Salivary Biomarker Analysis to Distinguish Between Health and Periodontitis Status: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
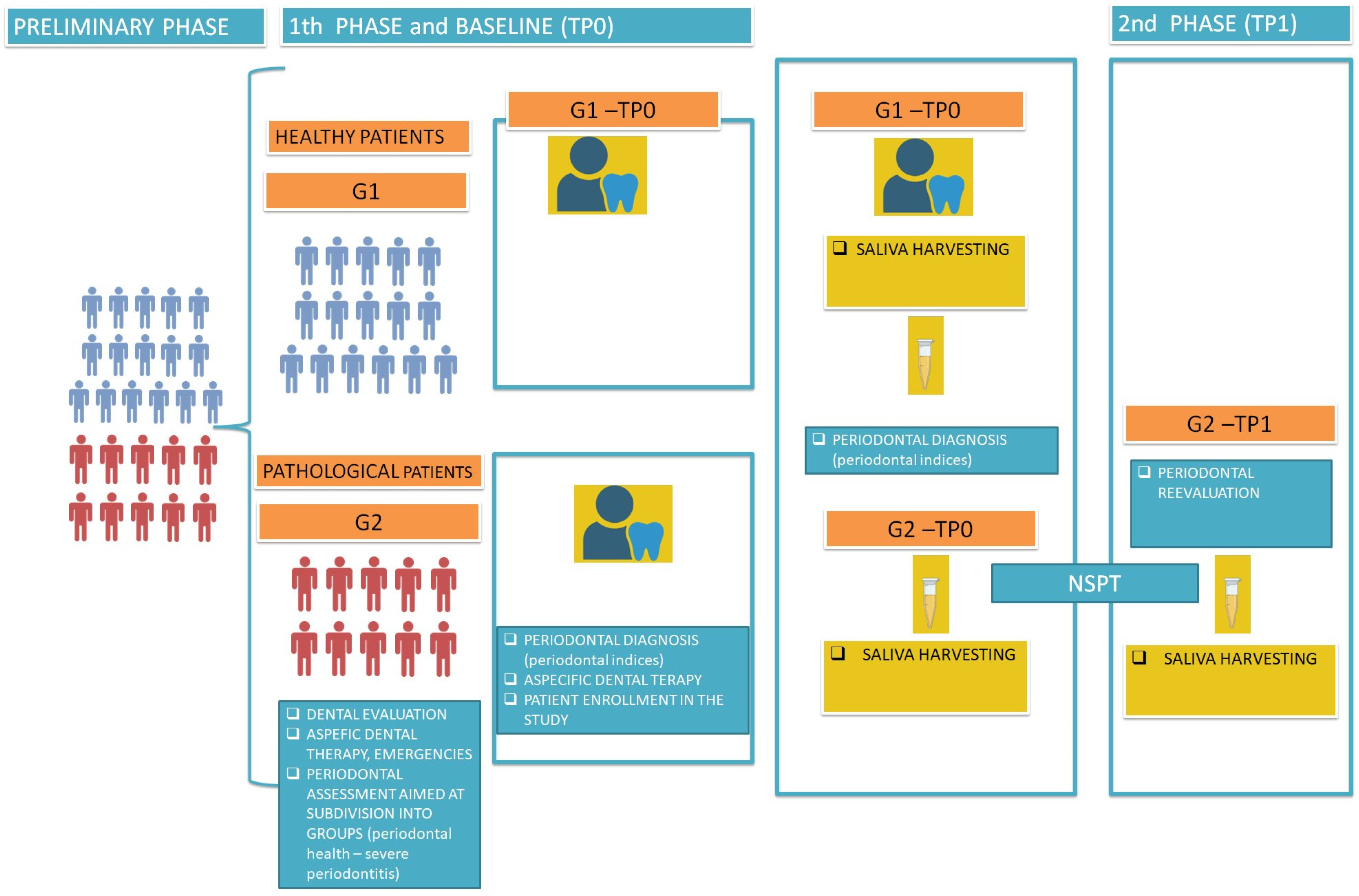
2.1. Participants
2.2. Clinical Measurements
2.3. Periodontal Procedures
2.4. Salivary Sample Collection
2.5. Matrix Metalloproteinase-8 Protein (MMP-8) Assay
2.6. Cytokine Immunological Assay
2.7. Study Outcomes
2.8. Statistical Analysis
3. Results
3.1. Examiner Calibration
3.2. Descriptive Clinical Analysis
3.3. Matrix Metalloproteinase-8 Protein (MMP-8) Assay
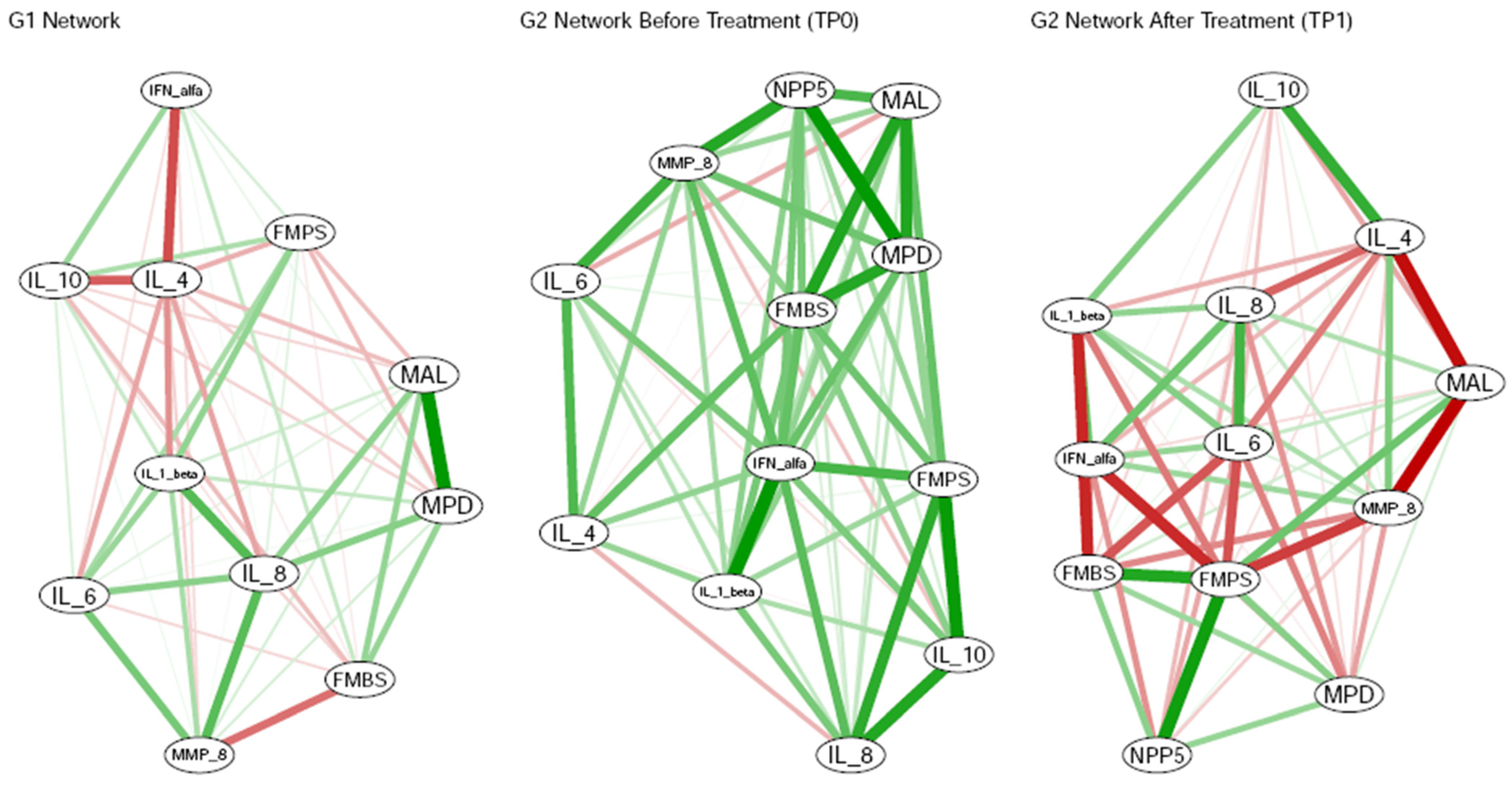
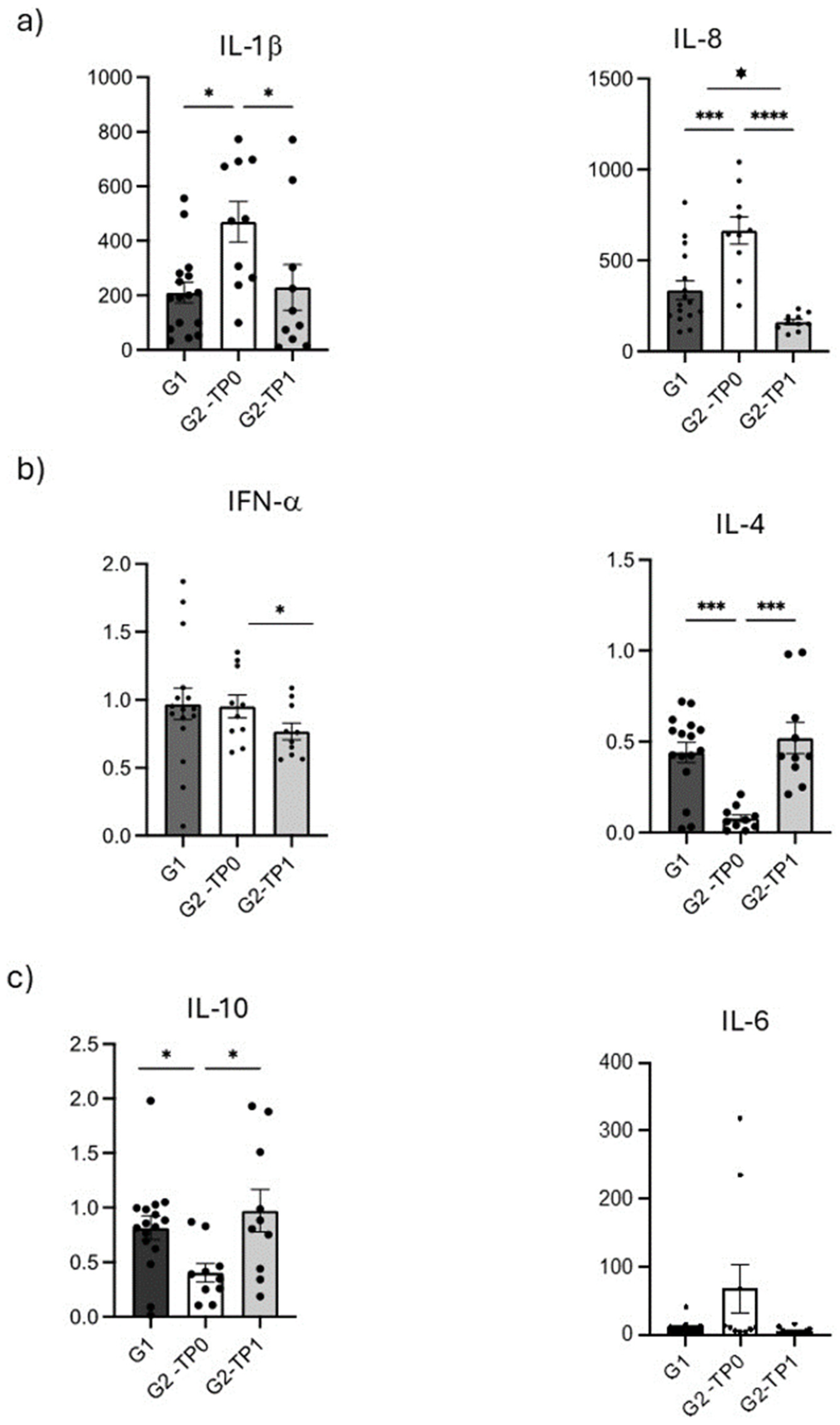
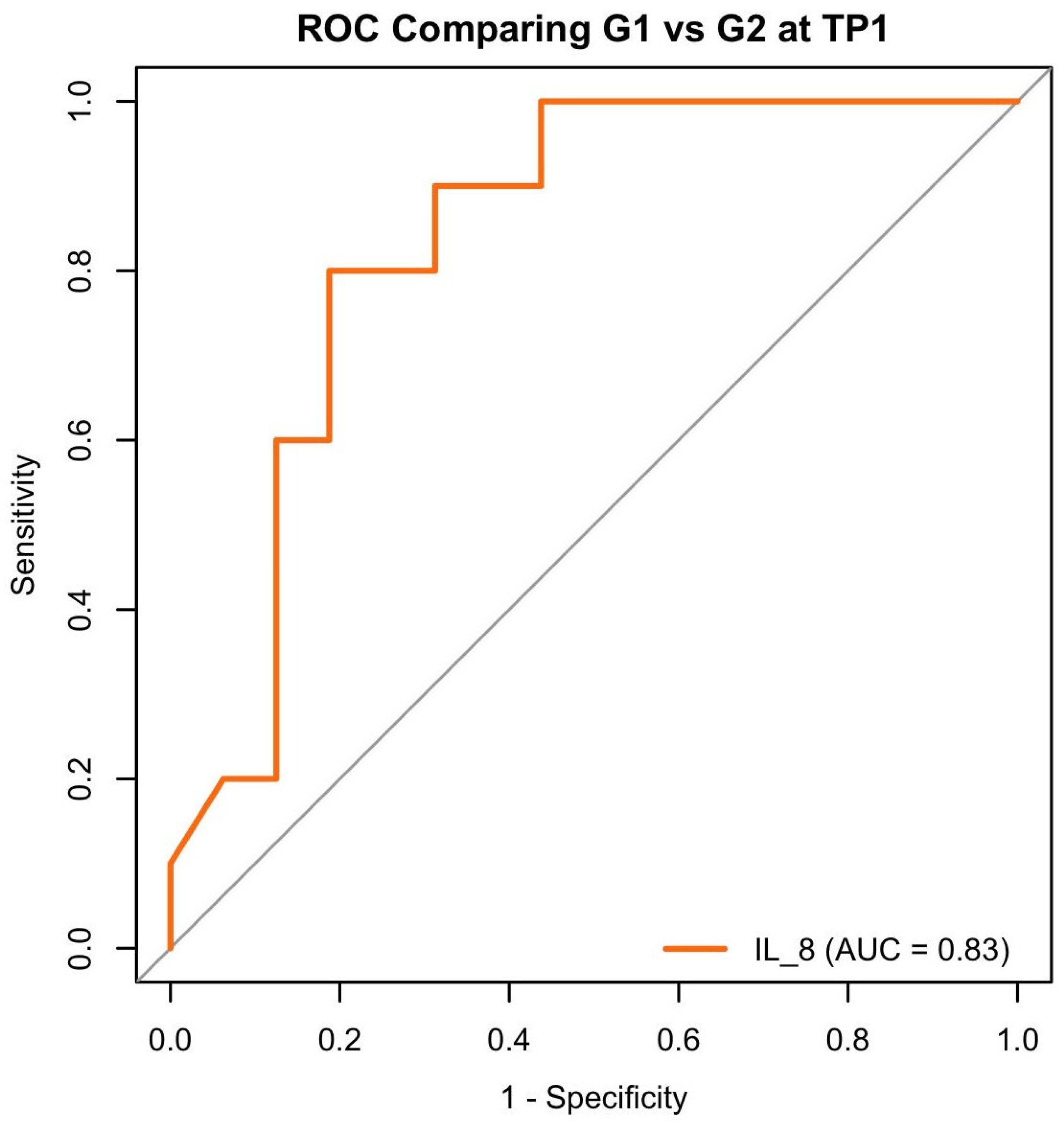
3.4. Cytokine Immunological Assay
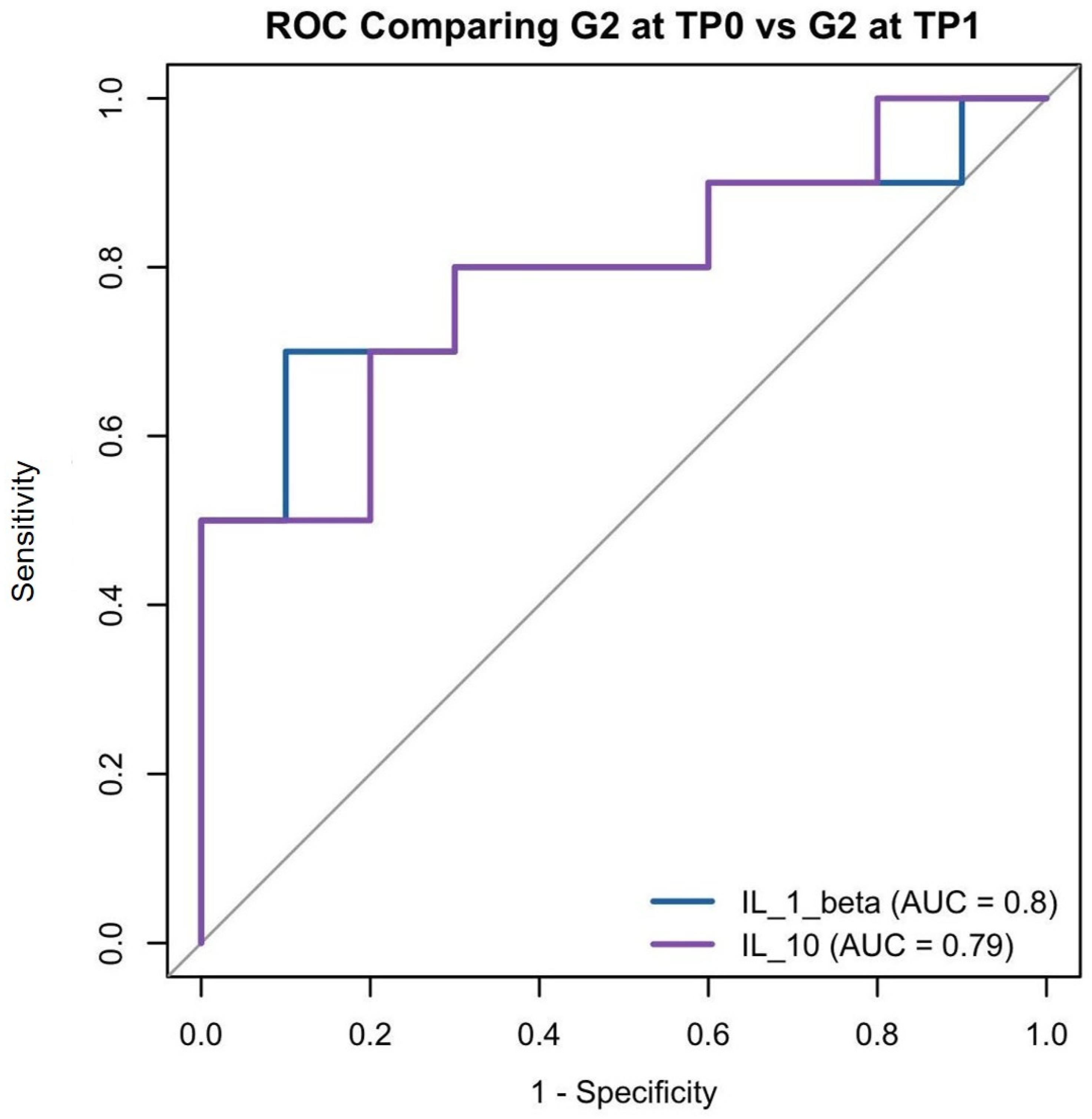
3.5. Logistic Regression
4. Discussion
5. Strengths and Limitations of the Study
6. Conclusions
- The NSPT produces clinically relevant effects, reducing the need for further, more invasive therapies.
- Molecular analysis, using validated procedures, provides objective and useful measurements.
- MMP-8 levels could reliably help differentiate between subjects with a healthy periodontium and those affected by severe PRD.
- The analysis of the biomarkers under consideration is at least a useful aid in differentiating between individuals with a healthy periodontium and patients with PRD. Assuming that changes in the biomarkers considered can be detected before periodontal damage occurs, this approach may serve as a valid starting point for effective early diagnosis of the disease, before tissue destruction becomes established. Moreover, the molecular network studied—including MMP-8—appears to have potential for monitoring the response to PRD therapy and provides a promising basis for future research. MMP-8, IL-1β, IL-4, IL-8, and IL-10 show the strongest statistical associations with clinical indices in both healthy and diseased states.
- Statistical evaluations reveal a clear distinction between the untreated PRD phase (G2 at TP0), the treated PRD phase (G2 at TP1), and periodontal health (G1), in terms of the behavior of clinical indices relative to the investigated biomarkers. These findings suggest that individuals with periodontal health or treated PRD could manage inflammation in a significantly different manner compared to those with untreated PRD.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area Under the Curve |
| CAL | Clinical Attachment Level |
| DM | Diabetes Mellitus |
| FMBS | Full-Mouth Bleeding Score |
| FMPS | Full-Mouth Plaque Score |
| G1 | Group 1: Healthy Controls |
| G2 | Group 2: Patients with PRD |
| GCF | Gingival Crevicular Fluid |
| IFN-α | Interferon Alpha |
| IL | Interleukin |
| MAL | Mean Attachment Level |
| MMP-8 | Matrix Metalloproteinase-8 |
| MPD | Mean Probing Depth |
| NPP5 | Number of Pockets with PPD ≥ 5 mm |
| NSPT | Non-Surgical Periodontal Therapy |
| OD | Optical Density |
| PPD | Probing Pocket Depth |
| PRD | Periodontitis |
| RA | Rheumatoid Arthritis |
| REC | Gingival Recession Depth |
| ROC | Receiver Operating Characteristic |
| SD | Standard Deviation |
| SE | Standard Error |
| TP0 | Time Point 0 (Baseline) |
| TP1 | Time Point 1 (Post-treatment Follow-Up) |
References
- Divaris, K. Searching Deep and Wide: Advances in the Molecular Understanding of Dental Caries and Periodontal Disease. Adv. Dent. Res. 2019, 30, 40–44. [Google Scholar] [CrossRef]
- Werner, N.; Heck, K.; Walter, E.; Ern, C.; Bumm, C.V.; Folwaczny, M. Probing Pocket Depth Reduction after Non-Surgical Periodontal Therapy: Tooth-Related Factors. J. Periodontol. 2024, 95, 29–39. [Google Scholar] [CrossRef]
- Kornman, K.S. Mapping the Pathogenesis of Periodontitis: A New Look. J. Periodontol. 2008, 79, 1560–1568. [Google Scholar] [CrossRef]
- Bertoldi, C.; Zaffe, D. In Vivo Comparison of Two Bone Substitutes in the Distal Femur of the Rabbit. Int. J. Oral Maxillofac. Implant. 2012, 27, 119–127. [Google Scholar]
- Bertoldi, C.; Lalla, M.; Pradelli, J.M.; Cortellini, P.; Lucchi, A.; Zaffe, D. Risk Factors and Socioeconomic Condition Effects on Periodontal and Dental Health: A Pilot Study among Adults over Fifty Years of Age. Eur. J. Dent. 2013, 7, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Monari, E.; Cuoghi, A.; Bellei, E.; Bergamini, S.; Lucchi, A.; Tomasi, A.; Cortellini, P.; Zaffe, D.; Bertoldi, C. Analysis of Protein Expression in Periodontal Pocket Tissue: A Preliminary Study. Proteome Sci. 2015, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Garlet, G.P. Critical Reviews in Oral Biology & Medicine: Destructive and Protective Roles of Cytokines in Periodontitis: A Re-Appraisal from Host Defense and Tissue Destruction Viewpoints. J. Dent. Res. 2010, 89, 1349–1363. [Google Scholar] [CrossRef]
- Gupta, M.; Chaturvedi, R.; Jain, A. Role of Cardiovascular Disease Markers in Periodontal Infection: Understanding the Risk. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2015, 26, 231–236. [Google Scholar] [CrossRef]
- Bertoldi, C.; Forabosco, A.; Lalla, M.; Generali, L.; Zaffe, D.; Cortellini, P. How Intraday Index Changes Influence Periodontal Assessment: A Preliminary Study. Int. J. Dent. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Huang, Y.K.; Tseng, K.F.; Tsai, P.H.; Wang, J.S.; Lee, C.Y.; Shen, M.Y. IL-8 as a Potential Therapeutic Target for Periodontitis and Its Inhibition by Caffeic Acid Phenethyl Ester In Vitro. Int. J. Mol. Sci. 2021, 22, 3641. [Google Scholar] [CrossRef]
- Abdullah, A.N.; Al-Habib, O.A.M.; Mohammed, S.A. Changes in the Level of Cytokines in the Saliva of Hypertensive Patients with Chronic Periodontitis after Scaling and Root Planning. Prostaglandins Other Lipid Mediat. 2023, 169, 106765. [Google Scholar] [CrossRef]
- Taylor, J.J.; Jaedicke, K.M.; Merwe, R.C.; Bissett, S.M.; Landsdowne, N.; Whall, K.M.; Pickering, K.; Thornton, V.; Lawson, V.; Yatsuda, H.; et al. A Prototype Antibody-Based Biosensor for Measurement of Salivary MMP-8 in Periodontitis Using Surface Acoustic Wave Technology. Sci. Rep. 2019, 9, 11034. [Google Scholar] [CrossRef]
- Liu, X.; Li, H. A Systematic Review and Meta-Analysis on Multiple Cytokine Gene Polymorphisms in the Pathogenesis of Periodontitis. Front. Immunol. 2022, 12, 713198. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Zonta, F.; Yang, H.; Pelekos, G.; Tonetti, M.S. Development of a Machine Learning Multiclass Screening Tool for Periodontal Health Status Based on Non-Clinical Parameters and Salivary Biomarkers. J. Clin. Periodontol. 2023, 11, 1547–1560. [Google Scholar] [CrossRef]
- Yilmaz, M.; Sorsa, T.; Demir, E.; Gürsoy, M.; Suominen, A.; Tervahartiala, T.; Räisänen, I.T.; Gürsoy, U.K. Accuracy of AMMP-8 Point-of-Care Test in Indicating Periodontal Treatment Outcomes in Stage III/IV Periodontitis: A 24-Week Follow-up Study. J. Periodontal Res. 2023, 58, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, E.A.; Ławicka, R.; Grygorczuk, P.; Nowosielska, M.; Kicman, A.; Ławicki, S. Importance of Metalloproteinase 8 (MMP-8) in the Diagnosis of Periodontitis. Int. J. Mol. Sci. 2024, 25, 2721. [Google Scholar] [CrossRef] [PubMed]
- Slavich, G.M. Understanding Inflammation, Its Regulation, and Relevance for Health: A Top Scientific and Public Priority. Brain Behav. Immun. 2015, 45, 13–14. [Google Scholar] [CrossRef]
- Bertoldi, C.; Salvatori, R.; Pinti, M.; Mattioli, A.V. Could the Periodontal Therapy Improve the Cardiologic Patient Health? A Narrative Review. Curr. Probl. Cardiol. 2024, 49, 102699. [Google Scholar] [CrossRef]
- GBD 2017 Causes of Death Collaborators. Global, Regional, and National Age-Sex-Specific Mortality for 282 Causes of Death in 195 Countries and Territories, 1980-2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of Lifetime Health around the Time of Conception: Causes and Consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef]
- Lockhart, P.B.; Bolger, A.F.; Papapanou, P.N.; Osinbowale, O.; Trevisan, M.; Levison, M.E.; Taubert, K.A.; Newburger, J.W.; Gornik, H.L.; Gewitz, M.H.; et al. American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, Council on Epidemiology and Prevention, Council on Peripheral Vascular Disease, and Council on Clinical Cardiol. Circulation 2012, 125, 2520–2544. [Google Scholar] [CrossRef]
- Monsarrat, P.; Blaizot, A.; Kémoun, P.; Ravaud, P.; Nabet, C.; Sixou, M.; Vergnes, J.N. Clinical Research Activity in Periodontal Medicine: A Systematic Mapping of Trial Registers. J. Clin. Periodontol. 2016, 43, 390–400. [Google Scholar] [CrossRef]
- Jain, P.; Mirza, M.A.; Iqbal, Z. Unraveling the Etiology of Periodontitis. Int. J. Biomed. Investig. 2021, 4, 131. [Google Scholar] [CrossRef]
- Herrera, D.; Sanz, M.; Shapira, L.; Brotons, C.; Chapple, I.; Frese, T.; Graziani, F.; Hobbs, F.D.R.; Huck, O.; Hummers, E.; et al. Association between Periodontal Diseases and Cardiovascular Diseases, Diabetes and Respiratory Diseases: Consensus Report of the Joint Workshop by the European Federation of Periodontology (EFP) and the European Arm of the World Organization of Family Doc. J. Clin. Periodontol. 2023, 50, 819–841. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, M.; Clark, D. Periodontal Disease as a Model to Study Chronic Inflammation in Aging. GeroScience 2024, 46, 3695–3709. [Google Scholar] [CrossRef]
- Leng, Y.; Hu, Q.; Ling, Q.; Yao, X.; Liu, M.; Chen, J.; Yan, Z.; Dai, Q. Periodontal Disease Is Associated with the Risk of Cardiovascular Disease Independent of Sex: A Meta-Analysis. Front. Cardiovasc. Med. 2023, 10, 1114927. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global Burden of Severe Periodontitis in 1990-2010: A Systematic Review and Meta-Regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef]
- Hasturk, H.; Kantarci, A. Activation and Resolution of Periodontal Inflammation and Its Systemic Impact. Periodontol. 2000 2015, 1, 255–273. [Google Scholar] [CrossRef]
- Sczepanik, F.S.C.; Grossi, M.L.; Casati, M.; Goldberg, M.; Glogauer, M.; Fine, N.; Tenenbaum, H.C. Periodontitis Is an Inflammatory Disease of Oxidative Stress: We Should Treat It That Way. Periodontology 2020, 84, 45–68. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T.; Lambris, J.D. Current Understanding of Periodontal Disease Pathogenesis and Targets for Host-Modulation Therapy. Periodontol. 2000 2020, 1, 14–34. [Google Scholar] [CrossRef]
- Martínez-García, M.; Hernández-Lemus, E. Periodontal Inflammation and Systemic Diseases: An Overview. Front. Physiol. 2021, 12, 709438. [Google Scholar] [CrossRef] [PubMed]
- Loos, B.G.; Van Dyke, T.E. The Role of Inflammation and Genetics in Periodontal Disease. Periodontol. 2000 2020, 83, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Page, R.C.; Eke, P.I. Case Definitions for Use in Population-Based Surveillance of Periodontitis. J. Periodontol. 2007, 78, 1387–1399. [Google Scholar] [CrossRef] [PubMed]
- Trombelli, L.; Farina, R.; Silva, C.O.; Tatakis, D.N. Plaque-Induced Gingivitis: Case Definition and Diagnostic Considerations. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S44–S67. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and Grading of Periodontitis: Framework and Proposal of a New Classification and Case Definition. J. Periodontol. 2018, 89 (Suppl. S1), S159–S172. [Google Scholar] [CrossRef]
- Mattey, D.L.; Nixon, N.B.; Dawes, P.T. Association of Circulating Levels of MMP-8 with Mortality from Respiratory Disease in Patients with Rheumatoid Arthritis. Arthritis Res. Ther. 2012, 14, 204. [Google Scholar] [CrossRef]
- Thirkettle, S.; Decock, J.; Hugh, A.; Pennington, C.J.; Jaworski, D.M.; Edwards, D.R. Matrix Metalloproteinase 8 (Collagenase 2) Induces the Expression of Interleukins 6 and 8 in Breast Cancer Cells. J. Biol. Chem. 2013, 288, 16282–16294. [Google Scholar] [CrossRef]
- Juurikka, K.; Butler, G.S.; Salo, T.; Nyberg, P.; Aström, P. The Role of MMP8 in Cancer: A Systematic Review. Int. J. Mol. Sci. 2019, 20, 4506. [Google Scholar] [CrossRef]
- American Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Duvina, M.; Barbato, L.; Brancato, L.; Glover, G.D.; Amunni, F.; Tonelli, P. Biochemical Markers as Predictors of Bone Remodelling in Dental Disorders: A Narrative Description of Literature. Clin. Cases Miner. Bone Metab. 2012, 9, 100–106. [Google Scholar]
- Zhang, Y.; Huang, L.; Zhang, J.; De Souza Rastelli, A.N.; Yang, J.; Deng, D. Anti-Inflammatory Efficacy of Curcumin as an Adjunct to Non-Surgical Periodontal Treatment: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 808460. [Google Scholar] [CrossRef]
- O’leary, T.J.; Drake, R.B.; Naylor, J.E. The Plaque Control Record. J. Periodontol. 1972, 43, 38. [Google Scholar] [CrossRef] [PubMed]
- Kaldahl, W.B.; Kalkwarf, K.L.; Patil, K.D.; Molvar, M.P.; Dyer, J.K. Long-Term Evaluation of Periodontal Therapy: I. Response to 4 Therapeutic Modalities. J. Periodontol. 1996, 67, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S.; EFP Workshop Participants and Methodological Consultants. Treatment of Stage I–III Periodontitis—The EFP S3 Level Clinical Practice Guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Sanz, M.; Kebschull, M.; Jepsen, S.; Sculean, A.; Berglundh, T.; Papapanou, P.N.; Chapple, I.; Tonetti, M.S. Treatment of Stage IV Periodontitis: The EFP S3 Level Clinical Practice Guideline. J. Clin. Periodontol. 2022, 49 (Suppl. S2), 4–71. [Google Scholar] [CrossRef]
- Suvan, J.; Leira, Y.; Moreno Sancho, F.M.; Graziani, F.; Derks, J.; Tomasi, C. Subgingival Instrumentation for Treatment of Periodontitis. A Systematic Review. J. Clin. Periodontol. 2020, 47, 155–175. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, N.D.; Gupta, A.; Khan, S.; Bansal, N. Role of Salivary Matrix Metalloproteinase-8 (MMP-8) in Chronic Periodontitis Diagnosis. Front. Med. 2015, 9, 72–76. [Google Scholar] [CrossRef]
- Nędzi-Góra, M.; Górska, R.; Górski, B. The Utility of Gingival Crevicular Fluid Matrix Metalloproteinase-8 Provides Site-Specific Diagnostic Value for Periodontal Grading. Cent. Eur. J. Immunol. 2021, 46, 236–243. [Google Scholar] [CrossRef]
- Wong, S.P.; Tan, S.M.; Lee, C.S.; Law, K.B.; Lim, Y.A.L.; Rajasuriar, R. Prospective Longitudinal Analysis of Clinical and Immunological Risk Factors Associated with Oral and Gastrointestinal Mucositis Following Autologous Stem Cell Transplant in Adults. Support. Care Cancer 2023, 31, 494. [Google Scholar] [CrossRef]
- Beheshti, R.; Halstead, E.S.; Cusack, B.; Hicks, S.D. Multi-Omic Factors Associated with Frequency of Upper Respiratory Infections in Developing Infants. Int. J. Mol. Sci. 2023, 24, 934. [Google Scholar] [CrossRef]
- Glantz, S.A. Primer of Biostatistic, 6th ed.; Mc-Graw Hill: New York, NY, USA, 2005. [Google Scholar]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. PROC: An Open-Source Package for R and S+ to Analyze and Compare ROC Curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Epskamp, S.; Cramer, A.O.J.; Waldorp, L.J.; Schmittmann, V.D.; Borsboom, D. Qgraph: Network Visualizations of Relationships in Psychometric Data. J. Stat. Softw. 2012, 48, 1–18. [Google Scholar] [CrossRef]
- Kosmidis, I.; Schumacher, D.; Schwendinger, F. Detect t and Check for Separation and Infinite Maximum Likelihood Estimates; R package version 0.8-3. 2025. Available online: https://cran.r-project.org/package=detectseparation (accessed on 22 July 2025).
- Offenbacher, S.; Barros, S.P.; Beck, J.D. Rethinking Periodontal Inflammation. J. Periodontol. 2008, 79 (Suppl. S8), 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Checchi, L.; Montevecchi, M.; Checchi, V.; Zappulla, F. The Relationship between Bleeding on Probing and Subgingival Deposits. An Endoscopical Evaluation. Open Dent. J. 2009, 28, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Morozumi, T.; Numabe, Y.; Ogata, Y.; Nakayama, Y.; Sugaya, T.; Nakamura, T.; Sato, S.; Takashiba, S.; Sekino, S.; et al. Estimation of the Periodontal Inflamed Surface Area by Simple Oral Examination. J. Clin. Med. 2021, 10, 723. [Google Scholar] [CrossRef]
- Lang, N.P.; Adler, R.; Joss, A.; Nyman, S. Absence of Bleeding on Probing. An Indicator of Periodontal Stability. J. Clin. Periodontol. 1990, 17, 714–721. [Google Scholar] [CrossRef]
- Dukka, H.; Saleh, M.H.A.; Ravidà, A.; Greenwell, H.; Wang, H.L. Is Bleeding on Probing a Reliable Clinical Indicator of Peri-Implant Diseases? J. Periodontol. 2021, 92, 1669–1674. [Google Scholar] [CrossRef]
- Herz, M.M.; Hoffmann, N.; Braun, S.; Lachmann, S.; Bartha, V.; Petsos, H. Periodontal Pockets: Predictors for Site-Related Worsening after Non-Surgical Therapy-A Long-Term Retrospective Cohort Study. J. Clin. Periodontol. 2024, 51, 680–690. [Google Scholar] [CrossRef]
- Castagnola, M.; Scarano, E.; Passali, G.C.; Messana, I.; Cabras, T.; Iavarone, F.; Di Cintio, G.; Fiorita, A.; De Corso, E.; Paludetti, G. Salivary Biomrakers and Proteomics: Future Diagnostics and Clinical Utilities. Acta Otorhinolaryngol. Ital. 2017, 37, 94–101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reddahi, S.; Bouziane, A.; Rida, S.; Tligui, H.; Ennibi, O. Salivary Biomarkers in Periodontitis Patients: A Pilot Study. Int. J. Dent. 2022, 2022, 3664516. [Google Scholar] [CrossRef]
- Impola, U.; Jeskanen, L.; Ravanti, L.; Syrjänen, S.; Baldursson, B.; Kähäri, V.M.; Saarialho-Kere, U. Expression of Matrix Metalloproteinase (MMP)-7 and MMP-13 and Loss of MMP-19 and P16 Are Associated with Malignant Progression in Chronic Wounds. Br. J. Dermatol. 2005, 152, 720–726. [Google Scholar] [CrossRef]
- Van Lint, P.; Libert, C. Matrix Metalloproteinase-8: Cleavage Can Be Decisive. Cytokine Growth Factor Rev. 2006, 17, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Balbín, M.; Fueyo, A.; Knäuper, V.; Pendás, A.M.; López, J.M.; Jiménez, M.G.; Murphy, G.; López-Otín, C. Collagenase 2 (MMP-8) Expression in Murine Tissue-Remodeling Processes. Analysis of Its Potential Role in Postpartum Involution of the Uterus. J. Biol. Chem. 1998, 273, 23959–23968. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Park, J.S.; Lee, Y.Y.; Kim, D.Y.; Kang, J.L.; Kim, H.S. Anti-Inflammatory and Anti-Oxidant Mechanisms of an MMP-8 Inhibitor in Lipoteichoic Acid-Stimulated Rat Primary Astrocytes: Involvement of NF-ΚB, Nrf2, and PPAR-γ Signaling Pathways. J Neuroinflammation 2018, 15, 326. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.J.; Matthews, J.B.; Chapple, I.L.; Ling-Mountford, N.; Cooper, P.R. Periodontitis Associates with a Type 1 IFN Signature in Peripheral Blood Neutrophils. J. Immunol. 2008, 181, 5775–5784. [Google Scholar] [CrossRef]
- Cafiero, C.; Spagnuolo, G.; Marenzi, G.; Martuscelli, R.; Colamaio, M.; Leuci, S. Predictive Periodontitis: The Most Promising Salivary Biomarkers for Early Diagnosis of Periodontitis. J. Clin. Med. 2021, 10, 1488. [Google Scholar] [CrossRef]
- Czuszak, C.A.; Sutherland, D.E.; Billman, M.A.; Stein, S.H. Prostaglandin E2 Potentiates Interleukin-1 Beta Induced Interleukin-6 Production by Human Gingival Fibroblasts. J. Clin. Periodontol. 1996, 23, 635–640. [Google Scholar] [CrossRef]
- Xing, Z.; Gauldie, J.; Cox, G.; Baumann, H.; Jordana, M.; Lei, X.F.; Achong, M.K. IL-6 Is an Anti-Inflammatory Cytokine Required for Controlling Local or Systemic Acute Inflammatory Responses. J. Clin. Investig. 1998, 101, 311–320. [Google Scholar] [CrossRef]
- Chalise, J.; Narendra, S.; Paudyal, B.; Magnusson, M. Interferon Alpha Inhibits Antigen-Specific Production of Proinflammatory Cytokines and Enhances Antigen-Specific Transforming Growth Factor Beta Production in Antigen-Induced Arthritis. Arthritis Res. Ther. 2013, 15, 143. [Google Scholar] [CrossRef]
- Wolf, J.; Rose-John, S.; Garbers, C. Interleukin-6 and Its Receptors: A Highly Regulated and Dynamic System. Cytokine 2014, 70, 11–20. [Google Scholar] [CrossRef]
- Adisa, A.; Bahrami-Hessari, M.; Bhangu, A.; George, C.; Ghosh, D.; Glasbey, J.; Haque, P.; Ingabire, J.C.A.; Kamarajah, S.K.; Kudrna, L.; et al. Reducing the Environmental Impact of Surgery on a Global Scale: Systematic Review and Co-Prioritization with Healthcare Workers in 132 Countries. Br. J. Surg. 2023, 110, 804–817. [Google Scholar] [CrossRef] [PubMed]
- Mazurek-Mochol, M.; Bonsmann, T.; Mochol, M.; Poniewierska-Baran, A.; Pawlik, A. The Role of Interleukin 6 in Periodontitis and Its Complications. Int. J. Mol. Sci. 2024, 25, 2146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; An, J. Cytokines, Inflammation, and Pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Michalowicz, B.; Castillo, M.; Aeppli, D. Interleukin-1 Alpha, Interleukin-8 and Interferon-Alpha Levels in Gingival Crevicular Fluid. J. Periodontal Res. 1996, 31, 489–495. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving Inflammation: Dual Anti-Inflammatory and pro-Resolution Lipid Mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef]
- Cordoba-David, G.; Garcia-Gimenez, J.; Cardoso Castelo-Branco, R.; Carrasco, S.; Cannata, P.; Ortiz, A.; Ramos, A.M. Crosstalk between TBK1/IKKepsilon and the Type I Interferon Pathway Contributes to Tubulointerstitial Inflammation and Kidney Tubular Injury. Front. Pharmacol. 2022, 13, 987979. [Google Scholar] [CrossRef]
- Ji, L.; Li, T.; Chen, H.; Yang, Y.; Lu, E.; Liu, J.; Qiao, W.; Chen, H. The Crucial Regulatory Role of Type I Interferon in Inflammatory Diseases. Cell Biosci. 2023, 13, 230. [Google Scholar] [CrossRef]
- Silva, A.C.; Faria, M.R.; Fontes, A.; Campos, M.S.; Cavalcanti, B.N. Interleukin-1 Beta and Interleukin-8 in Healthy and Inflamed Dental Pulps. J. Appl. Oral Sci. 2009, 17, 527–532. [Google Scholar] [CrossRef]
- Cheng, R.; Wu, Z.; Li, M.; Shao, M.; Hu, T. Interleukin-1β Is a Potential Therapeutic Target for Periodontitis: A Narrative Review. Int. J. Oral Sci. 2020, 12, 2. [Google Scholar] [CrossRef]
- Kim, G.Y.; Lee, J.W.; Ryu, H.C.; Wei, J.D.; Seong, C.M.; Kim, J.H. Proinflammatory Cytokine IL-1beta Stimulates IL-8 Synthesis in Mast Cells via a Leukotriene B4 Receptor 2-Linked Pathway, Contributing to Angiogenesis. J. Immunol. 2010, 184, 3946–3954. [Google Scholar] [CrossRef]
- Bréchard, S.; Bueb, J.L.; Tschirhart, E.J. Interleukin-8 Primes Oxidative Burst in Neutrophil-like HL-60 through Changes in Cytosolic Calcium. Cell Calcium 2005, 37, 531–540. [Google Scholar] [CrossRef]
- Uchida, Y.; Shiba, H.; Komatsuzawa, H.; Takemoto, T.; Sakata, M.; Fujita, T.; Kawaguchi, H.; Sugai, M.; Kurihara, H. Expression of IL-1 Beta and IL-8 by Human Gingival Epithelial Cells in Response to Actinobacillus Actinomycetemcomitans. Cytokine 2001, 14, 152–161. [Google Scholar] [CrossRef]
- Al-Qahtani, A.A.; Alhamlan, F.S.; Al-Qahtani, A.A. Pro-Inflammatory and Anti-Inflammatory Interleukins in Infectious Diseases: A Comprehensive Review. Trop. Med. Infect. Dis. 2024, 9, 13. [Google Scholar] [CrossRef]
- Noh, M.K.; Jung, M.; Kim, S.H.; Lee, S.R.; Park, K.H.; Kim, D.H.; Kim, H.H.; Park, Y.G. Assessment of IL-6, IL-8 and TNF-α Levels in the Gingival Tissue of Patients with Periodontitis. Exp. Ther. Med. 2013, 6, 847–851. [Google Scholar] [CrossRef]
- Uceyler, N.; Valenza, R.; Stock, M. Reduced Levels of Antiinflammatory Cytokines in Patients with Chronic Widespread Pain. Arthritis Rheum 2006, 54, 2656–2664. [Google Scholar] [PubMed]
- Sachdeva, S.; Harish, S.; Amit, M.; Tanupriya, S. Interleukins in Periodontics. J. Head Neck Physicians Surg. 2020, 8, 70–75. [Google Scholar]
- Bertoldi, C.; Pellacani, C.; Lalla, M.; Consolo, U.; Pinti, M.; Cortellini, P.; Cossarizza, A. Herpes Simplex i Virus Impairs Regenerative Outcomes of Periodontal Regenerative Therapy in Intrabony Defects. A Pilot Study. J. Clin. Periodontol. 2012, 39, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, A.; Gigante, I.; Colucci, S.; Grano, M. Periodontal Disease: Linking the Primary Inflammation to Bone Loss. Clin. Dev. Immunol. 2013, 2013, 503754. [Google Scholar] [CrossRef]
- Neurath, N.; Kesting, M. Cytokines in Gingivitis and Periodontitis: From Pathogenesis to Therapeutic Targets. Front. Immunol. 2024, 15, 1435054. [Google Scholar] [CrossRef]
- Deng, K.; Pelekos, G.; Jin, L.; Tonetti, M.S. Diagnostic Accuracy of Self-Reported Measures of Periodontal Disease: A Clinical Validation Study Using the 2017 Case Definitions. J. Clin. Periodontol. 2021, 48, 1037–1050. [Google Scholar] [CrossRef]
- Bertoldi, C.; Bellei, E.; Pellacani, C.; Ferrari, D.; Lucchi, A.; Cuoghi, A.; Bergamini, S.; Cortellini, P.; Tomasi, A.; Zaffe, D.; et al. Non-Bacterial Protein Expression in Periodontal Pockets by Proteome Analysis. J. Clin. Periodontol. 2013, 40, 573–582. [Google Scholar] [CrossRef]
- Bellei, E.; Bertoldi, C.; Monari, E.; Bergamini, S. Proteomics Disclose the Potential of Gingival Crevicular Fluid (GCF) as a Source of Biomarkers for Severe Periodontitis. Materials 2022, 15, 2161. [Google Scholar] [CrossRef]
- Headland, S.E.; Norling, L.V. The Resolution of Inflammation: Principles and Challenges. Semin. Immunol. 2015, 27, 149–160. [Google Scholar] [CrossRef]
- Sahni, V.; Dyke, T.E. Immunomodulation of Periodontitis with SPMs. Front. Oral Health 2023, 4, 1288722. [Google Scholar] [CrossRef]



| G1 | G2 | ||
|---|---|---|---|
| TP0 | TP0 | TP1 | |
| N (females) | 16 (8) | 10 (6) | |
| Age (years) | 24.9 ± 4.53 | 52.2 ± 8.28 * | |
| FMPS (%) | 8.12 ± 6.03 | 41.10 ± 22.20 | 20.72 ± 9.68 *° |
| FMBS (%) | 3.62 ± 3.12 | 33.80 ± 15.34 | 16.90 ± 10.82 *° |
| MPD (mm) | 1.24 ± 0.15 | 2.63 ± 0.65 | 1.94 ± 0.42 *° |
| MAL (mm) | 0 | 3.56 ± 0.75 | 2.95 ± 0.67 *° |
| NPP5 | 0 | 19.00 ± 12.76 | 7.30 ± 11.12 *° |
| Biomarker | Expression in Periodontitis | Function | Interaction |
|---|---|---|---|
| MMP-8 | Upregulated |
|
|
| IL-1β | Upregulated |
|
|
| IL-8 | Upregulated |
|
|
| IL-6 | Upregulated |
|
|
| IFN-α | Upregulated |
|
|
| IL-4 | Downregulated |
|
|
| IL-10 | Downregulated or insufficient |
|
|
| Biomarker | β (Coeff.) | St. Error | p-Value | OR (exp(β)) | 95% IC OR | Effect % on Odds | Biologic Significance |
|---|---|---|---|---|---|---|---|
| IL-1-β | 0.0067 | 0.0026 | 0.0108 | 1.0067 | [1.0016–1.0119] | ↑ +0.67% | Increased Risk |
| IL-8 | 0.0062 | 0.0024 | 0.0087 | 1.0062 | [1.0015–1.0110] | ↑ +0.62% | Increased Risk |
| IL-4 | −11.1641 | 4.4322 | 0.0118 | 0.0000142 | [6.19e−08–0.0334] | ↓ −99.99% | Reduced Risk |
| IL-10 | −3.5171 | 1.5243 | 0.0210 | 0.030 | [0.0021–0.4416] | ↓ –97% | Reduced Risk |
| Biomarker | β (Coeff.) | St. Error | p-Value | OR (exp(β)) | 95% IC OR | Effect % on Odds | Biologic Significance |
|---|---|---|---|---|---|---|---|
| IL-1-β | −0.0038 | 0.0020 | 0.059 | 0.9962 | [0.9923–1.0001] | ↑ +0.38% | Increased risk |
| IL-10 | 3.289 | 1.687 | 0.0511 | 26.81 | [2.67–268.33] | ↑ +2581% | Reduced Risk |
| Biomarker | β (Coeff.) | St. Error | p-Value | OR (exp(β)) | 95% IC OR | Effect % on Odds | Biologic Significance |
|---|---|---|---|---|---|---|---|
| IL-8 | −0.0175 | 0.0089 | 0.0472 | 0.9896 | [0.9657–0.9999] | ↑ 1.74% | Reduced Risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertoldi, C.; Nasi, M.; Salvatori, R.; Pinti, M.; Montagna, S.; Tonetti, M.; Generali, L.; Bellei, E.; Zaffe, D.; Selleri, V.; et al. Salivary Biomarker Analysis to Distinguish Between Health and Periodontitis Status: A Preliminary Study. Dent. J. 2025, 13, 436. https://doi.org/10.3390/dj13090436
Bertoldi C, Nasi M, Salvatori R, Pinti M, Montagna S, Tonetti M, Generali L, Bellei E, Zaffe D, Selleri V, et al. Salivary Biomarker Analysis to Distinguish Between Health and Periodontitis Status: A Preliminary Study. Dentistry Journal. 2025; 13(9):436. https://doi.org/10.3390/dj13090436
Chicago/Turabian StyleBertoldi, Carlo, Milena Nasi, Roberta Salvatori, Marcello Pinti, Silvia Montagna, Maurizio Tonetti, Luigi Generali, Elisa Bellei, Davide Zaffe, Valentina Selleri, and et al. 2025. "Salivary Biomarker Analysis to Distinguish Between Health and Periodontitis Status: A Preliminary Study" Dentistry Journal 13, no. 9: 436. https://doi.org/10.3390/dj13090436
APA StyleBertoldi, C., Nasi, M., Salvatori, R., Pinti, M., Montagna, S., Tonetti, M., Generali, L., Bellei, E., Zaffe, D., Selleri, V., & Bergamini, S. (2025). Salivary Biomarker Analysis to Distinguish Between Health and Periodontitis Status: A Preliminary Study. Dentistry Journal, 13(9), 436. https://doi.org/10.3390/dj13090436











