Technical Complications and Marginal Bone Loss Depending on the Crown Material of Dental Implants in the Posterior Region: A 3-Year Randomized Clinical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Ethical Considerations
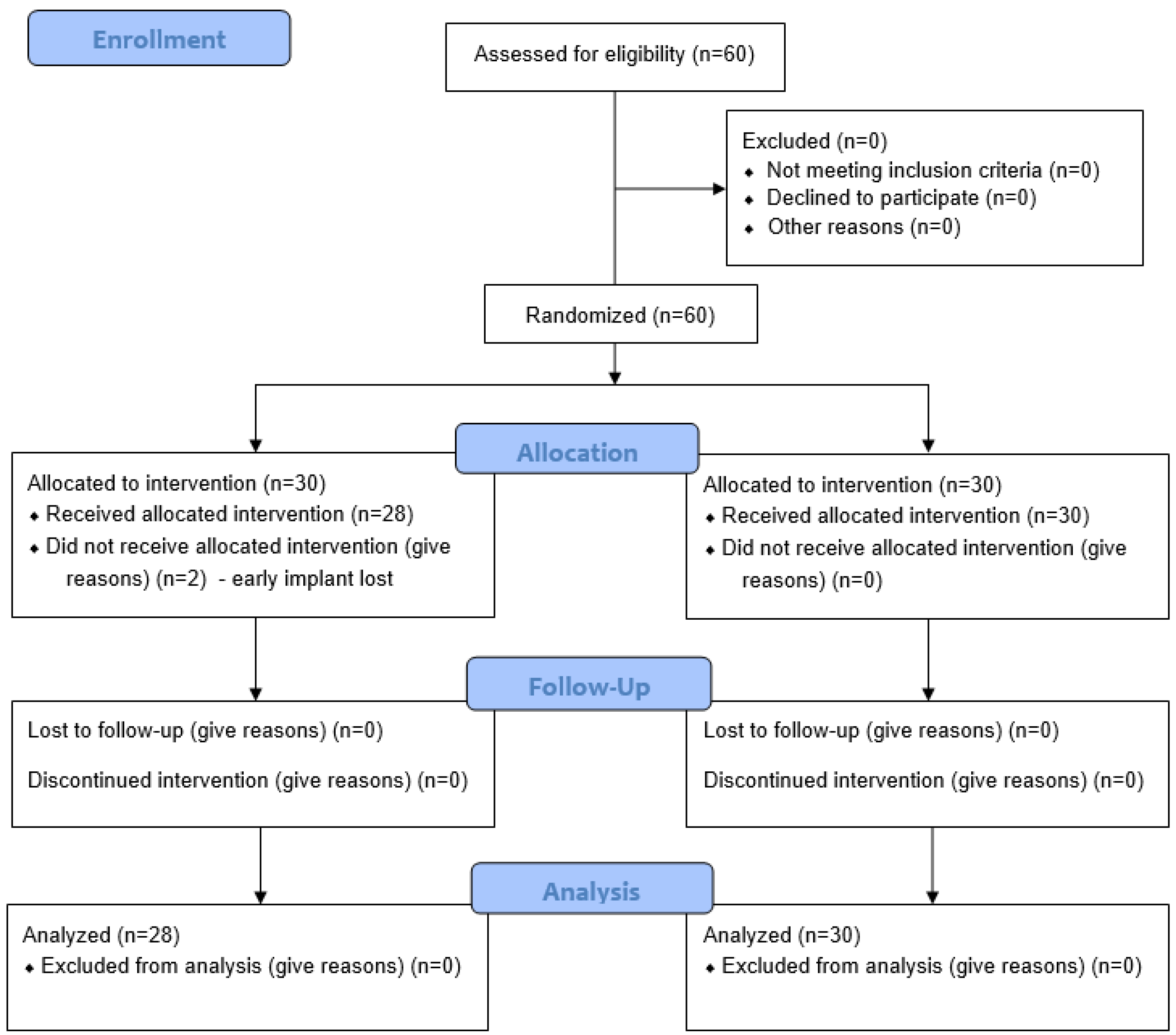
2.3. Sample Size, Randomization, and Blinding
2.3.1. Inclusion Criteria
- Presence of a single tooth gap in the upper or lower posterior region with indication for implant-supported prosthetic restoration;
- The tooth loss occurred at least three months ago (late or early implantation);
- The gap to be filled was bordered by adjacent natural teeth or implants;
- There was a natural tooth on the contralateral side for functional reference;
- Antagonistic dentition was completely present;
- Written informed consent to participate in this study was obtained.
2.3.2. Exclusion Criteria
- Age under 18 or lack of legal capacity;
- Presence of untreated periodontal disease with staging > stage II and/or grading B or C;
- Heavy nicotine use (more than 10 cigarettes per day);
- Taking bisphosphonates;
- Pregnancy;
- Alcohol or drug addiction;
- Diagnosed infectious diseases such as hepatitis B/C or HIV/AIDS;
- Uncontrolled or severe diabetes mellitus;
- Patients who wear mouth guards due to severe bruxism;
- Need for immediate implantation;
- Primarily recognizable extensive augmentation requirements, such as a sinus lift.
2.4. Clinical Procedure and Intervention Groups
2.5. Hybrid Abutment Crowns
- Lithium disilicate ceramic (IPS e.max CAD, Ivoclar Vivadent AG, Schaan, Liechtenstein);
- Hybrid ceramic with polymer content (VITA Enamic, VITA Zahnfabrik GmbH, Bad Säckingen, Germany).
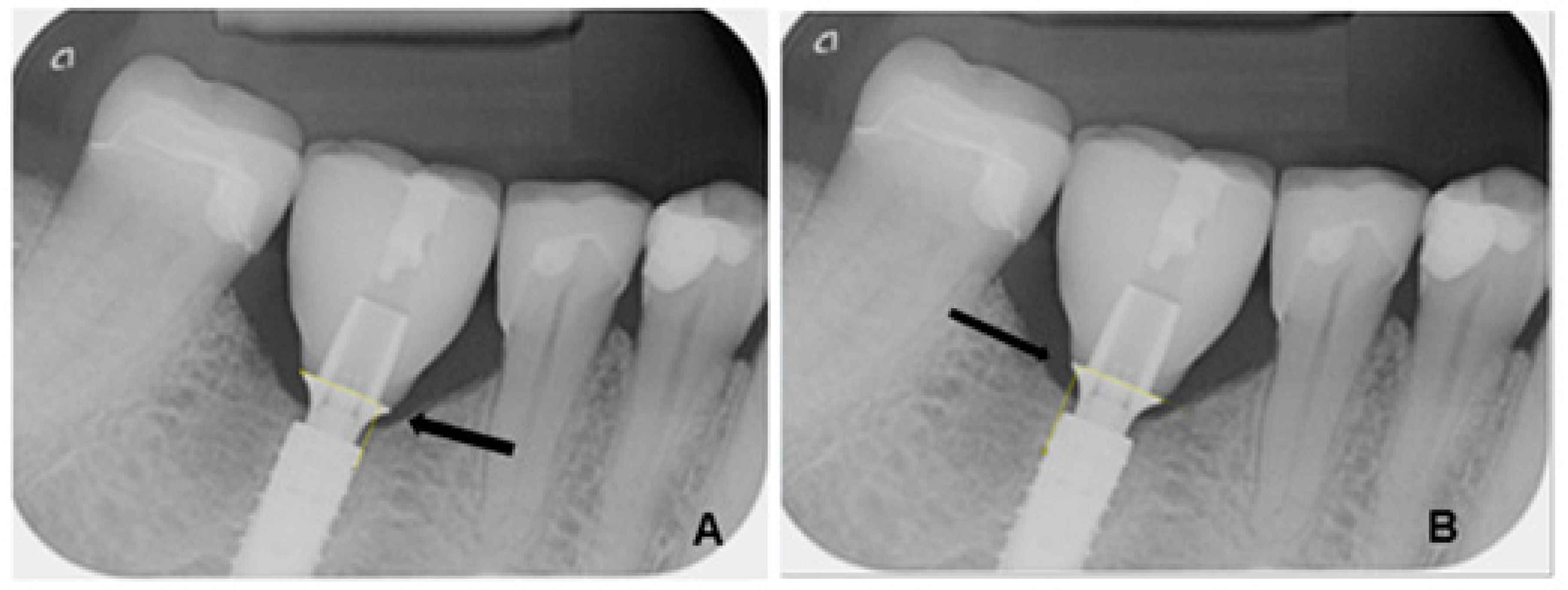
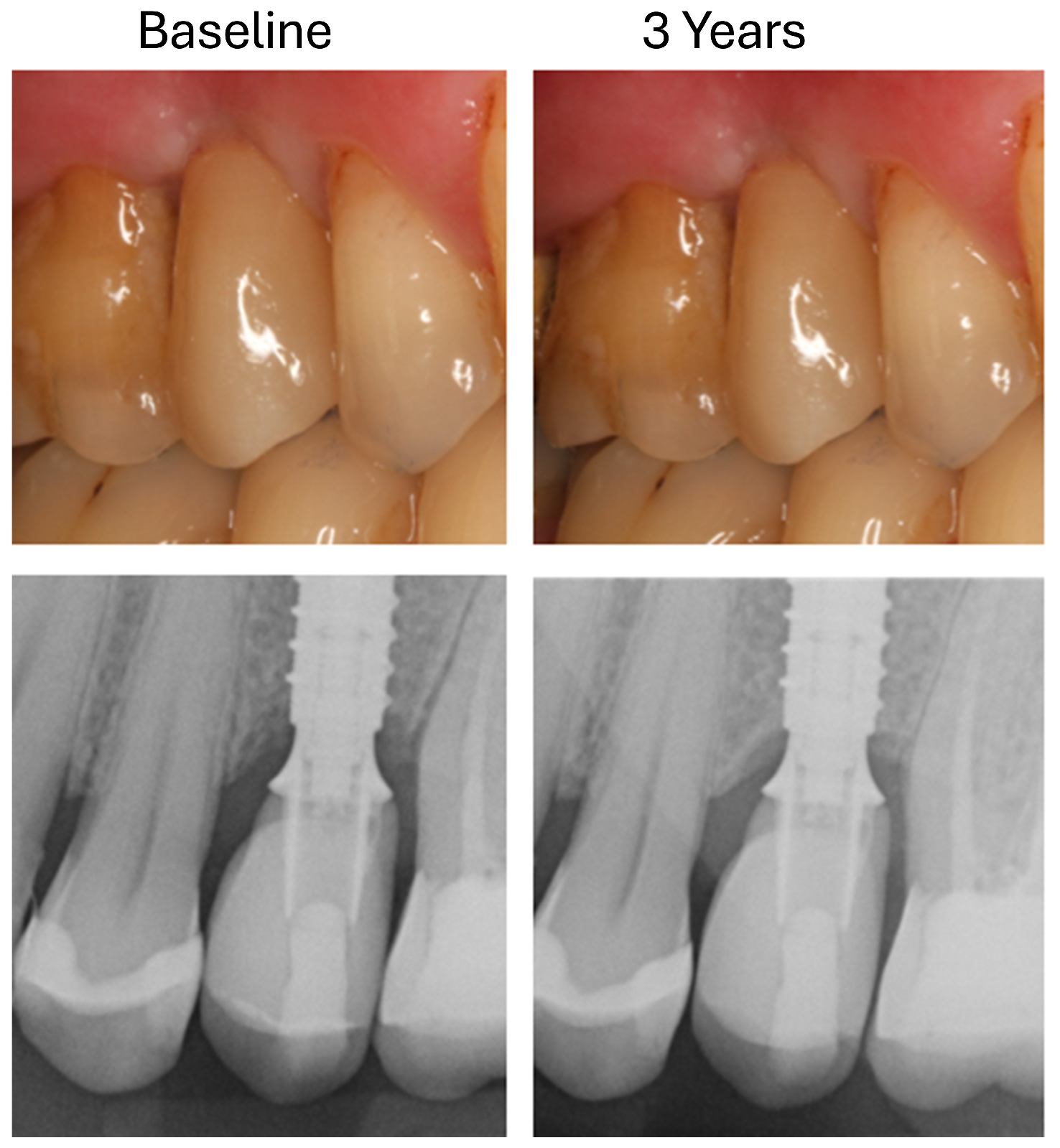
2.6. Radiological Analysis of Marginal Bone Loss
- T0: 1 week after insertion of the superstructure (baseline);
- T1: 3 years after implantation.
2.7. Recording Technical Complications
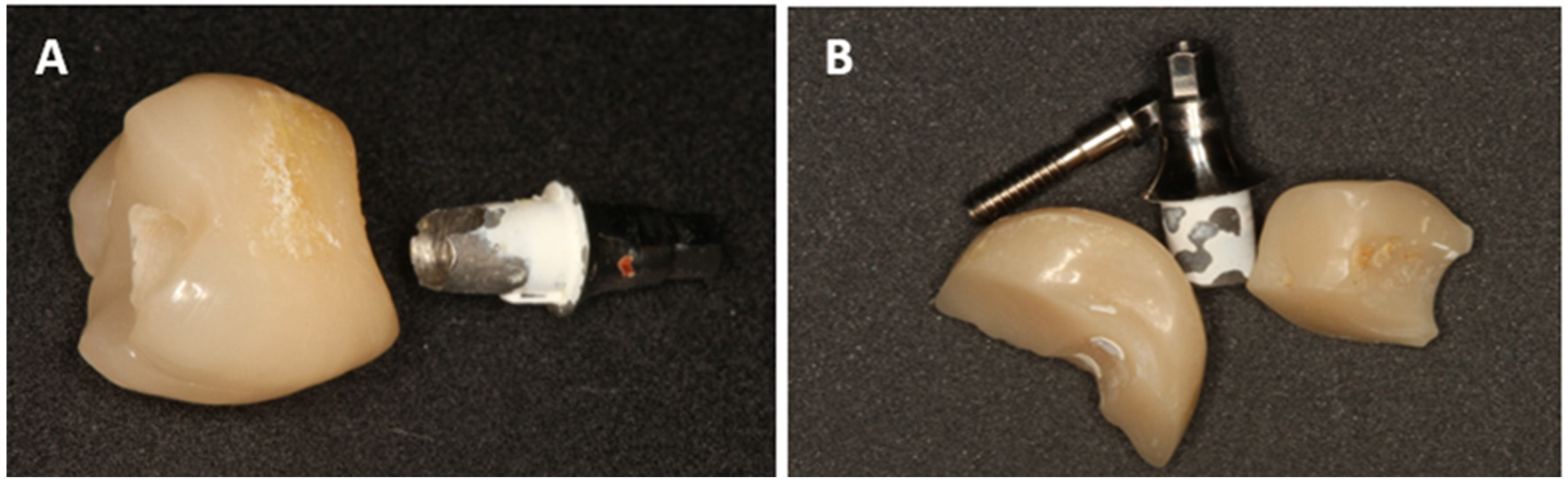
- Chipping or fractures of the all-ceramic superstructures (including photo documentation);
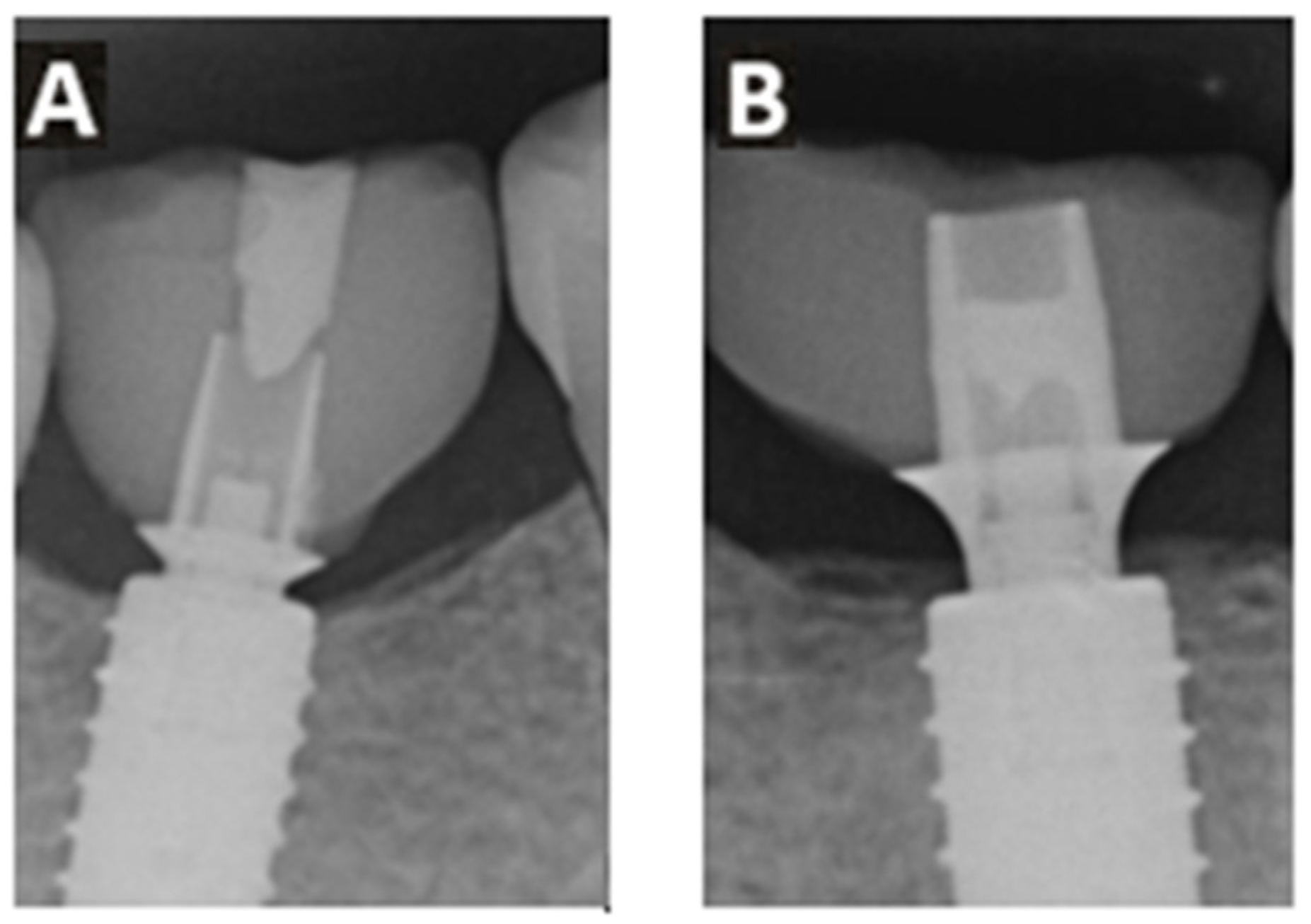
- Damage to the implant-abutment complex (Figure 2);
- Loosening or fractures of abutment screws.
2.8. Statistical Evaluation
3. Results
3.1. Patient Population and Implant Distribution
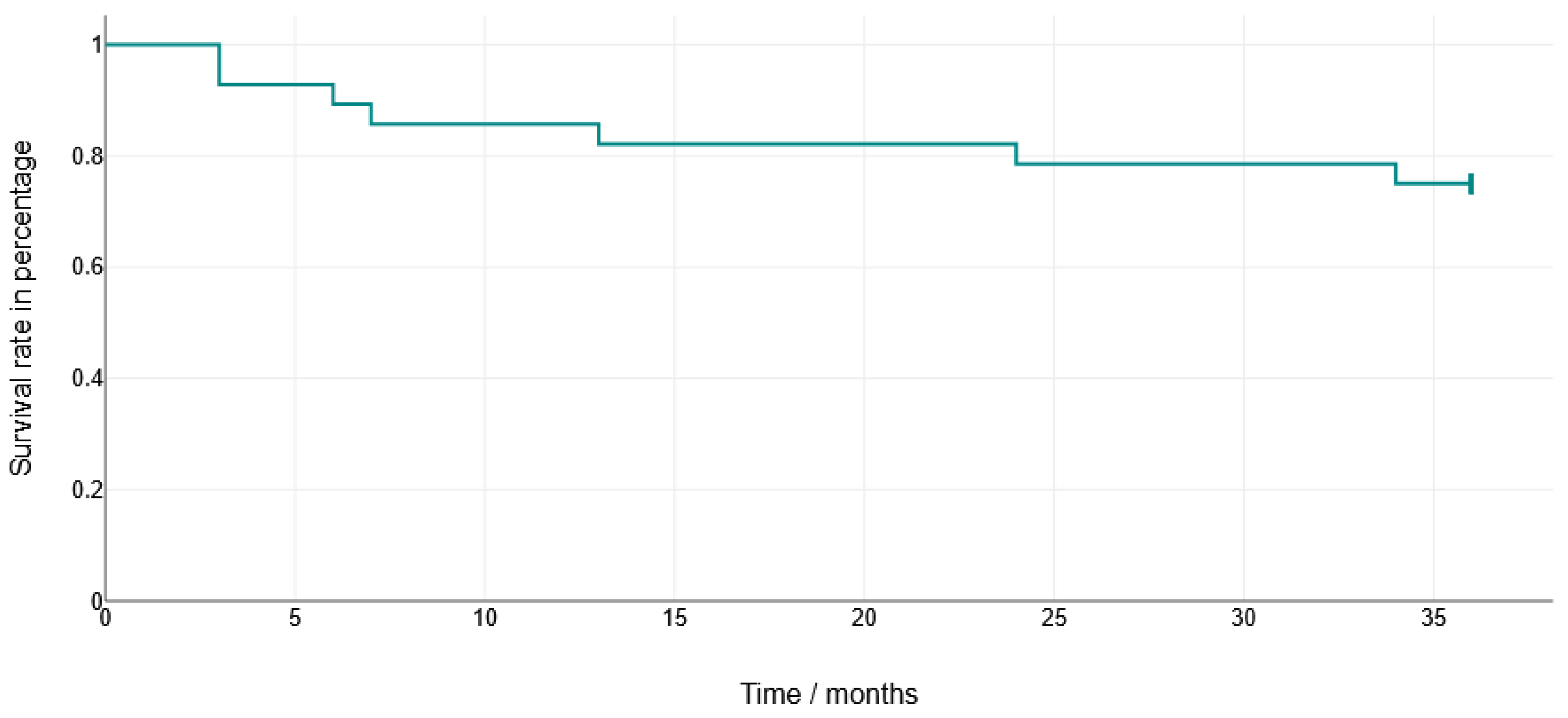
3.2. Implant Survival
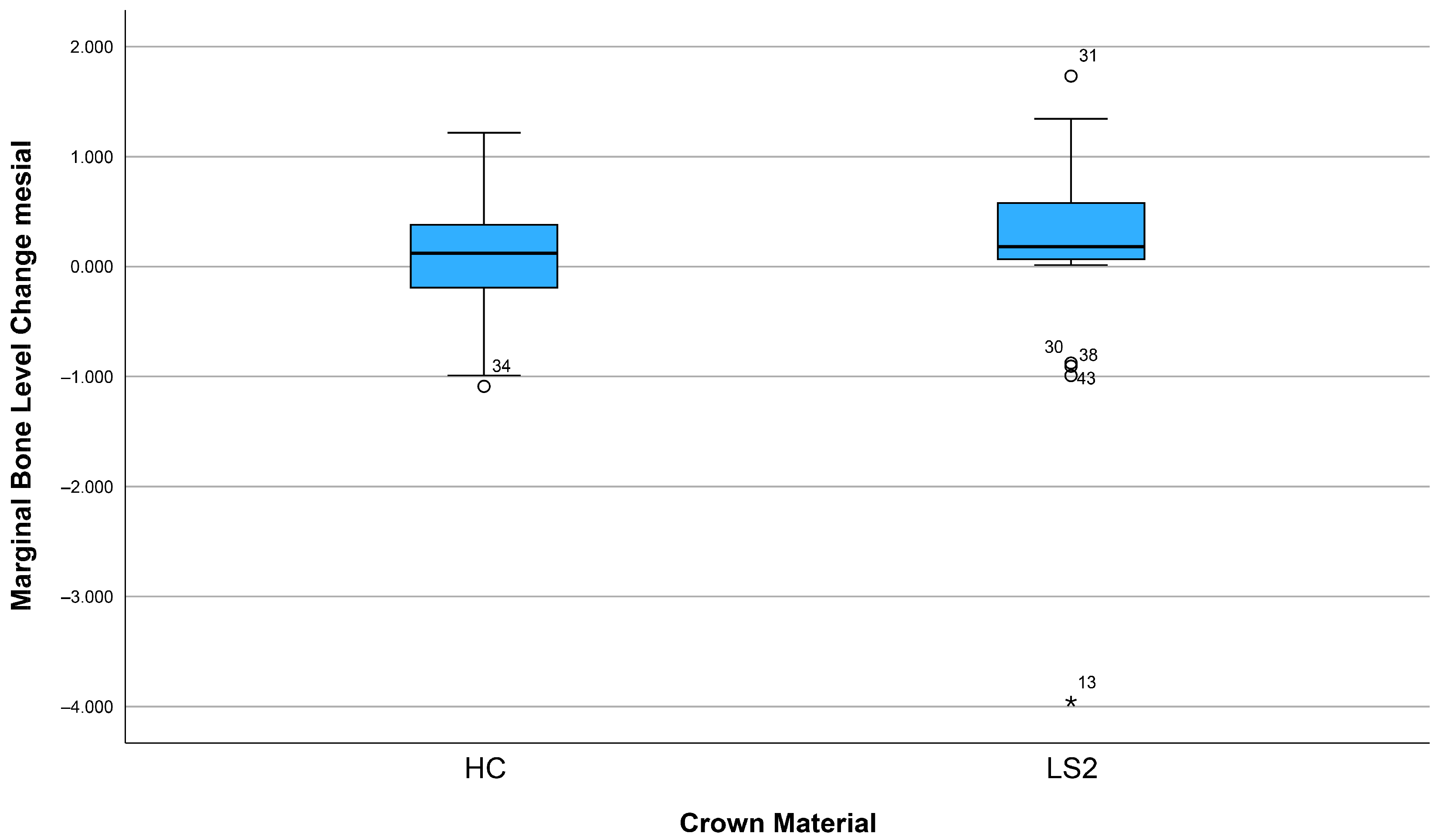
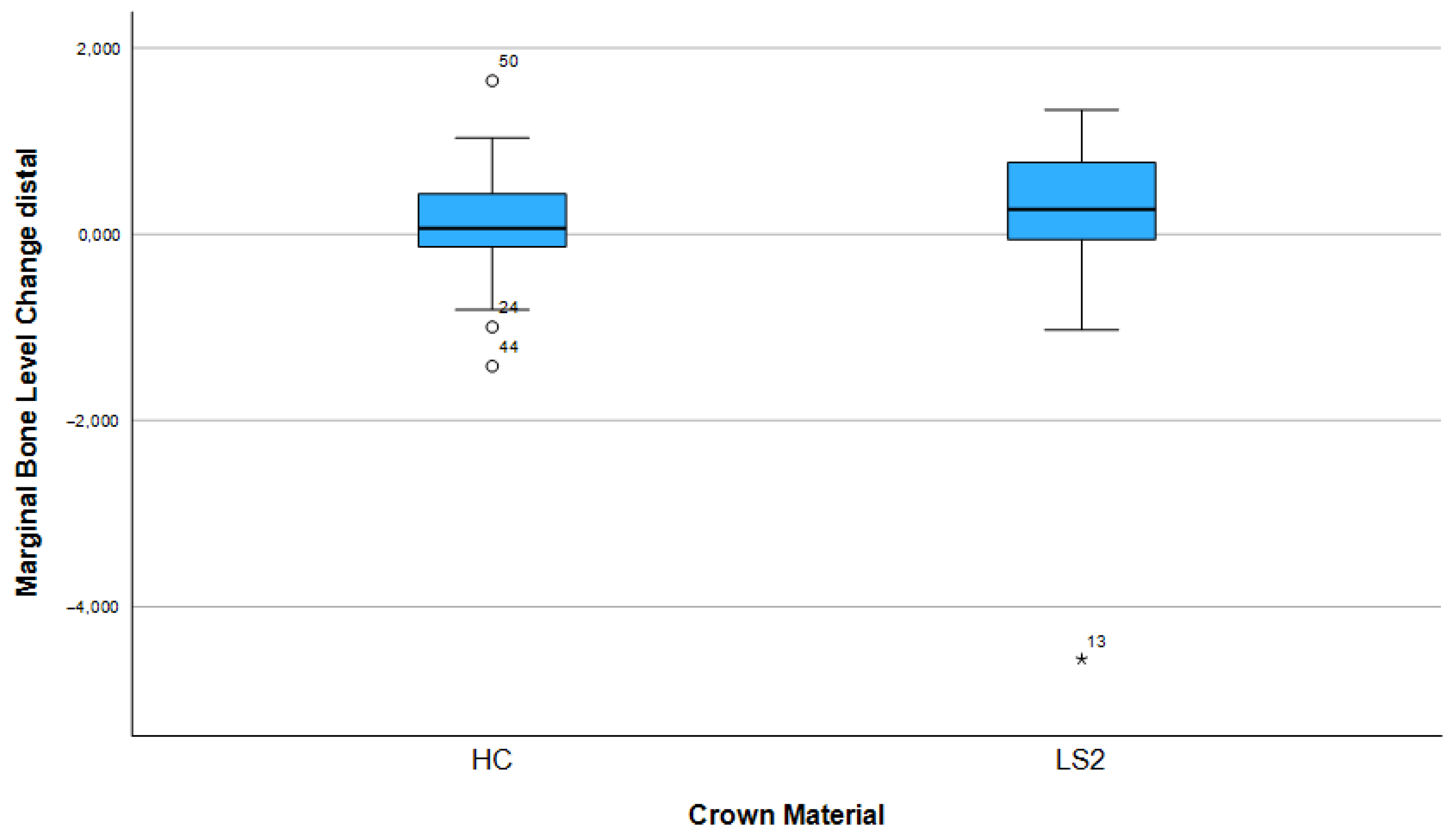
3.3. Changes in Marginal Bone Profile
3.4. Technical Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, R.E.; Zembic, A.; Pjetursson, B.E.; Zwahlen, M.; Thoma, D.S. Systematic review of the survival rate and the incidence of biological, technical, and aesthetic complications of single crowns on implants reported in longitudinal studies with a mean follow-up of 5 years. Clin. Oral Implant. Res. 2012, 23 (Suppl. S6), 2–21. [Google Scholar] [CrossRef]
- Smirani, R.; Chantler, J.G.; Endres, J.; Jung, R.E.; Naenni, N.; Strauss, F.J.; Thoma, D.S. Clinical outcomes of single implant supported crowns utilising the titanium base abutment: A 7.5-year prospective cohort study. J. Dent. 2024, 149, 105306. [Google Scholar] [CrossRef] [PubMed]
- Khijmatgar, S.; Tumedei, M.; Tartaglia, G.; Crescentini, M.; Isola, G.; Sidoti, E.; Sforza, C.; Del Fabbro, M.; Tartaglia, G.M. Fifteen-year recall period on zirconia-based single crowns and fixed dental prostheses. A prospective observational study. BDJ Open 2024, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.E.; Perel, M.L.; Wang, H.L.; Sammartino, G.; Galindo-Moreno, P.; Trisi, P.; Steigmann, M.; Rebaudi, A.; Palti, A.; Pikos, M.A.; et al. Implant success, survival, and failure: The International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant. Dent. 2008, 17, 5–15. [Google Scholar] [CrossRef]
- Afshari, Z.; Yaghini, J.; Naseri, R. Levels of Smoking and Peri-Implant Marginal Bone Loss: A Systematic Review and Meta-Analysis. J. Evid. Based Dent. Pract. 2022, 22, 101721. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chen, Z.; Pan, W.L.; Wang, H.L. Is History of Periodontal Disease Still a Negative Risk Indicator for Peri-implant Health Under Supportive Post-implant Treatment Coverage? A Systematic Review and Meta-analysis. Int. J. Oral Maxillofac. Implant. 2020, 35, 52–62. [Google Scholar] [CrossRef]
- Atieh, M.A.; AlAli, F.; Alsabeeha, N.H.M. Outcome of supportive peri-implant therapy on the rates of peri-implant diseases and marginal bone loss: A systematic review and meta-analysis. Quintessence Int. 2021, 52, 122–131. [Google Scholar]
- de Carvalho Barbara, J.G.; Luz, D.; Vianna, K.; Porto Barboza, E. The influence of abutment disconnections on peri-implant marginal bone: A systematic review. Int. J. Oral Implantol. 2019, 12, 283–296. [Google Scholar]
- Häggman-Henrikson, B.; Ali, D.; Aljamal, M.; Chrcanovic, B. Bruxism and dental implants: A systematic review and meta-analysis. J. Oral Rehabil. 2023, 51, 202–217. [Google Scholar] [CrossRef]
- Hamed, M.T.; Abdullah Mously, H.; Khalid Alamoudi, S.; Hossam Hashem, A.B.; Hussein Naguib, G. A Systematic Review of Screw versus Cement-Retained Fixed Implant Supported Reconstructions. Clin. Cosmet. Investig. Dent. 2020, 12, 9–16. [Google Scholar] [CrossRef] [PubMed]
- de Brandao, M.L.; Vettore, M.V.; Vidigal Junior, G.M. Peri-implant bone loss in cement- and screw-retained prostheses: Systematic review and meta-analysis. J. Clin. Periodontol. 2013, 40, 287–295. [Google Scholar] [CrossRef]
- Raval, N.C.; Wadhwani, C.P.; Jain, S.; Darveau, R.P. The Interaction of Implant Luting Cements and Oral Bacteria Linked to Peri-Implant Disease: An In Vitro Analysis of Planktonic and Biofilm Growth—A Preliminary Study. Clin. Implant. Dent. Relat. Res. 2015, 17, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.P.; Kim, D.M.; Ng, M.W.; Hwang, J.W.; Fiorellini, J.P. Peri-implant soft-tissue health surrounding cement- and screw-retained implant restorations: A multi-center, 3-year prospective study. Clin. Oral Implant. Res. 2006, 17, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.C., Jr.; Bonfante, E.A.; Rocha, E.P.; Silva, N.R.; Marotta, L.; Coelho, P.G. Effect of implant connection and restoration design (screwed vs. cemented) in reliability and failure modes of anterior crowns. Eur. J. Oral Sci. 2011, 119, 323–330. [Google Scholar] [CrossRef]
- Sailer, I.; Karasan, D.; Todorovic, A.; Ligoutsikou, M.; Pjetursson, B.E. Prosthetic failures in dental implant therapy. Periodontology 2000 2022, 88, 130–144. [Google Scholar] [CrossRef]
- Pitta, J.; Hjerppe, J.; Burkhardt, F.; Fehmer, V.; Mojon, P.; Sailer, I. Mechanical stability and technical outcomes of monolithic CAD/CAM fabricated abutment-crowns supported by titanium bases: An in vitro study. Clin. Oral Implant. Res. 2021, 32, 222–232. [Google Scholar] [CrossRef]
- Stimmelmayr, M.; Edelhoff, D.; Guth, J.F.; Erdelt, K.; Happe, A.; Beuer, F. Wear at the titanium-titanium and the titanium-zirconia implant-abutment interface: A comparative in vitro study. Dent. Mater. 2012, 28, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Alseddiek, A.; Al-Zordk, W.; Attia, A. Retention of hybrid-abutment-crowns with offset implant placement: Influence of Crown materials and Ti-base height. BMC Oral Health 2023, 23, 784. [Google Scholar] [CrossRef]
- Sevimay, M.; Turhan, F.; Kılıçarslan, M.A.; Eskitascioglu, G. Three-dimensional finite element analysis of the effect of different bone quality on stress distribution in an implant-supported crown. J. Prosthet. Dent. 2005, 93, 227–234. [Google Scholar] [CrossRef]
- Menicucci, G.; Mossolov, A.; Mozzati, M.; Lorenzetti, M.; Preti, G. Tooth-implant connection: Some biomechanical aspects based on finite element analyses. Clin. Oral Implant. Res. 1998, 9, 334–342. [Google Scholar] [CrossRef]
- Baggi, L.; Cappelloni, I.; Di Girolamo, M.; Maceri, F.; Vairo, G. The influence of implant diameter and length on stress distribution of osseointegrated implants related to crestal bone geometry: A three-dimensional finite element analysis. J. Prosthet. Dent. 2008, 100, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Robau-Porrua, A.; Pérez-Rodríguez, Y.; Soris-Rodríguez, L.M.; Pérez-Acosta, O.; González, J.E. The effect of diameter, length and elastic modulus of a dental implant on stress and strain levels in peri-implant bone: A 3D finite element analysis. Biomed Mater Eng. 2020, 30, 541–558. [Google Scholar] [CrossRef]
- Muhlemann, S.; Kraus, R.D.; Hammerle, C.H.F.; Thoma, D.S. Is the use of digital technologies for the fabrication of implant-supported reconstructions more efficient and/or more effective than conventional techniques: A systematic review. Clin. Oral Implant. Res. 2018, 29 (Suppl. S18), 184–195. [Google Scholar] [CrossRef]
- Derks, J.; Hakansson, J.; Wennstrom, J.L.; Tomasi, C.; Larsson, M.; Berglundh, T. Effectiveness of implant therapy analyzed in a Swedish population: Early and late implant loss. J. Dent. Res. 2015, 94 (Suppl. S3), 44S–51S. [Google Scholar] [CrossRef]
- Tomasi, C.; Derks, J. Etiology, occurrence, and consequences of implant loss. Periodontology 2000 2022, 88, 13–35. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Asgeirsson, A.G.; Zwahlen, M.; Sailer, I. Improvements in implant dentistry over the last decade: Comparison of survival and complication rates in older and newer publications. Int. J. Oral Maxillofac. Implant. 2014, 29, 308–324. [Google Scholar] [CrossRef]
- Hotinski, E.; Dudley, J. Abutment screw loosening in angulation-correcting implants: An in vitro study. J. Prosthet. Dent. 2019, 121, 151–155. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Bragger, U.; Lang, N.P.; Zwahlen, M. Comparison of survival and complication rates of tooth-supported fixed dental prostheses (FDPs) and implant-supported FDPs and single crowns (SCs). Clin. Oral Implant. Res. 2007, 18 (Suppl. S3), 97–113. [Google Scholar] [CrossRef] [PubMed]
- Hammerle, C.H.; Wagner, D.; Bragger, U.; Lussi, A.; Karayiannis, A.; Joss, A.; Lang, N.P. Threshold of tactile sensitivity perceived with dental endosseous implants and natural teeth. Clin. Oral Implant. Res. 1995, 6, 83–90. [Google Scholar] [CrossRef]
- Mikeli, A.; Walter, M.H.; Rau, S.A.; Raedel, M.; Raedel, M. Three-year clinical performance of posterior monolithic zirconia single crowns. J. Prosthet. Dent. 2022, 128, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Mangano, F.; Veronesi, G. Digital versus Analog Procedures for the Prosthetic Restoration of Single Implants: A Randomized Controlled Trial with 1 Year of Follow-Up. Biomed. Res. Int. 2018, 2018, 5325032. [Google Scholar] [CrossRef]
- Jemt, T.; Lekholm, U.; Adell, R. Osseointegrated implants in the treatment of partially edentulous patients: A preliminary study on 876 consecutively placed fixtures. Int. J. Oral Maxillofac. Implant. 1989, 4, 211–217. [Google Scholar]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Reasons for failures of oral implants. J. Oral Rehabil. 2014, 41, 443–476. [Google Scholar] [CrossRef]
- Cheng, Y.; Lai, Z.; Yu, W. Influencing factors and survival rates in immediate vs. delayed dental implant placement: A six-year retrospective analysis. Front. Dent. Med. 2025, 6, 1563641. [Google Scholar] [CrossRef] [PubMed]
- Khehra, A.; Zanner, O.; Samet, N.; Levin, L. The Influence of Patient-, Site-, and Implant-Related Factors on Marginal Bone Levels of Dental Implants in a Rural Population in China: A Retrospective Study. Clin. Exp. Dent. Res. 2024, 10, e70045. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sanchez, R.; Dopico, J.; Kalemaj, Z.; Buti, J.; Pardo Zamora, G.; Mardas, N. Comparison of clinical outcomes of immediate versus delayed placement of dental implants: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2022, 33, 231–277. [Google Scholar] [CrossRef] [PubMed]
- Linkevicius, T.; Apse, P.; Grybauskas, S.; Puisys, A. The influence of soft tissue thickness on crestal bone changes around implants: A 1-year prospective controlled clinical trial. Int. J. Oral Maxillofac. Implant. 2009, 24, 712–719. [Google Scholar]
- Tomar, S.; Saxena, D.; Kaur, N. Marginal bone loss around implants with platform switching and platform matched connection: A systematic review. J. Prosthet. Dent. 2025, 134, 109–113. [Google Scholar] [CrossRef]
- Pisoni, L.; Ordesi, P.; Siervo, P.; Bianchi, A.E.; Persia, M.; Siervo, S. Flapless Versus Traditional Dental Implant Surgery: Long-Term Evaluation of Crestal Bone Resorption. J. Oral Maxillofac. Surg. 2016, 74, 1354–1359. [Google Scholar] [CrossRef]
- Cai, H.; Liang, X.; Sun, D.Y.; Chen, J.Y. Long-term clinical performance of flapless implant surgery compared to the conventional approach with flap elevation: A systematic review and meta-analysis. World J. Clin. Cases 2020, 8, 1087–1103. [Google Scholar] [CrossRef]
- Vatenas, I.; Linkevicius, T. One abutment one time vs. repeatable abutment disconnections in implants, restored with cemented/screw retained fixed partial dentures: Marginal bone level changes. A systematic review and meta-analysis. Stomatologija 2021, 23, 35–40. [Google Scholar] [PubMed]
- Nunes, M.; Leitao, B.; Pereira, M.; Fernandes, J.C.H.; Fernandes, G.V.O. Final Prosthetic Abutment Insertion Time (One-Time Abutment Insertion Protocol Versus Conventional Protocol) and Related Outcomes: A Systematic Review of Randomized Controlled Trials with Meta-analysis. Int. J. Oral Maxillofac. Implant. 2025, 40, 162–170. [Google Scholar]
- Atieh, M.A.; Tawse-Smith, A.; Alsabeeha, N.H.M.; Ma, S.; Duncan, W.J. The One Abutment-One Time Protocol: A Systematic Review and Meta-Analysis. J. Periodontol. 2017, 88, 1173–1185. [Google Scholar] [CrossRef]
- Denry, I.; Kelly, J.R. Emerging ceramic-based materials for dentistry. J. Dent. Res. 2014, 93, 1235–1242. [Google Scholar] [CrossRef]
- Sailer, I.; Muhlemann, S.; Kohal, R.J.; Spies, B.C.; Pjetursson, B.E.; Lang, N.P.; Gotfredsen, K.L.; Ellingsen, J.E.; Francisco, H.; Ozcan, M.; et al. Reconstructive aspects: Summary and consensus statements of group 3. The 5(th) EAO Consensus Conference 2018. Clin. Oral Implant. Res. 2018, 29 (Suppl. S18), 237–242. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Sailer, I.; Latyshev, A.; Rabel, K.; Kohal, R.J.; Karasan, D. A systematic review and meta-analysis evaluating the survival, the failure, and the complication rates of veneered and monolithic all-ceramic implant-supported single crowns. Clin. Oral Implant. Res. 2021, 32 (Suppl. S21), 254–288. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, D.; Tian, J.; Zhao, Y.; Lin, Y.; Di, P. Clinical evaluation and quantitative occlusal change analysis of posterior implant-supported all-ceramic crowns: A 3-year randomized controlled clinical trial. Clin. Oral Implant. Res. 2023, 34, 1188–1197. [Google Scholar] [CrossRef]
- Spitznagel, F.; Balmer, M.; Wiedemeier, D.; Jung, R.; Gierthmuehlen, P. Clinical outcomes of all-ceramic single crowns and fixed dental prostheses supported by ceramic implants: A systematic review and meta-analyses. Clin. Oral Implant. Res. 2021, 33, 1–20. [Google Scholar] [CrossRef]
- Schepke, U.; Meijer, H.J.; Vermeulen, K.M.; Raghoebar, G.M.; Cune, M.S. Clinical Bonding of Resin Nano Ceramic Restorations to Zirconia Abutments: A Case Series within a Randomized Clinical Trial. Clin. Implant. Dent. Relat. Res. 2016, 18, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Sailer, I.; Feher, A.; Filser, F.; Gauckler, L.J.; Luthy, H.; Hammerle, C.H. Five-year clinical results of zirconia frameworks for posterior fixed partial dentures. Int. J. Prosthodont. 2007, 20, 383–388. [Google Scholar]
- Sailer, I.; Makarov, N.A.; Thoma, D.S.; Zwahlen, M.; Pjetursson, B.E. All-ceramic or metal-ceramic tooth-supported fixed dental prostheses (FDPs)? A systematic review of the survival and complication rates. Part I: Single crowns (SCs). Dent. Mater. 2015, 31, 603–623. [Google Scholar] [CrossRef] [PubMed]
- Spitznagel, F.A.; Horvath, S.D.; Gierthmuehlen, P.C. Prosthetic protocols in implant-based oral rehabilitations: A systematic review on the clinical outcome of monolithic all-ceramic single- and multi-unit prostheses. Eur. J. Oral Implantol. 2017, 10 (Suppl. S1), 89–99. [Google Scholar] [PubMed]
| Characteristic | Crown Material | ||
|---|---|---|---|
| Polymer-Infiltrated Hybrid Ceramic (HC) | Lithium Disilicate (LS2) | p-Value | |
| Gender | |||
| Male | 10 (36%) | 13 (43%) | 0.553 ns |
| Female | 18 (64%) | 17 (57%) | |
| Age in years | 51.8 ± 10.9 | 48.8 ± 12.9 | 0.400 ns |
| Site | |||
| Maxillary arch | 10 (36%) | 11 (37%) | 0.940 ns |
| Mandibular arch | 18 (64%) | 19 (63%) | |
| Premolars Maxillary | 6 (21%) | 8 (27%) | 0.971 ns |
| Premolars Mandibular | 4 (14%) | 3 (10%) | |
| Molars Maxillary | 6 (21%) | 6 (20%) | |
| Molars Mandibular | 12 (43%) | 13 (43%) |
| Factor | Mesial Bone Remodeling (Mean, 95% CI) | p-Value (Mesial) | Distal Bone Remodeling (Mean, 95% CI) | p-Value (Distal) |
|---|---|---|---|---|
| Crown material (LS2) | 0.15 (−0.21–0.51) | 0.24 | 0.13 (−0.26–0.53) | 0.35 |
| Crown material (HC) | 0.08 (−0.12–0.29) | 0.12 (−0.13–0.30) | ||
| Gender (female) | 0.31 (0.12–0.48) | * 0.024 | 0.21 (0.02–0.40) | 0.674 |
| Gender (male) | −0.17 (−0.60–0.27) | −0.01 (−0.53–0.52) | ||
| Jaw (upper jaw) | 0.11 (−0.11–0.32) | 0.639 | 0.11 (−0.18–0.40) | 0.622 |
| Jaw (lower jaw) | 0.12 (−0.18–0.43) | 0.13 (−0.20–0.46) | ||
| Ti–Base height (0.8 mm) | 0.04 (−0.37–0.45) | 0.64 | 0.02 (−0.42–0.46) | 0.99 |
| Ti–Base height (2.0 mm) | 0.18 (0.01–0.36) | 0.22 (−0.01–0.44) | ||
| Implant length (9 mm) | 0.33 (0.07–0.58) | 0.55 | 0.33 (0.08–0.58) | 0.12 |
| Implant length (11 mm) | −0.06 (−0.43–0.32) | 0.06 (−0.43–0.32) | ||
| Implant length (13 mm) | 0.54 (−0.09–1.18) | 0.54 (−0.09–1.18) | ||
| Implant diameter (3.8 mm) | 0.04 (−0.20–0.28) | 0.619 | 0.04 (−0.20–0.28) | 0.43 |
| Implant diameter (4.4 mm) | −0.24 (−0.07–0.54) | 0.24 (−0.07–0.54) | ||
| Implant diameter (5.0 mm) | 0.06 (–0.70–0.82) | 0.06 (−0.70–0.82) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnutenhaus, S.; Weinmann, M.; Römer, M.; Luthardt, R.G. Technical Complications and Marginal Bone Loss Depending on the Crown Material of Dental Implants in the Posterior Region: A 3-Year Randomized Clinical Study. Dent. J. 2025, 13, 430. https://doi.org/10.3390/dj13090430
Schnutenhaus S, Weinmann M, Römer M, Luthardt RG. Technical Complications and Marginal Bone Loss Depending on the Crown Material of Dental Implants in the Posterior Region: A 3-Year Randomized Clinical Study. Dentistry Journal. 2025; 13(9):430. https://doi.org/10.3390/dj13090430
Chicago/Turabian StyleSchnutenhaus, Sigmar, Marla Weinmann, Max Römer, and Ralph G. Luthardt. 2025. "Technical Complications and Marginal Bone Loss Depending on the Crown Material of Dental Implants in the Posterior Region: A 3-Year Randomized Clinical Study" Dentistry Journal 13, no. 9: 430. https://doi.org/10.3390/dj13090430
APA StyleSchnutenhaus, S., Weinmann, M., Römer, M., & Luthardt, R. G. (2025). Technical Complications and Marginal Bone Loss Depending on the Crown Material of Dental Implants in the Posterior Region: A 3-Year Randomized Clinical Study. Dentistry Journal, 13(9), 430. https://doi.org/10.3390/dj13090430







