Therapeutic Potential of Tanshinones in Osteolytic Diseases: From Molecular and Cellular Pathways to Preclinical Models
Abstract
1. Introduction
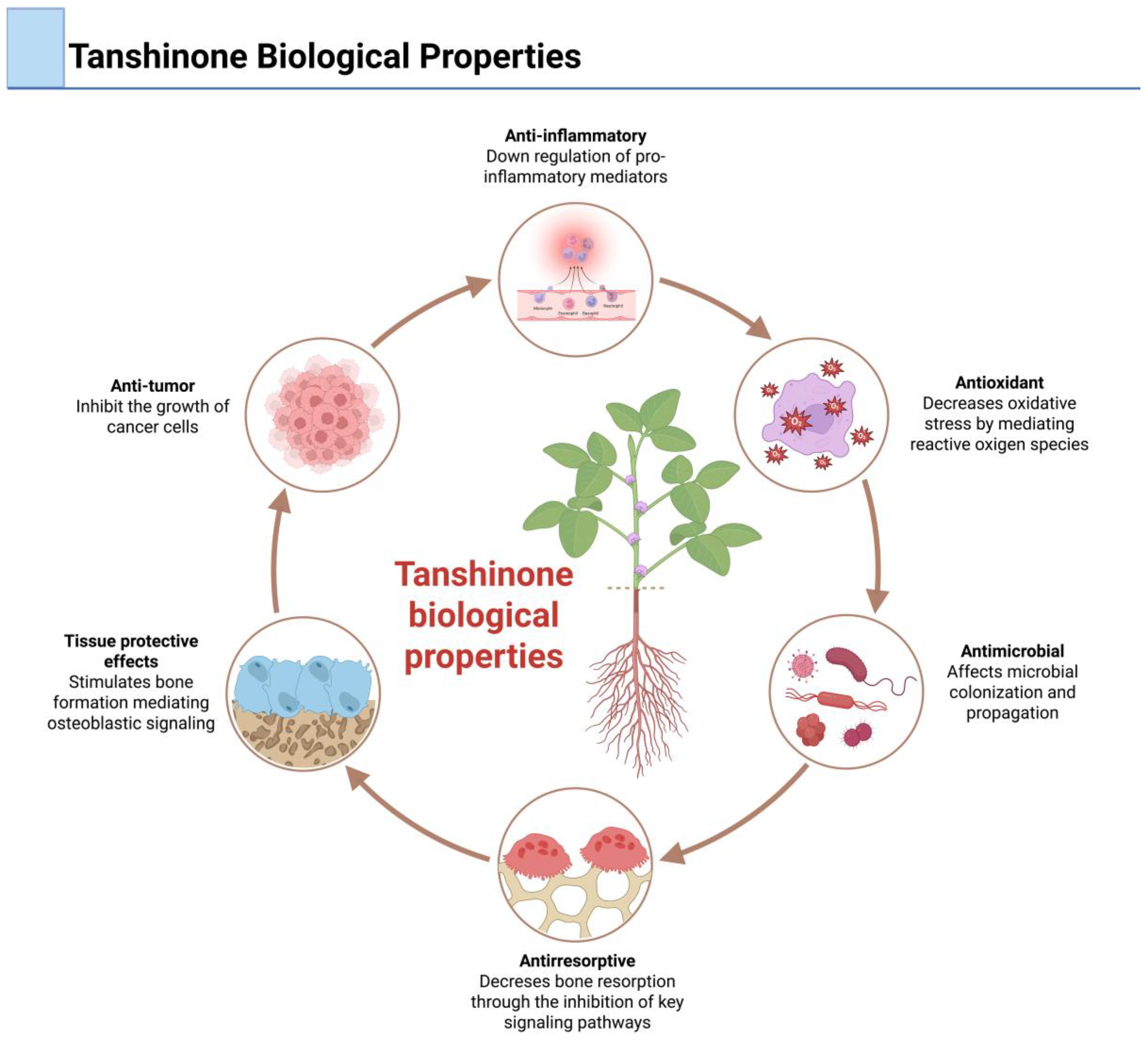
2. Tanshinones
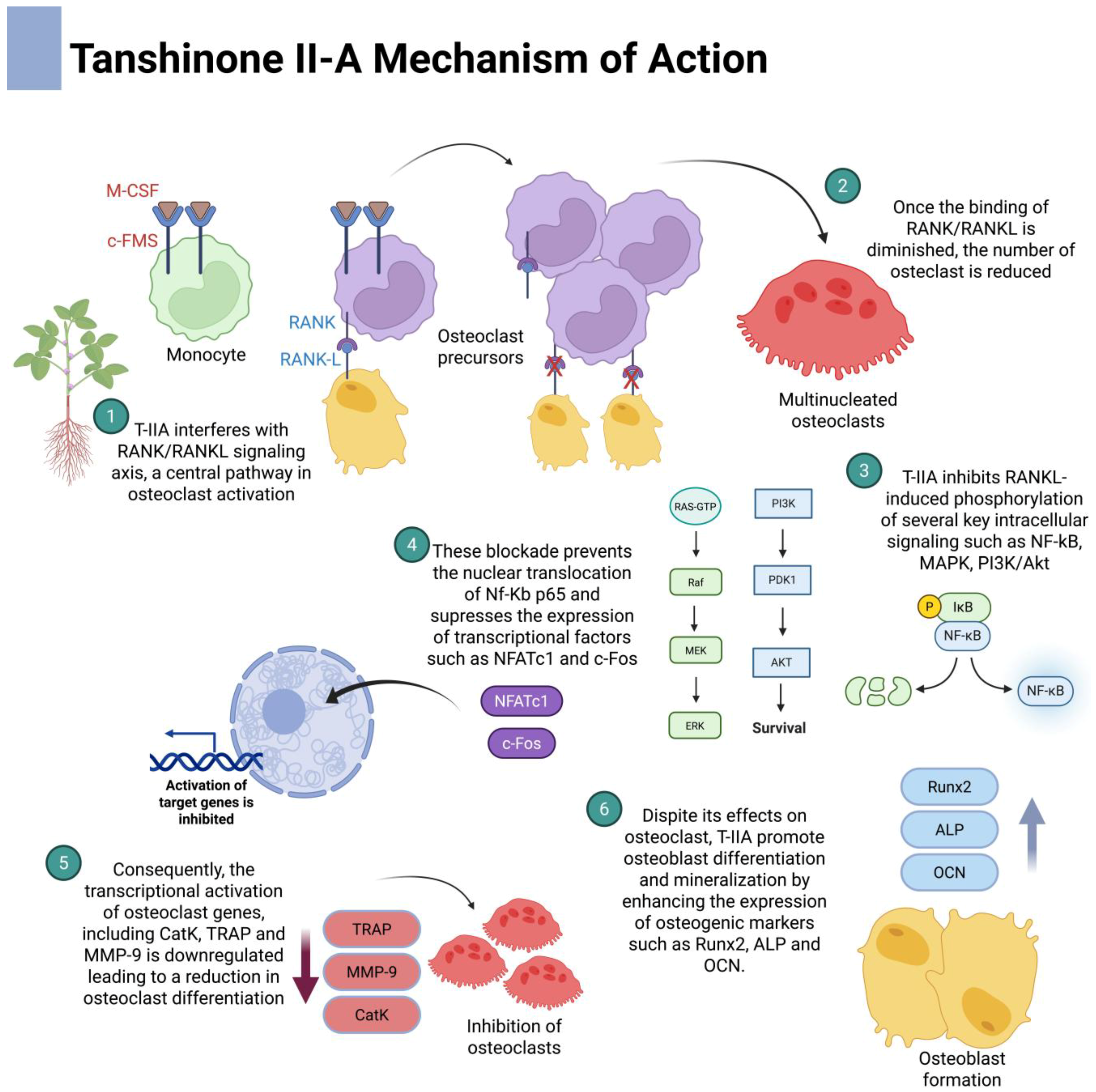
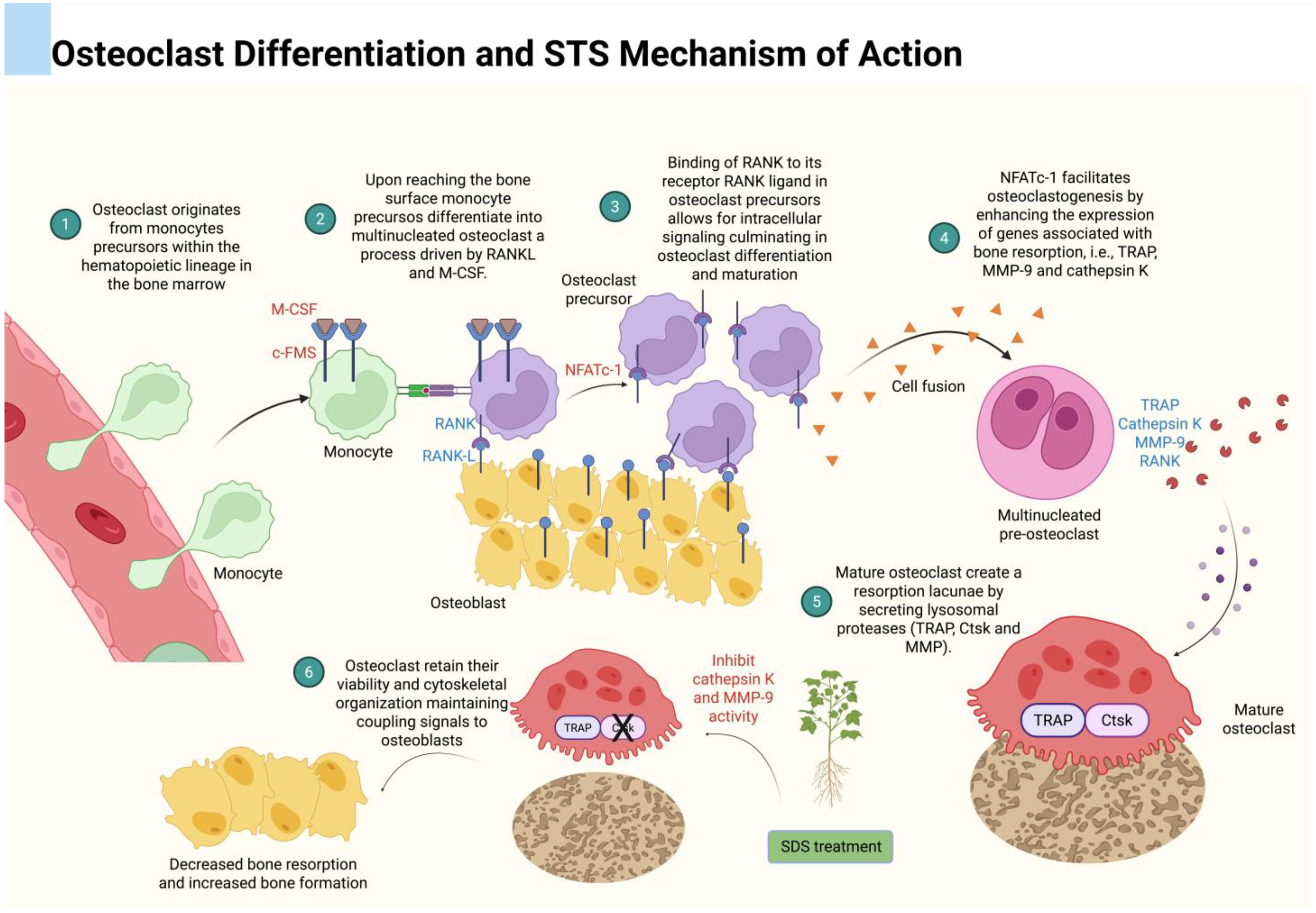
3. Tanshinone and Osteoclast Molecular Pathways
3.1. Nuclear-Factor-κB (NF-κB) Signaling
3.2. Mitogen-Activated Protein Kinase (MAPK) Signaling
3.3. Phosphoinositide 3-Kinase PI3K/Akt Signaling Pathway
3.4. RANK/RANKL/OPG Axis
4. Tanshinone and Osteoblast Signaling Pathways
4.1. Wnt/β-Catenin Signaling Pathway
4.2. Bone Morphogenetic Protein (BMP) Signaling Pathway
4.3. Fibroblast Growth Factor (FGF) Signaling Pathway
5. Preclinical Studies Investigating the Beneficial Effects of Tanshinone on Bone
5.1. Osteoporosis and Alveolar Bone Loss
5.2. Diabetes Mellitus
5.3. Rheumatoid Arthritis
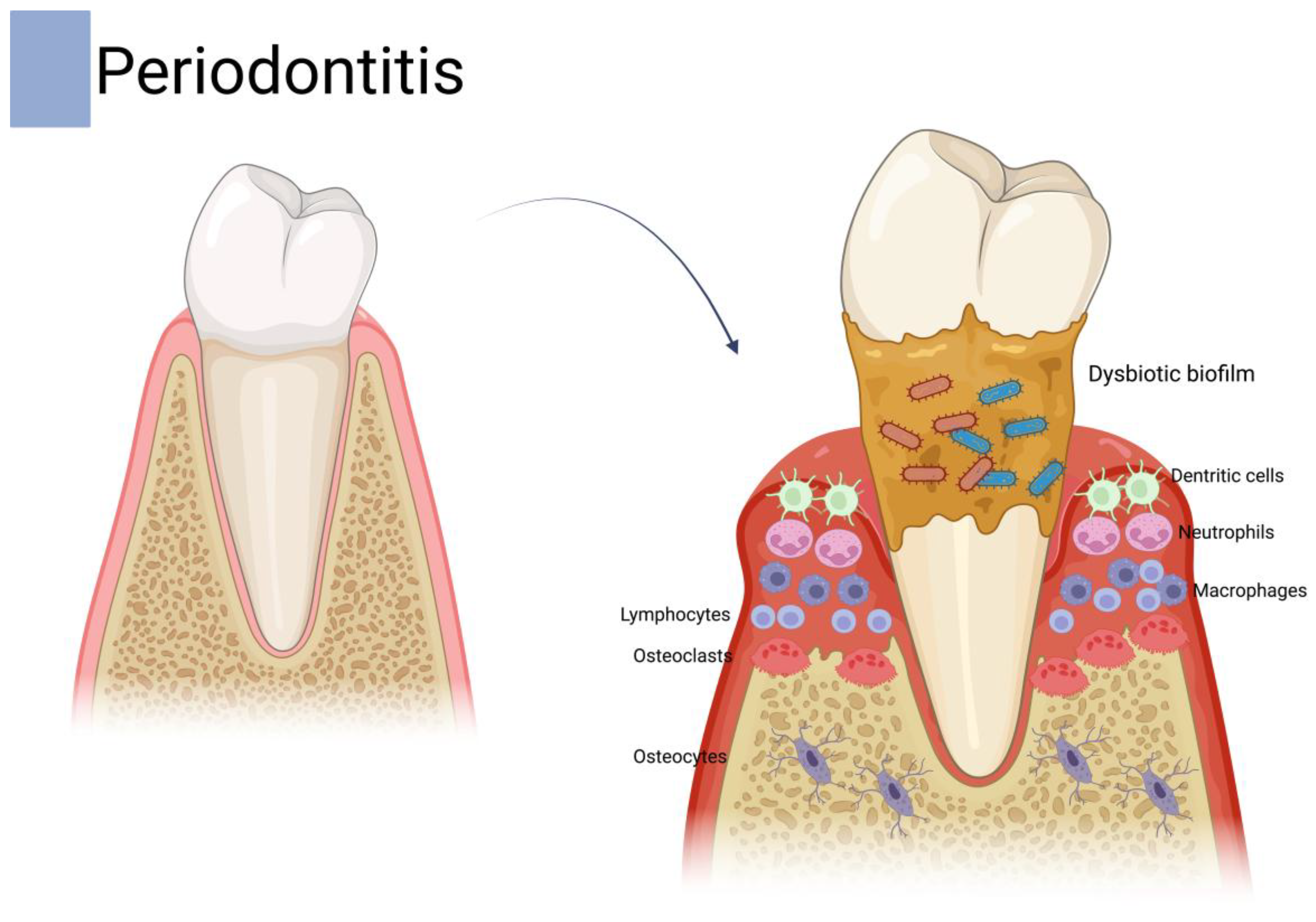
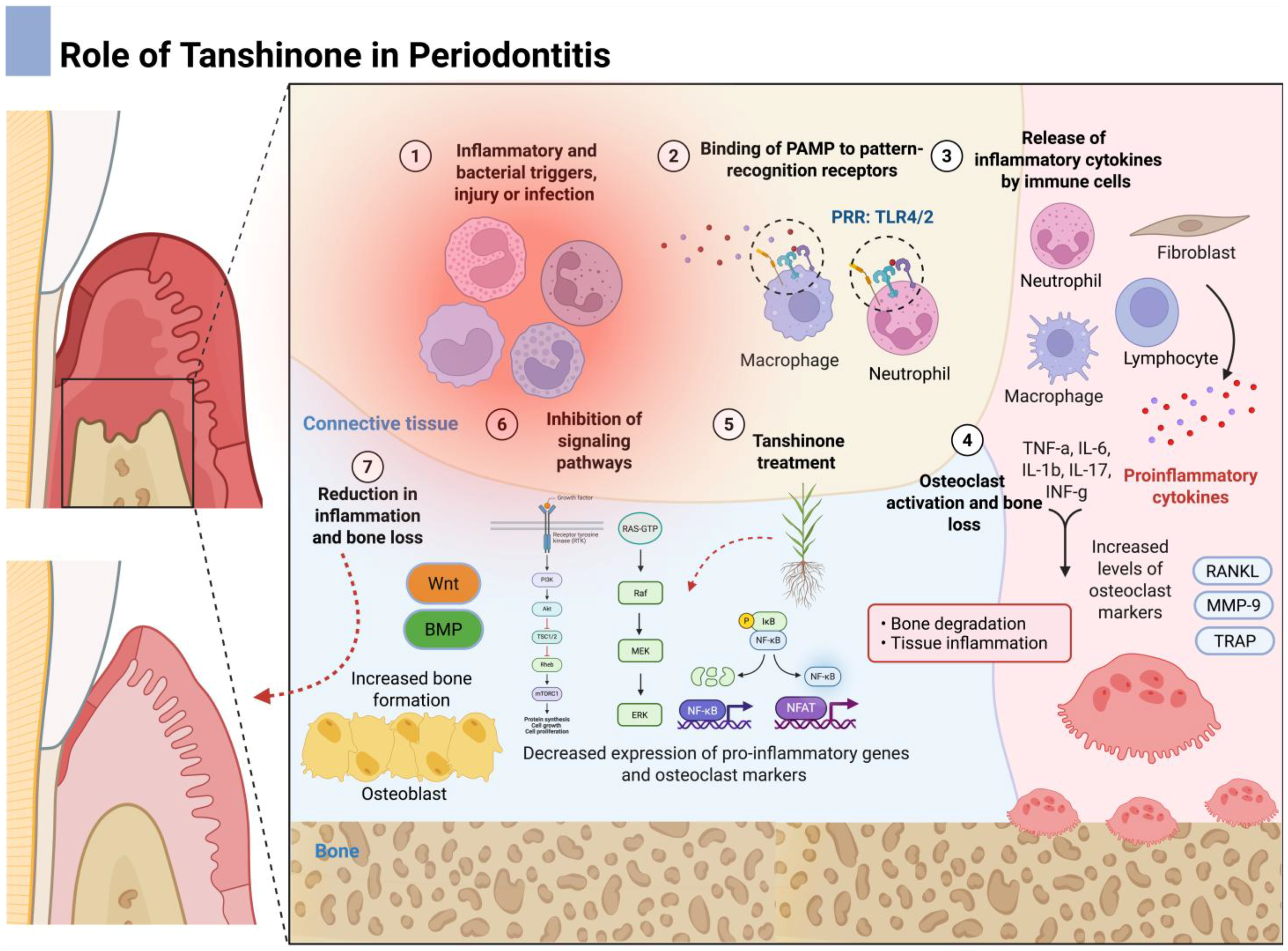
5.4. Periodontal Disease
5.5. Other Osteolytic Diseases
6. Concluding Remarks and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and classification of diabetes: Standards of care in diabetes-2024. Diabetes Care 2024, 47, S20–S42. [Google Scholar]
- Lane, N.E. Epidemiology, etiology, and diagnosis of osteoporosis. Am. J. Obstet. Gynecol. 2006, 194, S3–S11. [Google Scholar] [PubMed]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [PubMed]
- Hascoet, E.; Blanchard, F.; Blin-Wakkach, C.; Guicheux, J.; Lesclous, P.; Cloitre, A. New insights into inflammatory osteoclast precursors as therapeutic targets for rheumatoid arthritis and periodontitis. Bone Res. 2023, 11, 26. [Google Scholar]
- GBD 2019 Fracture Collaborators. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990-2019: A systematic analysis from the global burden of disease study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar]
- International Osteoporosis Foundation. On World Osteoporosis Day, Experts Warn of Growing Burden of Fragility Fractures; International Osteoporosis Foundation: Nyon, Switzerland, 2024. [Google Scholar]
- Fu, H.; Li, X.; Zhang, R.; Zhu, J.; Wang, X. Global burden of periodontal diseases among the working-age population from 1990-2021: Results from the global burden of disease study 2021. BMC Public Health 2025, 25, 1316. [Google Scholar]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American association of oral and maxillofacial surgeons’ position paper on medication-related osteonecrosis of the jaws-2022 update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar]
- Khan, A.A.; Morrison, A.; Hanley, D.A.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.R.; Ruggiero, S.L.; Taguchi, A.; Tetradis, S.; et al. Diagnosis and management of osteonecrosis of the jaw: A systematic review and international consensus. J. Bone Miner. Res. 2015, 30, 3–23. [Google Scholar]
- Munoz, M.; Robinson, K.; Shibli-Rahhal, A. Bone health and osteoporosis prevention and treatment. Clin. Obstet. Gynecol. 2020, 63, 770–787. [Google Scholar]
- Singh, J.A. Treatment guidelines in rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 2022, 48, 679–689. [Google Scholar]
- Pavanelli, A.L.R.; de Menezes, B.S.; Pereira, E.B.B.; de Souza Morais, F.A.; Cirelli, J.A.; de Molon, R.S. Pharmacological therapies for the management of inflammatory bone resorption in periodontal disease: A review of preclinical studies. Biomed. Res. Int. 2022, 2022, 5832009. [Google Scholar]
- Zhou, Z.Y.; Zhao, W.R.; Zhang, J.; Chen, X.L.; Tang, J.Y. Sodium tanshinone iia sulfonate: A review of pharmacological activity and pharmacokinetics. Biomed. Pharmacother. 2019, 118, 109362. [Google Scholar]
- Ke, L.; Zhong, C.; Chen, Z.; Zheng, Z.; Li, S.; Chen, B.; Wu, Q.; Yao, H. Tanshinone i: Pharmacological activities, molecular mechanisms against diseases and future perspectives. Phytomedicine 2023, 110, 154632. [Google Scholar] [PubMed]
- Oliveira, G.E.; da Silva Barbirato, D.; de Menezes, B.S.; Fuly, M.S.; Pelegrine, H.C.L.; Bonilha, D.C.; de Alencar, J.G.P.; Theodoro, L.H.; de Molon, R.S. Exploring the impact of biological agents on protecting against experimental periodontitis: A systematic review of animal-based studies. Biomed. Res. Int. 2024, 2024, 1716735. [Google Scholar]
- Usui, M.; Onizuka, S.; Sato, T.; Kokabu, S.; Ariyoshi, W.; Nakashima, K. Mechanism of alveolar bone destruction in periodontitis—Periodontal bacteria and inflammation. Jpn. Dent. Sci. Rev. 2021, 57, 201–208. [Google Scholar] [PubMed]
- Chen, Z.; Zhong, Y.; Chen, L.; Liu, W.; Lin, C.; Chen, Y.; Wang, X. Hgf aggravated periodontitis-associated gut barrier and microbial dysfunction: Implications for oral-gut axis regulation. Biology 2025, 14, 496. [Google Scholar]
- Hajishengallis, G.; Chavakis, T.; Lambris, J.D. Current understanding of periodontal disease pathogenesis and targets for host-modulation therapy. Periodontology 2000 2020, 84, 14–34. [Google Scholar]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar]
- Zhang, M.; Liu, Y.; Afzali, H.; Graves, D.T. An update on periodontal inflammation and bone loss. Front. Immunol. 2024, 15, 1385436. [Google Scholar]
- Lalla, E.; Papapanou, P.N. Diabetes mellitus and periodontitis: A tale of two common interrelated diseases. Nat. Rev. Endocrinol. 2011, 7, 738–748. [Google Scholar]
- Wu, C.Z.; Yuan, Y.H.; Liu, H.H.; Li, S.S.; Zhang, B.W.; Chen, W.; An, Z.J.; Chen, S.Y.; Wu, Y.Z.; Han, B.; et al. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health 2020, 20, 204. [Google Scholar]
- Belizario, L.C.G.; Figueredo, C.M.S.; Rodrigues, J.V.S.; Cirelli, T.; de Molon, R.S.; Garcia, V.G.; Theodoro, L.H. The impact of type 2 diabetes mellitus on non-surgical periodontal treatment: A non-randomized clinical trial. J. Clin. Med. 2024, 13, 5978. [Google Scholar] [PubMed]
- de Aquino, S.G.; Abdollahi-Roodsaz, S.; Koenders, M.I.; van de Loo, F.A.; Pruijn, G.J.; Marijnissen, R.J.; Walgreen, B.; Helsen, M.M.; van den Bersselaar, L.A.; de Molon, R.S.; et al. Periodontal pathogens directly promote autoimmune experimental arthritis by inducing a tlr2- and il-1-driven th17 response. J. Immunol. 2014, 192, 4103–4111. [Google Scholar] [PubMed]
- de Molon, R.S.; Rossa, C., Jr.; Thurlings, R.M.; Cirelli, J.A.; Koenders, M.I. Linkage of periodontitis and rheumatoid arthritis: Current evidence and potential biological interactions. Int. J. Mol. Sci. 2019, 20, 4541. [Google Scholar]
- de Aquino, S.G.; Talbot, J.; Sonego, F.; Turato, W.M.; Grespan, R.; Avila-Campos, M.J.; Cunha, F.Q.; Cirelli, J.A. The aggravation of arthritis by periodontitis is dependent of il-17 receptor a activation. J. Clin. Periodontol. 2017, 44, 881–891. [Google Scholar]
- Qiao, Y.; Wang, Z.; Li, Y.; Han, Y.; Zhou, Y.; Cao, X. Rheumatoid arthritis risk in periodontitis patients: A systematic review and meta-analysis. Jt. Bone Spine 2020, 87, 556–564. [Google Scholar]
- Chapple, I.L.C.; Hirschfeld, J.; Cockwell, P.; Dietrich, T.; Sharma, P. Interplay between periodontitis and chronic kidney disease. Nat. Rev. Nephrol. 2025, 21, 226–240. [Google Scholar]
- Rodrigues, J.V.S.; Claudio, M.M.; Franciscon, J.P.S.; Rosa, R.A.C.; Cirelli, T.; de Molon, R.S.; Figueredo, C.M.S.; Garcia, V.G.; Theodoro, L.H. The effect of non-surgical periodontal treatment on patients with combined refractory arterial hypertension and stage iii, grade b periodontitis: A preliminary prospective clinical study. J. Clin. Med. 2023, 12, 4277. [Google Scholar]
- Rosa, R.A.C.; Rodrigues, J.V.S.; Claudio, M.M.; Franciscon, J.P.S.; Mulinari-Santos, G.; Cirelli, T.; de Molon, R.S.; Gouveia Garcia, V.; Theodoro, L.H. The relationship between hypertension and periodontitis: A cross-sectional study. J. Clin. Med. 2023, 12, 5140. [Google Scholar]
- Khumaedi, A.I.; Purnamasari, D.; Wijaya, I.P.; Soeroso, Y. The relationship of diabetes, periodontitis and cardiovascular disease. Diabetes Metab. Syndr. 2019, 13, 1675–1678. [Google Scholar]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal disease: A risk factor for diabetes and cardiovascular disease. Int. J. Mol. Sci. 2019, 20, 1414. [Google Scholar] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; Participants, E.F.P.W.; Methodological, C. Treatment of stage i-iii periodontitis-the efp s3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 4–60. [Google Scholar] [PubMed]
- de Molon, R.S.; Rodrigues, J.V.S.; Deroide, M.B.; da Silva Barbirato, D.; Garcia, V.G.; Theodoro, L.H. The efficacy of topical or systemic antibiotics as adjuvants to non-surgical periodontal treatment in diabetic patients: A systematic review and meta-analysis of randomized clinical trials. J. Clin. Med. 2024, 13, 4763. [Google Scholar]
- Abdel-Fatah, R.; Mowafey, B.; Baiomy, A.; Elmeadawy, S. Efficacy of curcumin gel as an adjunct to scaling and root planing on salivary procalcitonin level in the treatment of patients with chronic periodontitis: A randomized controlled clinical trial. BMC Oral Health 2023, 23, 883. [Google Scholar]
- Nikniaz, S.; Vaziri, F.; Mansouri, R. Impact of resveratrol supplementation on clinical parameters and inflammatory markers in patients with chronic periodontitis: A randomized clinical trail. BMC Oral Health 2023, 23, 177. [Google Scholar]
- Bayer, J.; Petersen, N.K.; Hess, J.V.; Jockel-Schneider, Y.; Hogger, P. Impact of a dietary supplementation with french maritime pine bark extract pycnogenol((r)) on salivary and serum inflammatory biomarkers during non-surgical periodontal therapy-a randomized placebo-controlled double-blind trial. Nutrients 2025, 17, 1546. [Google Scholar]
- Zhu, Y.; Ali, A.; Mulinari Dos Santos, G.; Franciscon, J.P.S.; de Molon, R.S.; Goh, C.; Erovolino, E.; Theodoro, L.H.; Shrestha, A. A chitosan-based hydrogel to modulate immune cells and promote periodontitis healing in the high-fat diet-induced periodontitis rat model. Acta Biomater. 2025, 200, 452–463. [Google Scholar]
- Da Ponte Leguizamon, N.; de Molon, R.S.; Coletto-Nunes, G.; Nogueira, A.V.B.; Rocha, S.V.; Neo-Justino, D.M.; Soares-Costa, A.; Cerri, P.S.; Lerner, U.H.; Souza, P.P.C.; et al. Phytocystatin csincpi-2 reduces osteoclastogenesis and alveolar bone loss. J. Dent. Res. 2022, 101, 216–225. [Google Scholar]
- Fernandes, N.A.R.; Camilli, A.C.; Maldonado, L.A.G.; Pacheco, C.G.P.; Silva, A.F.; Molon, R.S.; Spolidorio, L.C.; Ribeiro de Assis, L.; Regasini, L.O.; Rossa Junior, C.; et al. Chalcone t4, a novel chalconic compound, inhibits inflammatory bone resorption in vivo and suppresses osteoclastogenesis in vitro. J. Periodontal Res. 2021, 56, 569–578. [Google Scholar]
- Laky, M.; Arslan, M.; Zhu, X.; Rausch-Fan, X.; Moritz, A.; Sculean, A.; Laky, B.; Ramseier, C.A.; Stahli, A.; Eick, S. Quercetin in the prevention of induced periodontal disease in animal models: A systematic review and meta-analysis. Nutrients 2024, 16, 735. [Google Scholar] [CrossRef]
- Taskan, M.M.; Gevrek, F. Quercetin decreased alveolar bone loss and apoptosis in experimentally induced periodontitis model in wistar rats. Antiinflamm. Antiallergy Agents Med. Chem. 2020, 19, 436–448. [Google Scholar] [PubMed]
- Casili, G.; Ardizzone, A.; Lanza, M.; Gugliandolo, E.; Portelli, M.; Militi, A.; Cuzzocrea, S.; Esposito, E.; Paterniti, I. Treatment with luteolin improves lipopolysaccharide-induced periodontal diseases in rats. Biomedicines 2020, 8, 442. [Google Scholar] [CrossRef] [PubMed]
- Pavanelli, A.L.R.; Vieira, S.M.; Marcantonio, C.C.; Faria, G.; Tetradis, S.; de Souza, P.P.C.; Cirelli, J.A.; de Molon, R.S. Anti-inflammatory and antiresorptive activities of tanshinone-iia mitigate alveolar bone destruction in mice with experimental periodontitis. J. Periodontol. 2025, accepted for publication. [Google Scholar]
- Ekeuku, S.O.; Pang, K.L.; Chin, K.Y. The skeletal effects of tanshinones: A review. Molecules 2021, 26, 2319. [Google Scholar] [CrossRef]
- Sudha, S.; Upmanyu, A.; Saraswat, D.; Singh, M. Pharmacological impacts of tanshinone on osteogenesis and osteoclastogenesis: A review. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 135–146. [Google Scholar]
- Wang, L.; Wang, S.; Dai, X.; Yue, G.; Yin, J.; Xu, T.; Shi, H.; Liu, T.; Jia, Z.; Bromme, D.; et al. Salvia miltiorrhiza in osteoporosis: A review of its phytochemistry, traditional clinical uses and preclinical studies (2014–2024). Front. Pharmacol. 2024, 15, 1483431. [Google Scholar]
- Ye, Z.; Liu, Y.; Song, J.; Gao, Y.; Fang, H.; Hu, Z.; Zhang, M.; Liao, W.; Cui, L.; Liu, Y. Expanding the therapeutic potential of salvia miltiorrhiza: A review of its pharmacological applications in musculoskeletal diseases. Front. Pharmacol. 2023, 14, 1276038. [Google Scholar]
- Huang, X.; Deng, H.; Shen, Q.K.; Quan, Z.S. Tanshinone iia: Pharmacology, total synthesis, and progress in structure-modifications. Curr. Med. Chem. 2022, 29, 1959–1989. [Google Scholar]
- Sung, H.J.; Choi, S.M.; Yoon, Y.; An, K.S. Tanshinone iia, an ingredient of salvia miltiorrhiza bunge, induces apoptosis in human leukemia cell lines through the activation of caspase-3. Exp. Mol. Med. 1999, 31, 174–178. [Google Scholar]
- Chen, X.; Guo, J.; Bao, J.; Lu, J.; Wang, Y. The anticancer properties of salvia miltiorrhiza bunge (danshen): A systematic review. Med. Res. Rev. 2014, 34, 768–794. [Google Scholar]
- Pang, H.; Wu, L.; Tang, Y.; Zhou, G.; Qu, C.; Duan, J.A. Chemical analysis of the herbal medicine salviae miltiorrhizae radix et rhizoma (danshen). Molecules 2016, 21, 51. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Li, L.; Su, J.; Li, S.; Duncan, S.E.; Liu, Z.; Fan, G. Pharmacological activity and mechanism of tanshinone iia in related diseases. Drug Des. Dev. Ther. 2020, 14, 4735–4748. [Google Scholar]
- Panwar, P.; Andrault, P.M.; Saha, D.; Bromme, D. Immune regulatory and anti-resorptive activities of tanshinone iia sulfonate attenuates rheumatoid arthritis in mice. Br. J. Pharmacol. 2024, 181, 5009–5027. [Google Scholar]
- Panwar, P.; Law, S.; Jamroz, A.; Azizi, P.; Zhang, D.; Ciufolini, M.; Bromme, D. Tanshinones that selectively block the collagenase activity of cathepsin k provide a novel class of ectosteric antiresorptive agents for bone. Br. J. Pharmacol. 2018, 175, 902–923. [Google Scholar] [PubMed]
- Panwar, P.; Xue, L.; Soe, K.; Srivastava, K.; Law, S.; Delaisse, J.M.; Bromme, D. An ectosteric inhibitor of cathepsin k inhibits bone resorption in ovariectomized mice. J. Bone Miner. Res. 2017, 32, 2415–2430. [Google Scholar]
- Ren, J.; Fu, L.; Nile, S.H.; Zhang, J.; Kai, G. Salvia miltiorrhiza in treating cardiovascular diseases: A review on its pharmacological and clinical applications. Front. Pharmacol. 2019, 10, 753. [Google Scholar]
- Ma, X.; Zhang, L.; Gao, F.; Jia, W.; Li, C. Salvia miltiorrhiza and tanshinone iia reduce endothelial inflammation and atherosclerotic plaque formation through inhibiting cox-2. Biomed. Pharmacother. 2023, 167, 115501. [Google Scholar]
- Xie, F.; Fu, X.; Li, W.; Bao, Y.; Chang, F.; Lu, Y.; Lu, Y. Effects of sodium tanshinone iia sulfonate injection on pro-inflammatory cytokines, adhesion molecules and chemokines in chinese patients with atherosclerosis and atherosclerotic cardiovascular disease: A meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 2025, 12, 1511747. [Google Scholar]
- Li, Z.M.; Xu, S.W.; Liu, P.Q. Salvia miltiorrhizaburge (danshen): A golden herbal medicine in cardiovascular therapeutics. Acta Pharmacol. Sin. 2018, 39, 802–824. [Google Scholar]
- Fu, L.; Han, B.; Zhou, Y.; Ren, J.; Cao, W.; Patel, G.; Kai, G.; Zhang, J. The anticancer properties of tanshinones and the pharmacological effects of their active ingredients. Front. Pharmacol. 2020, 11, 193. [Google Scholar]
- Luo, N.; Zhang, K.; Li, X.; Hu, Y.; Guo, L. Tanshinone iia destabilizes slc7a11 by regulating pias4-mediated sumoylation of slc7a11 through kdm1a, and promotes ferroptosis in breast cancer. J. Adv. Res. 2025, 69, 313–327. [Google Scholar] [PubMed]
- Zhou, J.; Jiang, Y.Y.; Wang, X.X.; Wang, H.P.; Chen, H.; Wu, Y.C.; Wang, L.; Pu, X.; Yue, G.Z.; Zhang, L. Tanshinone iia suppresses ovarian cancer growth through inhibiting malignant properties and angiogenesis. Ann. Transl. Med. 2020, 8, 1295. [Google Scholar] [PubMed]
- Liang, E.Y.; Huang, M.H.; Chen, Y.T.; Zhang, P.W.; Shen, Y.; Tu, X.X.; Chen, W.Y.; Wang, Y.; Yan, J.; Wang, H.Y.; et al. Tanshinone iia modulates cancer cell morphology and movement via rho gtpases-mediated actin cytoskeleton remodeling. Toxicol. Appl. Pharmacol. 2024, 483, 116839. [Google Scholar] [PubMed]
- Jia, Q.; Zhu, R.; Tian, Y.; Chen, B.; Li, R.; Li, L.; Wang, L.; Che, Y.; Zhao, D.; Mo, F.; et al. Salvia miltiorrhiza in diabetes: A review of its pharmacology, phytochemistry, and safety. Phytomedicine 2019, 58, 152871. [Google Scholar]
- Hu, C.H.; Chen, Y.; Jin, T.Y.; Wang, Z.; Jin, B.; Liao, J.; Ding, C.Y.; Zhang, A.; Tang, W.Y.; Zhang, L.X.; et al. A derivative of tanshinone iia and salviadione, 15a, inhibits inflammation and alleviates dss-induced colitis in mice by direct binding and inhibition of ripk2. Acta Pharmacol. Sin. 2025, 46, 672–686. [Google Scholar]
- Liu, L.; Gao, H.; Wen, T.; Gu, T.; Zhang, S.; Yuan, Z. Tanshinone iia attenuates aom/dss-induced colorectal tumorigenesis in mice via inhibition of intestinal inflammation. Pharm. Biol. 2021, 59, 89–96. [Google Scholar]
- Liu, X.; He, H.; Huang, T.; Lei, Z.; Liu, F.; An, G.; Wen, T. Tanshinone iia protects against dextran sulfate sodium-(dss-) induced colitis in mice by modulation of neutrophil infiltration and activation. Oxid. Med. Cell Longev. 2016, 2016, 7916763. [Google Scholar]
- Lei, W.; Li, X.; Li, L.; Huang, M.; Cao, Y.; Sun, X.; Jiang, M.; Zhang, B.; Zhang, H. Compound danshen dripping pill ameliorates post ischemic myocardial inflammation through synergistically regulating mapk, pi3k/akt and ppar signaling pathways. J. Ethnopharmacol. 2021, 281, 114438. [Google Scholar]
- Panwar, P.; Soe, K.; Guido, R.V.; Bueno, R.V.; Delaisse, J.M.; Bromme, D. A novel approach to inhibit bone resorption: Exosite inhibitors against cathepsin k. Br. J. Pharmacol. 2016, 173, 396–410. [Google Scholar]
- Lee, S.Y.; Choi, D.Y.; Woo, E.R. Inhibition of osteoclast differentiation by tanshinones from the root of salvia miltiorrhiza bunge. Arch. Pharm. Res. 2005, 28, 909–913. [Google Scholar]
- Cheng, L.; Zhou, S.; Zhao, Y.; Sun, Y.; Xu, Z.; Yuan, B.; Chen, X. Tanshinone iia attenuates osteoclastogenesis in ovariectomized mice by inactivating nf-kb and akt signaling pathways. Am. J. Transl. Res. 2018, 10, 1457–1468. [Google Scholar] [PubMed]
- Kim, H.H.; Kim, J.H.; Kwak, H.B.; Huang, H.; Han, S.H.; Ha, H.; Lee, S.W.; Woo, E.R.; Lee, Z.H. Inhibition of osteoclast differentiation and bone resorption by tanshinone iia isolated from salvia miltiorrhiza bunge. Biochem. Pharmacol. 2004, 67, 1647–1656. [Google Scholar] [PubMed]
- Ma, C.; Wang, Z.; Mo, L.; Wang, X.; Zhou, G.; Yi, C.; Niu, W.; Liu, Y. Tanshinone i attenuates estrogen-deficiency bone loss via inhibiting rankl-induced mapk and nf-kappab signaling pathways. Int. Immunopharmacol. 2024, 127, 111322. [Google Scholar] [PubMed]
- Peng, Q.; Wang, J.; Han, M.; Zhao, M.; Li, K.; Lu, T.; Guo, Q.; Jiang, Q. Tanshinone iia inhibits osteoclastogenesis in rheumatoid arthritis via ldhc-regulated ros generation. Chin. Med. 2023, 18, 54. [Google Scholar]
- Yang, W.; Han, J.; Gong, S.; Zhao, J.; Yu, T.; Ma, J. Cryptotanshinone suppressed postmenopausal osteoporosis by preventing rankl-mediated osteoclastogenesis against kidney injury. Evid. Based Complement. Altern. Med. 2022, 2022, 2821984. [Google Scholar]
- McClung, M.R.; O’Donoghue, M.L.; Papapoulos, S.E.; Bone, H.; Langdahl, B.; Saag, K.G.; Reid, I.R.; Kiel, D.P.; Cavallari, I.; Bonaca, M.P.; et al. Odanacatib for the treatment of postmenopausal osteoporosis: Results of the loft multicentre, randomised, double-blind, placebo-controlled trial and loft extension study. Lancet Diabetes Endocrinol. 2019, 7, 899–911. [Google Scholar]
- Feng, X.; Teitelbaum, S.L. Osteoclasts: New insights. Bone Res. 2013, 1, 11–26. [Google Scholar]
- Sabokbar, A.; Mahoney, D.J.; Hemingway, F.; Athanasou, N.A. Non-canonical (rankl-independent) pathways of osteoclast differentiation and their role in musculoskeletal diseases. Clin. Rev. Allergy Immunol. 2016, 51, 16–26. [Google Scholar]
- Sirisereephap, K.; Maekawa, T.; Tamura, H.; Hiyoshi, T.; Domon, H.; Isono, T.; Terao, Y.; Maeda, T.; Tabeta, K. Osteoimmunology in periodontitis: Local proteins and compounds to alleviate periodontitis. Int. J. Mol. Sci. 2022, 23, 5540. [Google Scholar]
- An, J.; Hao, D.; Zhang, Q.; Chen, B.; Zhang, R.; Wang, Y.; Yang, H. Natural products for treatment of bone erosive diseases: The effects and mechanisms on inhibiting osteoclastogenesis and bone resorption. Int. Immunopharmacol. 2016, 36, 118–131. [Google Scholar]
- AlQranei, M.S.; Chellaiah, M.A. Osteoclastogenesis in periodontal diseases: Possible mediators and mechanisms. J. Oral Biosci. 2020, 62, 123–130. [Google Scholar] [PubMed]
- Kohli, S.S.; Kohli, V.S. Role of rankl-rank/osteoprotegerin molecular complex in bone remodeling and its immunopathologic implications. Indian J. Endocrinol. Metab. 2011, 15, 175–181. [Google Scholar] [PubMed]
- Jiang, T.; Xia, T.; Qiao, F.; Wang, N.; Jiang, Y.; Xin, H. Role and regulation of transcription factors in osteoclastogenesis. Int. J. Mol. Sci. 2023, 24, 16175. [Google Scholar] [PubMed]
- Kwak, H.B.; Yang, D.; Ha, H.; Lee, J.H.; Kim, H.N.; Woo, E.R.; Lee, S.; Kim, H.H.; Lee, Z.H. Tanshinone iia inhibits osteoclast differentiation through down-regulation of c-fos and nfatc1. Exp. Mol. Med. 2006, 38, 256–264. [Google Scholar]
- Souza, J.A.; Rossa, C., Jr.; Garlet, G.P.; Nogueira, A.V.; Cirelli, J.A. Modulation of host cell signaling pathways as a therapeutic approach in periodontal disease. J. Appl. Oral Sci. 2012, 20, 128–138. [Google Scholar]
- Trares, K.; Ackermann, J.; Koch, I. The canonical and non-canonical nf-kappab pathways and their crosstalk: A comparative study based on petri nets. Biosystems 2022, 211, 104564. [Google Scholar]
- Sun, S.C. The non-canonical nf-kappab pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar]
- Gao, H.; Liu, X.; Sun, W.; Kang, N.; Liu, Y.; Yang, S.; Xu, Q.M.; Wang, C.; Chen, X. Total tanshinones exhibits anti-inflammatory effects through blocking tlr4 dimerization via the myd88 pathway. Cell Death Dis. 2017, 8, e3004. [Google Scholar]
- Jang, S.I.; Kim, H.J.; Kim, Y.J.; Jeong, S.I.; You, Y.O. Tanshinone iia inhibits lps-induced nf-kappab activation in raw 264.7 cells: Possible involvement of the nik-ikk, erk1/2, p38 and jnk pathways. Eur. J. Pharmacol. 2006, 542, 1–7. [Google Scholar]
- Tang, S.; Shen, X.Y.; Huang, H.Q.; Xu, S.W.; Yu, Y.; Zhou, C.H.; Chen, S.R.; Le, K.; Wang, Y.H.; Liu, P.Q. Cryptotanshinone suppressed inflammatory cytokines secretion in raw264.7 macrophages through inhibition of the nf-kappab and mapk signaling pathways. Inflammation 2011, 34, 111–118. [Google Scholar]
- Liu, C.; Zhang, J.; Ye, Z.; Luo, J.; Peng, B.; Wang, Z. Research on the role and mechanism of the pi3k/akt/mtor signalling pathway in osteoporosis. Front. Endocrinol. 2025, 16, 1541714. [Google Scholar]
- Kang, H.; Chang, W.; Hurley, M.; Vignery, A.; Wu, D. Important roles of pi3kgamma in osteoclastogenesis and bone homeostasis. Proc. Natl. Acad. Sci. USA 2010, 107, 12901–12906. [Google Scholar] [PubMed]
- Chiu, Y.C.; Lin, C.Y.; Chen, C.P.; Huang, K.C.; Tong, K.M.; Tzeng, C.Y.; Lee, T.S.; Hsu, H.C.; Tang, C.H. Peptidoglycan enhances il-6 production in human synovial fibroblasts via tlr2 receptor, focal adhesion kinase, akt, and ap-1-dependent pathway. J. Immunol. 2009, 183, 2785–2792. [Google Scholar] [PubMed]
- Yuan, P.; Qin, H.Y.; Wei, J.Y.; Chen, G.; Li, X. Proteomics reveals the potential mechanism of tanshinone iia in promoting the ex vivo expansion of human bone marrow mesenchymal stem cells. Regen. Ther. 2022, 21, 560–573. [Google Scholar]
- Liu, X.; Niu, Y.; Xie, W.; Wei, D.; Du, Q. Tanshinone iia promotes osteogenic differentiation of human periodontal ligament stem cells via erk1/2-dependent runx2 induction. Am. J. Transl. Res. 2019, 11, 340–350. [Google Scholar]
- Lacey, D.L.; Boyle, W.J.; Simonet, W.S.; Kostenuik, P.J.; Dougall, W.C.; Sullivan, J.K.; San Martin, J.; Dansey, R. Bench to bedside: Elucidation of the opg-rank-rankl pathway and the development of denosumab. Nat. Rev. Drug Discov. 2012, 11, 401–419. [Google Scholar]
- Hofbauer, L.C.; Khosla, S.; Dunstan, C.R.; Lacey, D.L.; Boyle, W.J.; Riggs, B.L. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J. Bone Miner. Res. 2000, 15, 2–12. [Google Scholar]
- Kwak, H.B.; Sun, H.M.; Ha, H.; Kim, H.N.; Lee, J.H.; Kim, H.H.; Shin, H.I.; Lee, Z.H. Tanshinone iia suppresses inflammatory bone loss by inhibiting the synthesis of prostaglandin e2 in osteoblasts. Eur. J. Pharmacol. 2008, 601, 30–37. [Google Scholar]
- Zhang, S.; Liu, J.; Zhao, G. Tanshinone type iia inhibits osteoprotegerin and osteoclast differentiation factor expression at relapse stage after orthodontic tooth movement. Chin. J. Tissue Eng. Res. 2014, 53, 1730–1736. [Google Scholar]
- Yao, J.; Ma, S.; Feng, W.; Wei, Y.; Lu, H.; Zhong, G.; Wu, Z.; Wang, H.; Su, W.; Li, J. Tanshinone iia protects against polyethylene particle-induced osteolysis response in a mouse calvarial model. Int. J. Clin. Exp. Pathol. 2018, 11, 4461–4471. [Google Scholar]
- Baron, R.; Kneissel, M. Wnt signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [PubMed]
- Krishnan, V.; Bryant, H.U.; Macdougald, O.A. Regulation of bone mass by wnt signaling. J. Clin. Investig. 2006, 116, 1202–1209. [Google Scholar] [PubMed]
- Yang, Y.J.; Zhu, Z.; Wang, D.T.; Zhang, X.L.; Liu, Y.Y.; Lai, W.X.; Mo, Y.L.; Li, J.; Liang, Y.L.; Hu, Z.Q.; et al. Tanshinol alleviates impaired bone formation by inhibiting adipogenesis via klf15/ppargamma2 signaling in gio rats. Acta Pharmacol. Sin. 2018, 39, 633–641. [Google Scholar] [PubMed]
- Miyazono, K.; Kamiya, Y.; Morikawa, M. Bone morphogenetic protein receptors and signal transduction. J. Biochem. 2010, 147, 35–51. [Google Scholar]
- Kim, H.J.; Kim, S.H. Tanshinone iia enhances bmp-2-stimulated commitment of c2c12 cells into osteoblasts via p38 activation. Amino Acids 2010, 39, 1217–1226. [Google Scholar]
- Chen, G.; Deng, C.; Li, Y.P. Tgf-beta and bmp signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar]
- Marie, P.J.; Miraoui, H.; Severe, N. Fgf/fgfr signaling in bone formation: Progress and perspectives. Growth Factors 2012, 30, 117–123. [Google Scholar]
- Su, N.; Du, X.; Chen, L. Fgf signaling: Its role in bone development and human skeleton diseases. Front. Biosci. 2008, 13, 2842–2865. [Google Scholar]
- Compston, J.; Cooper, A.; Cooper, C.; Gittoes, N.; Gregson, C.; Harvey, N.; Hope, S.; Kanis, J.A.; McCloskey, E.V.; Poole, K.E.S.; et al. Uk clinical guideline for the prevention and treatment of osteoporosis. Arch. Osteoporos. 2017, 12, 43. [Google Scholar]
- Wang, L.; Cheng, L.; Zhang, B.; Wang, N.; Wang, F. Tanshinone prevents alveolar bone loss in ovariectomized osteoporosis rats by up-regulating phosphoglycerate dehydrogenase. Toxicol. Appl. Pharmacol. 2019, 376, 9–16. [Google Scholar]
- Wei, W.; Heng, Y.Y.; Wu, F.F.; Dong, H.Y.; Zhang, P.F.; Li, J.X.; Liu, C.Y.; Yang, B.J.; Fu, J.N.; Liang, X.Y. Sodium tanshinone iia sulfonate alleviates vascular senescence in diabetic mice by modulating the a20-nfkappab-nlrp3 inflammasome-catalase pathway. Sci. Rep. 2024, 14, 17665. [Google Scholar]
- Zhang, J.; Cai, Z.; Yang, M.; Tong, L.; Zhang, Y. Inhibition of tanshinone iia on renin activity protected against osteoporosis in diabetic mice. Pharm. Biol. 2020, 58, 219–224. [Google Scholar] [PubMed]
- Wang, P.; Zhao, Z.; Li, Z.; Li, X.; Huang, B.; Lu, X.; Dai, S.; Li, S.; Man, Z.; Li, W. Attenuation of osteoarthritis progression via locoregional delivery of klotho-expressing plasmid DNA and tanshinon iia through a stem cell-homing hydrogel. J. Nanobiotechnol. 2024, 22, 325. [Google Scholar]
- Wang, X.; Fan, J.; Ding, X.; Sun, Y.; Cui, Z.; Liu, W. Tanshinone i inhibits il-1beta-induced apoptosis, inflammation and extracellular matrix degradation in chondrocytes chon-001 cells and attenuates murine osteoarthritis. Drug Des. Dev. Ther. 2019, 13, 3559–3568. [Google Scholar]
- Cui, L.; Wu, T.; Liu, Y.Y.; Deng, Y.F.; Ai, C.M.; Chen, H.Q. Tanshinone prevents cancellous bone loss induced by ovariectomy in rats. Acta Pharmacol. Sin. 2004, 25, 678–684. [Google Scholar]
- Li, H.Z.; Han, D.; Ao, R.F.; Cai, Z.H.; Zhu, G.Z.; Wu, D.Z.; Gao, J.W.; Zhuang, J.S.; Tu, C.; Zhao, K.; et al. Tanshinone iia attenuates osteoarthritis via inhibiting aberrant angiogenesis in subchondral bone. Arch. Biochem. Biophys. 2024, 753, 109904. [Google Scholar]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar]
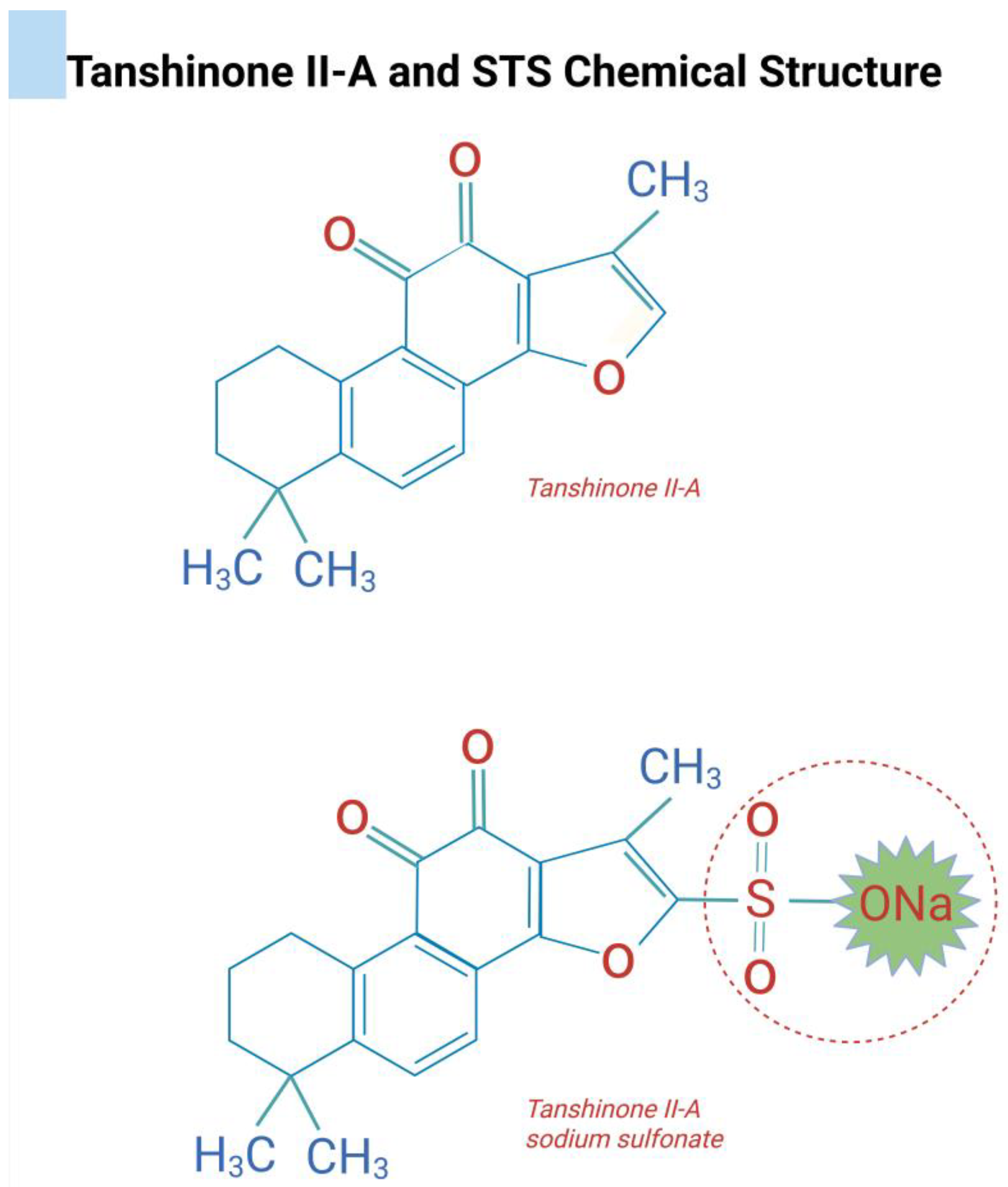
| Tanshinone Type | Biological Effects | Key Properties |
|---|---|---|
| Tanshinone IIA |
| Lipophilic diterpenoid quinone; suppresses inflammatory cytokines (IL-1β, IL-17); modulates signaling pathways; and improves bone density and architecture in vivo. |
| Sodium Tanshinone IIA Sulfonate (STS) |
| Water-soluble derivative of T-IIA; protects endothelial and smooth muscle cells from oxidative stress; and downregulates cathepsin K. Binds ectosteric site on CatK; reversible inhibition; reduces inflammatory cytokines and osteoclast activity; and avoids side effects of active-site inhibitors. |
| Tanshinone I |
| Lipophilic diterpenoid; modulates NF-κB and apoptosis pathways; and protects cartilage in vitro and in vivo models. |
| Cryptotanshinone |
| Diterpenoid quinone; modulates inflammation and osteoclast activity; and exact mechanisms less studied independently. |
| dihydrotanshinone I |
| Principal lipophilic phenanthraquinone compound found in Salvia miltiorrhiza. It has a broad range of biological roles, including antibacterial and anti-inflammatory, antioxidant, and regulation of immune cells. |
| Authors (Year) | Tanshinone Type | Experimental Design | Animal Model/Methodology | Main Results |
|---|---|---|---|---|
| Wang et al. (2019) [111] | T-IIA | Investigate the effects and molecular mechanisms of tanshinone on osteoporosis. T-IIA was administered by tail vein injection at a dose of 10 mg/kg daily for two weeks. | Osteoporosis was induced by bilateral ovariectomy (OVX) in adult female rats treated with or without T-IIA. Trabecular bone structure was assessed by micro-CT, and levels of age-related genes were measured by mRNA. | T-IIA preserved bone volume and microarchitecture, enhanced trabecular number and thickness, and reduced trabecular separation. Mechanistically, it rejuvenated stromal cells by upregulating PHGDH, countering estrogen-deficiency-induced senescence. Tanshinone potently suppresses OVX-induced osteoporosis and BMSC senescence through upregulation of PHGDH. |
| Panwar et al. (2017) [56] | STS | In vitro and in vivo study using human and mouse osteoclasts and OVX mice. Adult C57BL/6 mice received 40 mg/kg/d STS by oral gavage for 3 months. | STS was tested for collagen degradation inhibition, osteoclast activity, bone resorption, and in vivo bone parameters in OVX mice. | STS selectively inhibited collagen degradation and suppressed bone resorption in human and mouse osteoclasts without affecting osteoclastogenesis or metabolism. In OVX mice, 3-month STS treatment reduced plasma CTx-1 by 20%, increased osteoblasts and P1NP (~28%), and improved femoral BMD by 35%. |
| Wei et al. (2024) [112] | STS | Examined vascular senescence in diabetic mice treated with STS. Focused on the NFκB–NLRP3–catalase axis. In vivo (diabetic mice) and in vitro (primary ECs and VSMCs under high glucose) study. Diabetic mice were treated with intravenous injections of 10 mg/kg/day STS for 90 days. | Diabetic mice and primary vascular cells were treated with STS and transfected with NLRP3 and A20 overexpression/knockout plasmids; senescence markers and signaling pathways were assessed. | STS reduced vascular senescence in diabetic mice by maintaining catalase levels, improving vascular relaxation, and lowering oxidative stress and senescence markers (p21, SA-β-gal, and collagen). Mechanistically, STS inhibited NLRP3 phosphorylation, dimerization, and inflammasome activation while preserving A20 and CAT expression and suppressing NFκB signaling in ECs and VSMCs under high glucose. |
| Zhang et al. (2020) [113] | T-IIA | Assessed T-IIA’s impact on diabetic osteoporosis through RAS modulation. In vitro (renin-expressing HEK-293 cells) and in vivo (STZ-induced diabetic mice) study. | T-IIA was screened for renin inhibition in engineered HEK-293 cells; diabetic C57BL/6 mice were treated with T-IIA (10 or 30 mg/kg) or aliskiren. ANG II levels and bone parameters were assessed. | T-IIA inhibited renin activity and reduced ANG II expression in vitro. In diabetic mice, it decreased serum ANG II levels and expression in bone and improved trabecular bone mineral density and structure in the tibia and femur. Findings suggest T-IIA as a potential renin inhibitor with osteoprotective effects in diabetic osteoporosis. |
| Wang et al. (2024) [114] | T-IIA | Developed a peptide-hydrogel (pPNP+TIIA@PFS) for OA therapy targeting senescent chondrocytes using in vitro and in vivo (surgically induced osteoarthritis in rats) models. The pPNP + TIIA@PFS was injected into the rat knee joint to assess its therapeutic efficacy against OA. | Developed an injectable peptide hydrogel (pPNP + TIIA@PFS) delivering T-IIA and DNA-loaded nanoparticles; evaluated effects on chondrocyte senescence, cartilage regeneration, and OA progression | pPNP + TIIA@PFS increased anti-aging protein Klotho, blocked senescence signaling, reduced chondrocyte senescence, and improved cartilage integrity. It recruited bone mesenchymal stem cells and promoted chondrogenesis. In OA rats, it reduced osteophyte formation and cartilage degeneration, indicating therapeutic potential for OA. |
| Pavanelli et al. (2025) [44] | T-IIA and STS | Explored T-IIA and STS effects in a murine model of ligature-induced periodontitis in C57BL/6 mice. | C57BL/6J mice assigned to control, periodontitis, T-IIA, and STS groups; treated with 40 mg/kg tanshinones via oral gavage for 10 days; assessed via micro-CT, histology, immunohistochemistry, and RT-qPCR. | Both T-IIA and STS reduced inflammatory cell infiltration, increased fibroblast count, prevented alveolar bone loss, improved bone architecture and mineral density, reduced osteoclast numbers, and suppressed IL-1β, IL-17, and MMP-13. STS significantly reduced cathepsin K expression. Findings support their anti-inflammatory and antiresorptive potential in periodontitis. |
| Panwar et al. (2024) [54] | STS | Evaluated anti-inflammatory and anti-resorptive actions of STS in collagen-induced arthritis (CIA). CIA was induced by injecting 200 μg of bovine type II collagen emulsified with complete Freund adjuvant with 0.5 mg/mL of M. tuberculosis into the tail base, followed by 200 μg of immunization with bovine type II collagen and IFA. STS (40 mg/kg/d) was mixed with food powder (3 g chow per mouse). | Compared STS with active site inhibitor odanacatib (ODN); assessed joint pathology, cytokines, osteoclasts, and NF-κB signaling using histopathology, flow cytometry, and biochemical assays. | STS reduced immune cell infiltration, inflammatory cytokines (incl. IL-17), Th17 cells, and osteoclasts in joints. It selectively inhibited CatK collagenolytic activity via oligomerization blockade and suppressed the NF-κB pathway by inhibiting IκBα phosphorylation. ODN showed only antiresorptive activity without anti-inflammatory effects. T06 demonstrated dual therapeutic action in RA. |
| Wang et al. (2019) [115] | T-I | Investigated anti-inflammatory and cartilage-protective effects in vitro (IL-1β-induced OA model in CHON-001 cells) and in vivo using an anterior cruciate ligament transection mouse model (ACLT-induced OA in mice). Mice were treated with T-I via intraperitoneal injection once daily for 8 weeks after surgery using 10 mg/kg or 30 mg/kg T-I. | CHON-001 cells pretreated with T-I (20 μM) and stimulated with IL-1β; mice treated with T-I (10 or 30 mg/kg) for 8 weeks post-ACLT. Cell viability, apoptosis, ECM degradation, and inflammation were assessed via CCK-8, flow cytometry, Western blot, and histological staining. | T-I reduced IL-1β-induced apoptosis, preserved collagen II and aggrecan, suppressed MMP-13, cleaved caspase 1, Gasdermin D, and p-NF-κB, and restored SOX11 expression in vitro. In vivo, it alleviated cartilage degradation, synovitis, and subchondral bone loss and reduced OARSI scores, suggesting therapeutic potential in OA. |
| Panwar et al. (2018) [55] | STS | Screened tanshinone derivatives for cathepsin K (CatK) inhibition. In vitro enzymatic and cell-based assays using human osteoclasts. | Screened 31 tanshinones from Salvia miltiorrhiza for CatK inhibition; assessed collagen degradation, bone resorption, cell viability, osteoclastogenesis, reversibility, and binding sites via enzymatic assays, SEM, mechanical testing, and molecular docking. | Twelve tanshinones showed selective anti-collagenase activity without affecting non-collagenous substrates. Six compounds strongly inhibited osteoclast-mediated bone resorption (IC50 < 500 nM) without impacting cell viability or osteoclastogenesis. The core pharmacophore was identified as a three-ring structure with para- or ortho-quinone. Findings support ectosteric CatK inhibition as a safer therapeutic approach. |
| Cheng et al. (2018) [72] | T-IIA | In vivo study using ovariectomized (OVX) C57BL/6 mice to model postmenopausal osteoporosis; in vitro study using bone marrow-derived macrophages (BMMs) to evaluate osteoclastogenesis. T-IIA (10 mg/kg) was given by intraperitoneal injection daily for 6 weeks. | OVX mice were treated with T-IIA to assess bone loss prevention. In vitro, BMMs were stimulated with RANKL in the presence or absence of T-IIA to evaluate osteoclast formation. Western blot, TRAP staining, and immunofluorescence were used to analyze signaling pathways and osteoclast markers. | T-IIA prevented bone loss in OVX mice and inhibited RANKL-induced osteoclast differentiation in vitro. It blocked NF-κB, Akt, and MAPK signaling pathways, reducing phosphorylation of IκB, ERK, p38, and Akt, and nuclear NF-κB p65 translocation. Osteoclast-related gene expression was also decreased. Suggests therapeutic potential in postmenopausal osteoporosis. |
| Cui et al. (2004) [116] | Total tanshinone (containing 17% of T-IIA and 8% of cryptotanshinone) | In vivo study using OVX Sprague–Dawley female rats: four groups including sham-operated control, OVX + vehicle, OVX + total tanshinone, and OVX + 17α-ethynylestradiol (positive control). Treatments started 1 day post-OVX and continued daily for 10 weeks. | Rats were administered total tanshinone (200 mg/kg/day, equivalent doses of T-IIA and cryptotanshinone), vehicle, or estrogen. Bone histomorphometry of lumbar vertebrae (LV4) and proximal tibial metaphyses (PTM) was performed. | Tanshinone prevented OVX-induced decreases in trabecular bone volume and number and increases in osteoclast surface in LV4 and partially protected PTM. Tanshinone increased trabecular thickness and did not affect mineralizing surface, body, or uterine weight. Estrogen increased bone volume but decreased mineralizing surface and increased uterine weight. Tanshinone prevented bone loss by inhibiting bone resorption without estrogenic side effects. |
| Li et al. (2024) [117] | T-IIA | In vivo study using a monosodium iodoacetate (MIA)-induced osteoarthritis (OA) mouse model; in vitro assays with primary CD31hiEmcnhi endothelial cells T-IIA (20 mg/kg) was administered daily via intragastric administration for 2 weeks. | MIA was injected to induce OA in mice, followed by T-IIA treatment to evaluate effects on cartilage degeneration, subchondral bone remodeling, and angiogenesis. Endothelial cell angiogenesis assays and hypertrophic chondrocyte culture supernatant were used to study mechanisms. | TIIA attenuated cartilage degeneration, normalized subchondral bone remodeling, and suppressed aberrant angiogenesis in vivo. It reduced hypertrophic chondrocyte numbers and VEGFA secretion, inhibited tube formation of endothelial cells, and downregulated VEGFR2 and MAPK signaling. Results highlight TIIA as a potential anti-angiogenic therapeutic agent for OA. |
| Yao et al. (2018) [101] | T-IIA | In vivo study using a polyethylene (PE) particle-induced osteolysis mouse calvarial model: C57BL/6J male mice divided into sham, PE+PBS, PE+low-dose T- IIA, and PE+high-dose T-IIA groups T-IIA (1 or 2 µg/g) was locally injected into the mouse skull for 21 days. | PE particles implanted on calvaria to induce osteolysis; mice treated with T-IIA (1 or 2 µg/g) or PBS for 21 days. Bone resorption assessed by micro-CT and histomorphometry; osteoclast activity markers (OSCAR and CTX-1) and OPG measured by ELISA. | T-IIA dose-dependently reduced PE particle-induced bone resorption and osteoclast formation/activity. It decreased OSCAR and CTX-1 levels and increased OPG expression, protecting bone around implants. Suggests potential to prevent aseptic loosening post-joint replacement. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Molon, R.S. Therapeutic Potential of Tanshinones in Osteolytic Diseases: From Molecular and Cellular Pathways to Preclinical Models. Dent. J. 2025, 13, 309. https://doi.org/10.3390/dj13070309
de Molon RS. Therapeutic Potential of Tanshinones in Osteolytic Diseases: From Molecular and Cellular Pathways to Preclinical Models. Dentistry Journal. 2025; 13(7):309. https://doi.org/10.3390/dj13070309
Chicago/Turabian Stylede Molon, Rafael Scaf. 2025. "Therapeutic Potential of Tanshinones in Osteolytic Diseases: From Molecular and Cellular Pathways to Preclinical Models" Dentistry Journal 13, no. 7: 309. https://doi.org/10.3390/dj13070309
APA Stylede Molon, R. S. (2025). Therapeutic Potential of Tanshinones in Osteolytic Diseases: From Molecular and Cellular Pathways to Preclinical Models. Dentistry Journal, 13(7), 309. https://doi.org/10.3390/dj13070309






