for in situ tissue regeneration. FASEB J. 2012, 26, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Walboomers, X.F.; Jansen, J.A. The formation of tertiary dentin after pulp capping with a calcium phosphate cement, loaded with PLGA microparticles containing TGF-β1. J. Biomed. Mater. Res. Part A 2008, 85, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.-M.; Lee, E.-S.; Kim, M.; Kim, J.-J.; Lee, J.-H.; Lee, H.-H.; Park, K.-R.; Yi, J.-K.; Kim, H.-W.; Kim, E. Magnetic Nanocomposite Scaffold-Induced Stimulation of Migration and Odontogenesis of Human Dental Pulp Cells through Integrin Signaling Pathways. PLoS ONE 2015, 10, e0138614. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Widbiller, M.; Buchalla, W.; Eidt, A.; Hiller, K.-A.; Hoffer, P.C.; Schmalz, G. EDTA conditioning of dentine promotes adhesion, migration and differentiation of dental pulp stem cells. Int. Endod. J. 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]


| Chemotactic Molecule | Receptor | Inflammation | Regeneration | Role | Reference |
|---|---|---|---|---|---|
| HGF | c-Met | x | Recruitment of DPSC | [13] | |
| FGF-2 | FGF receptors 1 and 2 | x | Recruitment of DPSC and pulp cell proliferation | [9,16,17] | |
| TGFβ-1 | TGF-β1 receptors I and II | x | Recruitment of DPSC and odontoblastic differentiation | [9,16,18,19] | |
| MCP-1 | CCR2 | x | Recruitment of DPSC and stem cell homing* | [10,20] | |
| CXCL14 | C-X-C chemokine receptor type 4 (CXCR4) | x | x | Recruitment of DPSC and mediator of immune cell migration* | [10,21] |
| G-CSF | G-CSF receptor | x | x | DPSC mobilization and anti-inflammatory properties | [12,22,23] |
| S1P | S1P receptor 1–3 | x | x | Pleiotropic actions including recruitment of DPSC and inflammatory effects* | [9,24,25] |
| SDF-1 | CXCR4 | x | Recruitment of DPSC and stem cell homing* | [11,20,26,27] | |
| C5a | C5a receptor | x | x | Recruitment of DPCS and inflammatory cells* | [8,28,29] |
| HMGB-1 | receptor for advanced glycation end products (RAGE) | x | x | Recruitment of DPCS and production of inflammatory cytokines* | [14,30] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rombouts, C.; Jeanneau, C.; Bakopoulou, A.; About, I. Dental Pulp Stem Cell Recruitment Signals within Injured Dental Pulp Tissue. Dent. J. 2016, 4, 8. https://doi.org/10.3390/dj4020008
Rombouts C, Jeanneau C, Bakopoulou A, About I. Dental Pulp Stem Cell Recruitment Signals within Injured Dental Pulp Tissue. Dentistry Journal. 2016; 4(2):8. https://doi.org/10.3390/dj4020008
Chicago/Turabian StyleRombouts, Charlotte, Charlotte Jeanneau, Athina Bakopoulou, and Imad About. 2016. "Dental Pulp Stem Cell Recruitment Signals within Injured Dental Pulp Tissue" Dentistry Journal 4, no. 2: 8. https://doi.org/10.3390/dj4020008
APA StyleRombouts, C., Jeanneau, C., Bakopoulou, A., & About, I. (2016). Dental Pulp Stem Cell Recruitment Signals within Injured Dental Pulp Tissue. Dentistry Journal, 4(2), 8. https://doi.org/10.3390/dj4020008





