Effect of Storage Temperature and Storage Time on the pH and Oxidation–Reduction Potential of Commercial Oral Moisturizers
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples, Storage Temperature, and Storage Time
2.2. pH and ORP Measurement
2.3. Statistical Analysis
3. Results
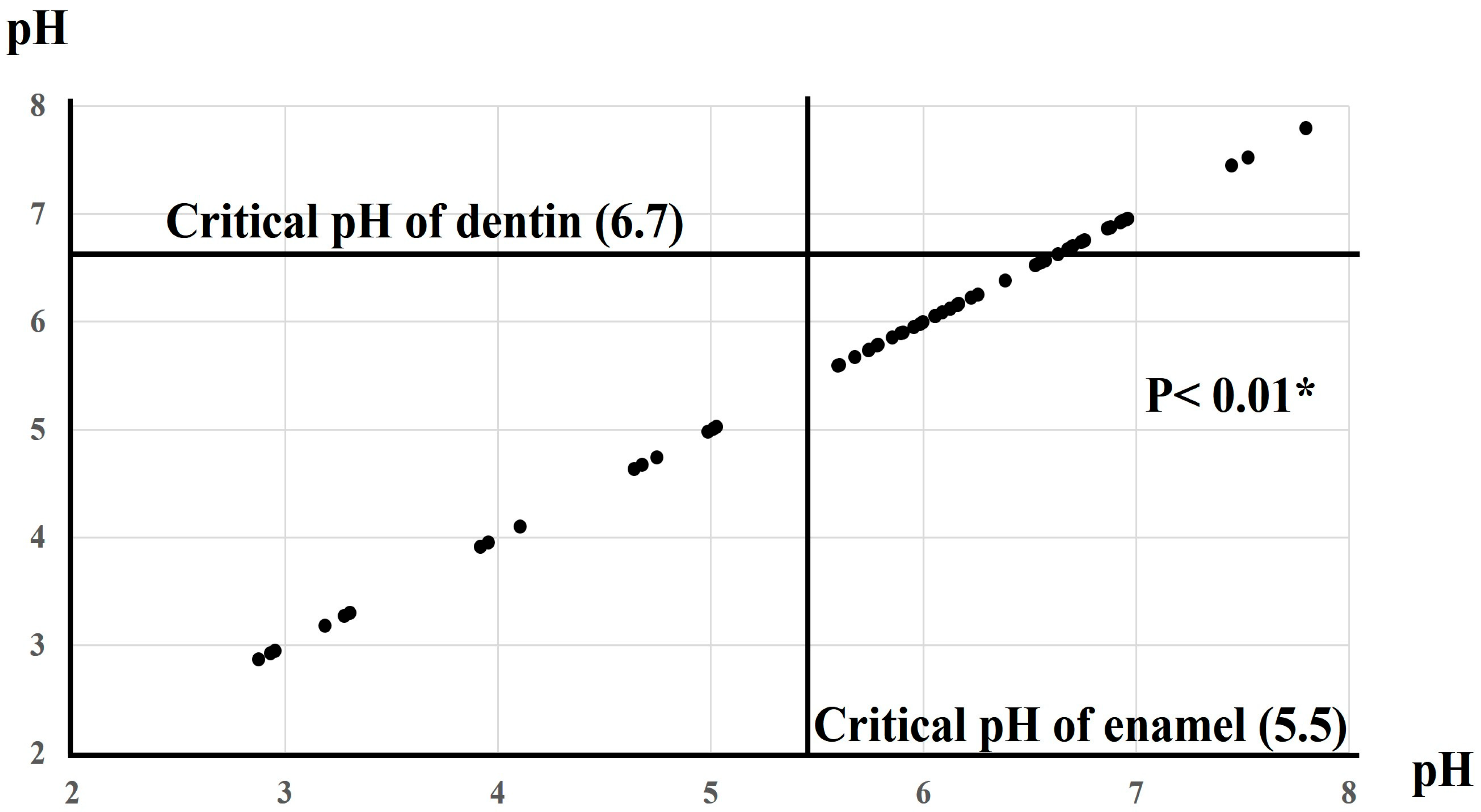
3.1. pH of Oral Moisturizers
3.2. Effects of Storage Temperature and Time on pH and ORP
4. Discussion
4.1. Study Design and Measurement Methods
4.2. pH of Oral Moisturizers
4.3. Effects of Storage Temperature and Time on pH and ORP
4.4. Limitations of the Study and Future Prospects
5. Conclusions
- Because many oral moisturizers are acidic, products with a pH of 6.7 or higher should be prioritized.
- To maintain quality and minimize degradation, oral moisturizers should be stored at 4 °C and used within 3 months after opening.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edgar, M.; Dawes, C.; O’Mullane, D. Saliva and Oral Health, 4th ed.; Stephen Hancocks Limited: Little Steine, UK, 2014; pp. 49–68. [Google Scholar]
- Saito, I. Pathogenesis and Management of Dry Mouth; Ishiyaku Publisher Inc.: Tokyo, Japan, 2007; pp. 3–4. [Google Scholar]
- Diaz-Arnold, A.M.; Marek, C.A. The impact of saliva on patients: A literature review. J. Prosthet. Dent. 2002, 88, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Suarez-Durall, P.; Mulligan, R. Dry mouth: A critical topic for older adult patients. J. Prosthodont. Res. 2015, 59, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Nishi, Y.; Seto, K.; Kamashita, Y.; Nagaoka, E. Dry mouth and denture plaque microflora in complete denture and palatal obturator prosthesis wearers. Gerodontology 2015, 32, 188–194. [Google Scholar] [CrossRef]
- Torres, S.R.; Peixoto, C.B.; Caldas, D.M.; Silva, E.B.; Akiti, T.; Nucci, M.; de Uzeda, M. Relationship between salivary flow rates and Candida counts in subjects with xerostomia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 93, 149–154. [Google Scholar] [CrossRef]
- Radfar, L.; Shea, Y.; Fischer, S.H.; Sankar, V.; Leakan, R.A.; Baum, B.J.; Pillemer, S.R. Fungal load and candidiasis in Sjögren’s syndrome. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 96, 283–287. [Google Scholar] [CrossRef]
- Macfarlane, T.W.; Mason, D.K. Changes in the oral flora in Sjögren’s syndrome. J. Clin. Path 1974, 27, 416–419. [Google Scholar] [CrossRef]
- Almståh, I.A.; Wikström, M.; Stenberg, I.; Jakobsson, A.; Fagerberg-Mohlin, B. Oral microbiota associated with hyposalivation of different origins. Oral Microbiol. Immunol. 2003, 18, 1–8. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Gallo, S.; Zampetti, P.; Scribante, A. Oral Microbiota in Patients with Peri-Implant Disease: A Narrative Review. Appl. Sci. 2022, 12, 3250. [Google Scholar] [CrossRef]
- Chambers, M.S.; Posner, M.; Jones, C.U.; Biel, M.A.; Hodge, K.M.; Vitti, R.; Armstrong, I.; Yen, C.; Weber, R.S. Cevimeline for the treatment of postirradiation xerostomia in patients with head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1102–1109. [Google Scholar] [CrossRef]
- Jha, N.; Seikaly, H.; Harris, J.; Williams, D.; Sultanem, K.; Hier, M.; Ghosh, S.; Black, M.; Butler, J.; Sutherland, D.; et al. Phase III randomized study: Oral pilocarpine versus submandibular salivary gland transfer protocol for the management of radiation-induced xerostomia. Head Neck 2009, 31, 234–243. [Google Scholar] [CrossRef]
- Okura, E.; Ishii, H.; Takamoto, Y.; Takayama, Y.; Makihira, S.; Kumagai, H.; Sasaki, M.; Taji, T.; Nikawa, H. Evaluation of physical properties of commercial mouth moisturizer. Jpn. J. Dent. Mater. 2012, 31, 258–265. [Google Scholar]
- Chinen, M.; Kuroki, M.; Kijima, S.; Maeda, T.; Hidaka, S. The relation between viscosity and water retainability in oral moisturizers. Jpn. J. Gerodontol. 2013, 28, 3–9. [Google Scholar]
- Kano, H.; Kurogi, T.; Shimizu, T.; Nishimura, M.; Murata, H. Viscosity and adhesion strength of cream-type denture adhesives and mouth moisturizers. Dent. Mater. J. 2012, 31, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Güneri, P.; Alpöz, E.; Epstein, J.B.; Çankaya, H.; Ates, M. In vitro antimicrobial effects of commercially available mouth-wetting agents. Spec. Care Dent. 2011, 31, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Rhodus, N.L.; Bereuter, J. Clinical evaluation of a commercially available oral moisturizer in relieving signs and symptoms of xerostomia in postirradiation head and neck cancer patients and patients with Sjögren’s syndrome. J. Otolaryngol. 2000, 29, 28–34. [Google Scholar]
- Gil-Montoya, J.A.; Guardia-López, I.; González-Moles, M.A. Evaluation of the clinical efficacy of a mouthwash and oral gel containing the antimicrobial proteins lactoperoxidase, lysozyme and lactoferrin in elderly patients with dry mouth—A pilot study. Gerodontology 2008, 25, 3–9. [Google Scholar] [CrossRef]
- Motoyama, S.; Murakami, M.; Nishi, Y.; Nishimura, M. A questionnaire survey on recognition and instruction of oral moisturizer for medical staff. Ronen Shika Igaku 2019, 34, 399–405. [Google Scholar]
- Dawes, C.; Pedersen, A.M.; Villa, A.; Ekström, J.; Proctor, G.B.; Vissink, A.; Aframian, D.; McGowan, R.; Aliko, A.; Narayana, N.; et al. The functions of human saliva: A review sponsored by the World Workshop on Oral Medicine VI. Arch. Oral Biol. 2015, 60, 863–874. [Google Scholar] [CrossRef]
- Bardow, A.; Madsen, J.; Nauntofte, B. The bicarbonate concentration in human saliva does not exceed the plasma level under normal physiological conditions. Clin. Oral Investig. 2000, 4, 245–253. [Google Scholar] [CrossRef]
- Tarapan, S.; Matangkasombut, O.; Trachootham, D.; Sattabanasuk, V.; Talungchit, S.; Paemuang, W.; Phonyiam, T.; Chokchaitam, O.; Mungkung, O.O.; Lam-Ubol, A. Oral Candida colonization in xerostomic postradiotherapy head and neck cancer patients. Oral Dis. 2019, 25, 1798–1808. [Google Scholar] [CrossRef]
- Delgado, A.J.; Olafsson, V.G.; Donovan, T.E. pH and Erosive Potential of Commonly Used Oral Moisturizers. J. Prosthodont. 2016, 25, 39–43. [Google Scholar] [CrossRef]
- Murakami, M.; Fujishima, K.; Nishi, Y.; Minemoto, Y.; Kanie, T.; Taguchi, N.; Nishimura, M. Impact of type and duration of application of commercially available oral moisturizers on their antifungal effects. J. Prosthodont. 2018, 27, 52–56. [Google Scholar] [CrossRef]
- Claros, J.; Serralta, J.; Seco, A.; Ferrer, J.; Aguado, D. Monitoring pH and ORP in a SHARON reactor. Water Sci. Technol. 2011, 63, 2505–2512. [Google Scholar] [CrossRef]
- Lin, W.-C.; Brondum, K.; Monroe, C.W.; Burns, M.A. Multifunctional Water Sensors for pH, ORP, and Conductivity Using Only Microfabricated Platinum Electrodes. Sensors 2017, 17, 1655. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; An, D.; Ren, N.; Zhang, Y.; Chen, Y. Effects of pH and ORP on microbial ecology and kinetics for hydrogen production in continuously dark fermentation. Bioresour. Technol. 2011, 102, 10875–10880. [Google Scholar] [CrossRef] [PubMed]
- Okouchi, S.; Suzuki, M.; Sugano, K.; Kagamimori, S.; Ikeda, S. Water Desirable for the Human Body in Terms of Oxidation-Reduction Potential (ORP) to pH Relationship. J. Food Sci. 2002, 67, 1594–1598. [Google Scholar] [CrossRef]
- Murakami, M.; Harada, K.; Nishi, Y.; Shimizu, T.; Motoyama, S.; Nishimura, M. Effects of Storage Temperature and pH on the Antifungal Effects of Commercial Oral Moisturizers against Candida Albicans and Candida Glabrata. Medicina 2020, 56, 525. [Google Scholar] [CrossRef]
- Austin, W.; Hdeib, M.; Fraser, P.; Goldchtaub, M.; Shams, E.; Han, T.; Michaud, P.-L.; Adibnia, V. Oral Lubrication, Xerostomia, and Advanced Macromolecular Lubricants for Treatment of Dry Mouth. Lubricants 2024, 12, 126. [Google Scholar] [CrossRef]
- Han, T.; Zhang, S.; Zhang, C. Unlocking the secrets behind liquid superlubricity: A state-of-the-art review on phenomena and mechanisms. Friction 2022, 10, 1137–1165. [Google Scholar] [CrossRef]
- Yamagaki, K.; Kitagawa, N.; Sato, Y.; Okane, M.; Mashimo, J. The relation between the physical properties of oral moisturizer and denture retention force. Jpn. J. Gerodontol. 2012, 26, 402–411. [Google Scholar]
- Suzuki, H.; Furuya, J.; Matsubara, C.; Aoyagi, M.; Shirobe, M.; Sato, Y.; Tohara, H.; Minakuchi, S. Comparison of the Amount of Used and the Ease of Oral Care between Liquid and Gel-Type Oral Moisturizers Used with an Oral Care Simulators. Int. J. Environ. Res. Public Health 2022, 19, 8158. [Google Scholar] [CrossRef]
- Horiba. Ways of Measuring pH. Available online: https://www.horiba.com/usa/water-quality/support/electrochemistry/the-story-of-ph/ways-of-measuring-ph/ (accessed on 10 October 2024).
- Houri, D.; Yoshioka, S.; Matsumoto, K.; Amikawa, K.; Tanaka, S.; Nagata, N. Elements and physical properties of green tea decoction using Hakusan-meisui mineral water. Yonago Acta Medica 2008, 51, 61–67. [Google Scholar]
- Edwards, M.; Creanor, S.L.; Foye, R.H.; Gilmour, W.H. Buffering capacities of soft drinks: The potential influence on dental erosion. J. Oral Rehabil. 1999, 26, 923–927. [Google Scholar] [CrossRef]
- Butera, A.; Maiorani, C.; Gallo, S.; Pascadopoli, M.; Quintini, M.; Lelli, M.; Tarterini, F.; Foltran, I.; Scribante, A. Biomimetic Action of Zinc Hydroxyapatite on Remineralization of Enamel and Dentin: A Review. Biomimetics 2023, 8, 71. [Google Scholar] [CrossRef]
- Butera, A.; Scribante, A. Editorial for Oral Microbes and Human Health. Microorganisms 2025, 13, 922. [Google Scholar] [CrossRef]

| Materials | Code | Manufacturer (City, Country) | Main/Active Ingredients |
|---|---|---|---|
| Stoppers for | A | Sun Dental Co., Ltd. (Osaka, Japan) | Water, Glycerin, Xylitol, Lysozyme, Lactoferrin |
| Oral Wet Spray | B | Yoshida Co., Ltd. (Tokyo, Japan) | Water, Xylitol, Sodium benzoate, Hyaluronate sodium |
| ConCool Mouth Rinse | C | Weltec Co., Ltd. (Osaka, Japan) | Water, PG, Sorbitol, Xylitol, Lactoferrin, Whey protein |
| Pepti Sal Mouth Wash | D | T&K Co., Ltd. (Tokyo, Japan) | Water, PG, Xylitol, Polyglycitol, Nisin, Lactoferrin |
| Wet Keeping Mist | E | Oral Care. Inc. (Tokyo, Japan) | Glycerin, Betaine, Xylitol, Methylparaben, Sodium citrate |
| Biotene Mouth Wash | F | GSK Co., Ltd. (Tokyo, Japan) | Water, Glycerin, Xylitol, Sorbitol, Propylene glycol |
| Clean & Moisture Spray | G | Trife Inc. (Yokohama, Japan) | Water, Glycerin, Lactococcus culture extract, Ume extract |
| SMILE HONEY Gel Spray | H | Nippon Zettoc Co., Ltd. (Tokyo, Japan) | Water, Glycerin, Propanediol, Hyaluronate sodium, Xylitol |
| Sunstar Gel Spray | I | SUNSTAR Co., Ltd. (Osaka, Japan) | Water, Glycerin, Glycosyl trehalose, BG |
| Materials | Code | Manufacturer (City, Country) | Main/Active Ingredients |
|---|---|---|---|
| Wet aid | a | Dent care. Inc. (Osaka, Japan) | Water, Maltitol, Trehalose, BG, Hyaluronate sodium |
| Oral Moisturizer Ai Gel | b | Ryoka Dental Inc. (Mie, Japan) | Water, Glycerin, Carboxymethyl cellulose, Xylitol |
| Oral Aquagel | c | GC Co., Ltd. (Tokyo, Japan) | Diglycerine, Water, Carboxymethyl cellulose |
| Denture Gel | d | Kamemizu Chem. Ind. (Osaka, Japan) | Maltitol, Water, Glycerin, Propylene glycol |
| SMILE HONEY Gel | e | Nippon Zettoc Co., Ltd. (Tokyo, Japan) | Honey, Xylitol, Sucralose, Lemon juice, Tea extract |
| ConCool Mouth Gel | f | Weltec Co., Ltd. (Osaka, Japan) | Water, Maltitol, Sorbitol, Glycerin, Xylitol, Whey protein |
| Oral Balance Gel | g | GSK Co., Ltd. (Tokyo, Japan) | Glycerin, Water, Sorbitol, Xylitol, Hydroxyethyl cellulose |
| Terumo Oral Gel | h | Nippon Zettoc Co., Ltd. (Tokyo, Japan) | Water, Sorbitol, Glycerin, Xylitol, Dried egg yolk, Maltitol |
| Butler Moisturizing Gel | i | SUNSTAR Co., Ltd. (Osaka, Japan) | Water, Glycerin, Sorbitol, Hydrogenated starch hydrolysate |
| Clean & Moisture Gel | j | Trife Inc. (Yokohama, Japan) | Glycerin, Water, Lactococcus culture extract, Ume extract |
| Care Heart Gel | k | Tamagawa Eizai Co., Ltd. (Tokyo, Japan) | Water, Glycerin, Xylitol, Hydroxyethyl cellulose |
| 4 °C | 25 °C | 37 °C | |||
|---|---|---|---|---|---|
| Liquid * | 5.94 (1.22) | 5.89 (1.20) | 5.89 (1.25) | ||
| Gel * | 5.74 (1.34) | 5.67 (1.30) | 5.66 (1.28) | ||
| Liquid and Gel * | 5.83 (1.26) | 5.77 (1.23) | 5.76 (1.24) | ||
| Minimum value | 2.87 | 2.93 | 2.95 | ||
| Maximum value | 7.80 | 7.52 | 7.45 | ||
| Acidic/Neutral/Alkaline (Acid%) | 13/6/1 (65%) | 13/6/1 (65%) | 13/7/0 (65%) | ||
| Source | Sum of Squares | Degrees of Freedom | Mean Square | f-Value | p-Value |
| Type of moisturizers (A) | 0.68 | 1 | 0.68 | 0.42 | 0.52 |
| Storage temperature (B) | 0.05 | 2 | 0.02 | 0.01 | 0.99 |
| (A) × (B) | 0.00 | 2 | 0.00 | 0.00 | 0.99 |
| Error | 87.16 | 54 | 1.61 | ||
| Total | 2096.78 | 60 | |||
| 0M | 1M | 3M | 6M | ||
|---|---|---|---|---|---|
| 4 °C | 5.83 (1.26) | 5.73 (1.30) | 5.60 (1.20) | 5.58 (1.24) | |
| 25 °C | 5.77 (1.23) | 5.65 (1.22) | 5.58 (1.21) | 5.56 (1.21) | |
| 37 °C | 5.76 (1.24) | 5.69 (1.22) | 5.56 (1.21) | 5.49 (1.20) | |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | f-Value | p-Value |
| Storage time (A) | 2.17 | 2.21 | 0.98 | 67.99 | 0.00 * |
| Storage temperature (B) | 0.15 | 2 | 0.08 | 0.01 | 0.99 |
| (A) × (B) | 0.07 | 4.42 | 0.02 | 1.03 | 0.40 |
| Error | 343.75 | 182.83 | 1.88 | ||
| Total | 346.14 | 191.50 | |||
| 0M | 1M | 3M | 6M | ||
| 0M | - | 0.00 * | 0.00 * | 0.00 * | |
| 1M | - | - | 0.00 * | 0.00 * | |
| 3M | - | - | - | 0.57 | |
| 6M | - | - | - | - | |
| 0M | 1M | 3M | 6M | ||
| 4 °C | 255.31 (28.50) | 245.69 (29.42) | 221.03 (34.11) | 217.66 (44.41) | |
| 25 °C | 237.01 (48.44) | 205.10 (43.58) | 173.44 (33.07) | 210.67 (43.27) | |
| 37 °C | 213.54 (38.69) | 198.20 (39.62) | 171.04 (36.80) | 188.12 (36.41) | |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | f-Value | p-Value |
| Storage time (A) | 69,248.52 | 2.38 | 29,043.16 | 66.07 | 0.00 * |
| Storage temperature (B) | 74,043.01 | 2 | 37,021.51 | 7.59 | 0.00 * |
| (A) × (B) | 11,160.08 | 4.769 | 2340.30 | 5.32 | 0.00 * |
| Error | 337,664.50 | 192.91 | 1750.40 | ||
| Total | 492,116.12 | 202.06 | |||
| 0M | 1M | 3M | 6M | ||
| 4 °C | # A | # A | # A | # A | |
| 25 °C | # A | $ B | $ B | # B | |
| 37 °C | $ A | $ B | $ B | $ B | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murakami, M.; Komabashiri, S.; Harada, K.; Shimizu, T.; Nishimura, M. Effect of Storage Temperature and Storage Time on the pH and Oxidation–Reduction Potential of Commercial Oral Moisturizers. Dent. J. 2025, 13, 344. https://doi.org/10.3390/dj13080344
Murakami M, Komabashiri S, Harada K, Shimizu T, Nishimura M. Effect of Storage Temperature and Storage Time on the pH and Oxidation–Reduction Potential of Commercial Oral Moisturizers. Dentistry Journal. 2025; 13(8):344. https://doi.org/10.3390/dj13080344
Chicago/Turabian StyleMurakami, Mamoru, Sara Komabashiri, Kae Harada, Takaharu Shimizu, and Masahiro Nishimura. 2025. "Effect of Storage Temperature and Storage Time on the pH and Oxidation–Reduction Potential of Commercial Oral Moisturizers" Dentistry Journal 13, no. 8: 344. https://doi.org/10.3390/dj13080344
APA StyleMurakami, M., Komabashiri, S., Harada, K., Shimizu, T., & Nishimura, M. (2025). Effect of Storage Temperature and Storage Time on the pH and Oxidation–Reduction Potential of Commercial Oral Moisturizers. Dentistry Journal, 13(8), 344. https://doi.org/10.3390/dj13080344






