Therapeutic Applications of Dental Mesenchymal Stem Cells in Alzheimer’s Disease—A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Sources and Search Strategy
2.4. Study Selection
2.5. Data Extraction and Charting
2.6. Synthesis of Results
3. Results
3.1. Selection of Sources
3.2. Characteristics of the Included Studies
3.3. Types of DMSCs
3.4. Therapeutic Properties of DMSCs Useful in the Treatment of AD
3.4.1. Neural Differentiation
3.4.2. Neuroprotection
Increasing the Neuron Number and Vitality
Mitochondrial Repair
Anti-Neuroinflammation and Neuroimmunomodulation
3.4.3. Positive Effect on Cognitive Function and Memory
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| AD | Alzheimer’s disease |
| MSC | Mesenchymal stem cells |
| DMSC | Mesenchymal stem cells of dental origin |
| DPSC | Dental pulp stem cells |
| SHED | Human-exfoliated deciduous teeth |
| PDLSC | Periodontal ligament stem cells |
| BMSC | Bone marrow stem cells |
| CNS | Central nervous system |
| ROS | Reactive oxygen species |
| NFT | Neurofibrillary Tangles |
| CM | Conditioned media |
| NGF | Nerve growth factor |
| bFGF | Basic fibroblastic growth factor |
| SHH | Sonic hedgehog |
| RA | Retinoic acid |
| D609 | Tricyclodecan-9-yl-xanthogenate |
| NeuN | Neuronal nuclei |
| BDNF | Brain-derived neurotrophic factor |
| GDNF | Glial cell-derived neurotropic factors |
| NF | Neurofilament |
References
- International, A.D.; Guerchet, M.; Prince, M.; Prina, M. Numbers of People with Dementia Worldwide: An Update to the Estimates in the World Alzheimer Report 2015; Alzheimer’s Disease International (ADI): London, UK, 2020. [Google Scholar]
- Singh, B.; Day, C.M.; Abdella, S.; Garg, S. Alzheimer’s Disease Current Therapies, Novel Drug Delivery Systems and Future Directions for Better Disease Management. J. Control. Release 2024, 367, 402–424. [Google Scholar] [CrossRef] [PubMed]
- Haddad, H.W.; Malone, G.W.; Comardelle, N.J.; Degueure, A.E.; Kaye, A.M.; Kaye, A.D. Aducanumab, a Novel Anti-Amyloid Monoclonal Antibody, for the Treatment of Alzheimer’s Disease: A Comprehensive Review. Health Psychol. Res. 2022, 10, 31925. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Lyu, L.; Zhan, S. Stem Cell Therapy for Alzheimer’s Disease: A Scoping Review for 2017–2022. Biomedicines 2023, 11, 120. [Google Scholar] [CrossRef]
- Song, C.-G.; Zhang, Y.-Z.; Wu, H.-N.; Cao, X.-L.; Guo, C.-J.; Li, Y.-Q.; Zheng, M.-H.; Han, H. Stem Cells: A Promising Candidate to Treat Neurological Disorders. Neural Regen. Res. 2018, 13, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, G. In Vivo Reprogramming for CNS Repair: Regenerating Neurons from Endogenous Glial Cells. Neuron 2016, 91, 728–738. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, J.-W.; Shi, H.-Y.; Ma, Y.-M. Neural Stem Cell Therapy for Brain Disease. World J. Stem Cells 2021, 13, 1278–1292. [Google Scholar] [CrossRef]
- Mulvey, R. Stem Cells Slow Cognitive Decline in Alzheimer’s Disease via Neurotrophin Action. Biosci. Horiz. Int. J. Stud. Res. 2014, 7, hzu013. [Google Scholar] [CrossRef][Green Version]
- Blurton-Jones, M.; Kitazawa, M.; Martinez-Coria, H.; Castello, N.A.; Müller, F.-J.; Loring, J.F.; Yamasaki, T.R.; Poon, W.W.; Green, K.N.; LaFerla, F.M. Neural Stem Cells Improve Cognition via BDNF in a Transgenic Model of Alzheimer Disease. Proc. Natl. Acad. Sci. USA 2009, 106, 13594–13599. [Google Scholar] [CrossRef]
- Chen, H.; Jacobs, E.; Schwarzschild, M.A.; McCullough, M.L.; Calle, E.E.; Thun, M.J.; Ascherio, A. Nonsteroidal Antiinflammatory Drug Use and the Risk for Parkinson’s Disease. Ann. Neurol. 2005, 58, 963–967. [Google Scholar] [CrossRef]
- Tuszynski, M.H.; Thal, L.; Pay, M.; Salmon, D.P.; U, H.S.; Bakay, R.; Patel, P.; Blesch, A.; Vahlsing, H.L.; Ho, G.; et al. A Phase 1 Clinical Trial of Nerve Growth Factor Gene Therapy for Alzheimer Disease. Nat. Med. 2005, 11, 551–555. [Google Scholar] [CrossRef]
- Rahbaran, M.; Zekiy, A.O.; Bahramali, M.; Jahangir, M.; Mardasi, M.; Sakhaei, D.; Thangavelu, L.; Shomali, N.; Zamani, M.; Mohammadi, A.; et al. Therapeutic Utility of Mesenchymal Stromal Cell (MSC)-Based Approaches in Chronic Neurodegeneration: A Glimpse into Underlying Mechanisms, Current Status, and Prospects. Cell. Mol. Biol. Lett. 2022, 27, 56. [Google Scholar] [CrossRef] [PubMed]
- Regmi, S.; Liu, D.D.; Shen, M.; Kevadiya, B.D.; Ganguly, A.; Primavera, R.; Chetty, S.; Yarani, R.; Thakor, A.S. Mesenchymal Stromal Cells for the Treatment of Alzheimer’s Disease: Strategies and Limitations. Front. Mol. Neurosci. 2022, 15, 1011225. [Google Scholar] [CrossRef] [PubMed]
- Pourhadi, M.; Zali, H.; Ghasemi, R.; Vafaei-Nezhad, S. Promising Role of Oral Cavity Mesenchymal Stem Cell-Derived Extracellular Vesicles in Neurodegenerative Diseases. Mol. Neurobiol. 2022, 59, 6125–6140. [Google Scholar] [CrossRef]
- Apel, C.; Forlenza, O.; Paula, V.; Talib, L.; Denecke, B.; Eduardo, C.; Gattaz, W. The Neuroprotective Effect of Dental Pulp Cells in Models of Alzheimer’s and Parkinson’s Disease. J. NEURAL Transm. 2009, 116, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-M.; Ouyang, Y.-J.; Yu, B.-Q.; Li, W.; Yu, M.-Y.; Li, J.-Y.; Jiao, Z.-M.; Yang, D.; Li, N.; Shi, Y.; et al. Therapeutic Potential of Dental Pulp Stem Cell Transplantation in a Rat Model of Alzheimer’s Disease. Neural Regen. Res. 2021, 16, 893–898. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, T.; Wang, D.; Cai, S.; Hang, Z.; Yang, Y.; Bi, W.; Xiao, Z.; Du, H. Stem Cells from Human Exfoliated Deciduous Teeth Relieves Alzheimer’s Disease Symptoms in SAMP8 Mice by up-Regulating the PPARγ Pathway. Biomed. Pharmacother. 2022, 152, 113169. [Google Scholar] [CrossRef]
- Guo, W.; Zeng, Z.; Xing, C.; Zhang, J.; Bi, W.; Yang, J.; Shah, R.; Wang, D.; Li, Y.; Zhang, X.; et al. Stem Cells from Human Exfoliated Deciduous Teeth Affect Mitochondria and Reverse Cognitive Decline in a Senescence-Accelerated Mouse Prone 8 Model. Cytotherapy 2022, 24, 59–71. [Google Scholar] [CrossRef]
- Mohebichamkhorami, F.; Niknam, Z.; Khoramjouy, M.; Heidarli, E.; Ghasemi, R.; Hosseinzadeh, S.; Mohseni, S.S.; Hajikarim-Hamedani, A.; Heidari, A.; Ghane, Y.; et al. Brain Homogenate of a Rat Model of Alzheimer Disease Modifies the Secretome of 3D Cultured Periodontal Ligament Stem Cells: A Potential Neuroregenerative Therapy. Iran. J. Pharm. Res. 2022, 21, e133668. [Google Scholar] [CrossRef]
- Xiong, W.; Liu, Y.; Zhou, H.; Jing, S.; He, Y.; Ye, Q. Alzheimer’s Disease: Pathophysiology and Dental Pulp Stem Cells Therapeutic Prospects. Front. Cell Dev. Biol. 2022, 10, 999024. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping Studies: Towards a Methodological Framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.J.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for Conducting Systematic Scoping Reviews. Int. J. Evid. Based Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Yalvaç, M.E.; Yarat, A.; Mercan, D.; Rizvanov, A.A.; Palotás, A.; Şahin, F. Characterization of the Secretome of Human Tooth Germ Stem Cells (hTGSCs) Reveals Neuro-Protection by Fine-Tuning Micro-Environment. Brain. Behav. Immun. 2013, 32, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Malekzadeh, S.; Edalatmanesh, M.A.; Mehrabani, D.; Shariati, M. Dental Pulp Stem Cells Transplantation Improves Passive Avoidance Memory and Neuroinflammation in Trimethyltin-Induced Alzheimer’s Disease Rat Model. Galen Med. J. 2021, 10, e2254. [Google Scholar] [CrossRef]
- Ahmed, N.E.-M.B.; Murakami, M.; Hirose, Y.; Nakashima, M. Therapeutic Potential of Dental Pulp Stem Cell Secretome for Alzheimer’s Disease Treatment: An In Vitro Study. Stem Cells Int. 2016, 2016, 8102478. [Google Scholar] [CrossRef]
- Wang, F.; Jia, Y.; Liu, J.; Zhai, J.; Cao, N.; Yue, W.; He, H.; Pei, X. Dental Pulp Stem Cells Promote Regeneration of Damaged Neuron Cells on the Cellular Model of Alzheimer’s Disease. Cell Biol. Int. 2017, 41, 639–650. [Google Scholar] [CrossRef]
- Kang, Y.-H.; Shivakumar, S.B.; Son, Y.-B.; Bharti, D.; Jang, S.-J.; Heo, K.-S.; Park, W.-U.; Byun, J.-H.; Park, B.-W.; Rho, G.-J. Comparative Analysis of Three Different Protocols for Cholinergic Neuron Differentiation in Vitro Using Mesenchymal Stem Cells from Human Dental Pulp. Anim. Cells Syst. 2019, 23, 275–287. [Google Scholar] [CrossRef]
- Goudarzi, G.; Hamidabadi, H.G.; Bojnordi, M.N.; Hedayatpour, A.; Niapour, A.; Zahiri, M.; Absalan, F.; Darabi, S. Role of Cerebrospinal Fluid in Differentiation of Human Dental Pulp Stem Cells into Neuron-like Cells. Anat. Cell Biol. 2020, 53, 292–300. [Google Scholar] [CrossRef]
- Mishra, M.; Raik, S.; Rattan, V.; Bhattacharyya, S. Mitochondria Transfer as a Potential Therapeutic Mechanism in Alzheimer’s Disease-like Pathology. Brain Res. 2023, 1819, 148544. [Google Scholar] [CrossRef]
- Howlader, M.S.I.; Prateeksha, P.; Hansda, S.; Naidu, P.; Das, M.; Barthels, D.; Das, H. Secretory Products of DPSC Mitigate Inflammatory Effects in Microglial Cells by Targeting MAPK Pathway. Biomed. Pharmacother. 2024, 170, 115971. [Google Scholar] [CrossRef]
- Mita, T.; Furukawa-Hibi, Y.; Takeuchi, H.; Hattori, H.; Yamada, K.; Hibi, H.; Ueda, M.; Yamamoto, A. Conditioned Medium from the Stem Cells of Human Dental Pulp Improves Cognitive Function in a Mouse Model of Alzheimer’s Disease. Behav. Brain Res. 2015, 293, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, C.; Shobha, K.; Rai, K.S.; Dhanushkodi, A. Neurogenic and Cognitive Enhancing Effects of Human Dental Pulp Stem Cells and Its Secretome in Animal Model of Hippocampal Neurodegeneration. Brain Res. Bull. 2022, 180, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Liu, Y.; Zhou, H.; Li, J.; Jing, S.; Jiang, C.; Li, M.; He, Y.; Ye, Q. Human Dental Pulp Stem Cells Mitigate the Neuropathology and Cognitive Decline via AKT-GSK3β-Nrf2 Pathways in Alzheimer’s Disease. Int. J. Oral Sci. 2024, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; She, W.; Liu, Y.; Zhou, H.; Wang, X.; Li, F.; Li, R.; Wang, J.; Qin, D.; Jing, S.; et al. Clinical-Grade Human Dental Pulp Stem Cells Improve Adult Hippocampal Neural Regeneration and Cognitive Deficits in Alzheimer’s Disease. Theranostics 2025, 15, 894–914. [Google Scholar] [CrossRef]
- Man, R.C.; Sulaiman, N.; Idrus, R.B.H.; Ariffin, S.H.Z.; Wahab, R.M.A.; Yazid, M.D. Insights into the Effects of the Dental Stem Cell Secretome on Nerve Regeneration: Towards Cell-Free Treatment. Stem Cells Int. 2019, 2019, 4596150. [Google Scholar] [CrossRef]
- Bar, J.K.; Lis-Nawara, A.; Grelewski, P.G. Dental Pulp Stem Cell-Derived Secretome and Its Regenerative Potential. Int. J. Mol. Sci. 2021, 22, 2018. [Google Scholar] [CrossRef]
- Dong, Z.; Wu, L.; Zhao, L. A Concise Review of the Orofacial Mesenchymal Stromal Cells as a Novel Therapy for Neurological Diseases and Injuries. J. Tissue Eng. Regen. Med. 2022, 16, 775–787. [Google Scholar] [CrossRef]
- Mohebichamkhorami, F.; Fattahi, R.; Niknam, Z.; Aliashrafi, M.; Khakpour Naeimi, S.; Gilanchi, S.; Zali, H. Periodontal Ligament Stem Cells as a Promising Therapeutic Target for Neural Damage. Stem Cell Res. Ther. 2022, 13, 273. [Google Scholar] [CrossRef]
- Stefańska, K.; Volponi, A.A.; Kulus, M.; Waśko, J.; Farzaneh, M.; Grzelak, J.; Azizidoost, S.; Mozdziak, P.; Bukowska, D.; Antosik, P.; et al. Dental Pulp Stem Cells—A Basic Research and Future Application in Regenerative Medicine. Biomed. Pharmacother. 2024, 178, 116990. [Google Scholar] [CrossRef]
- Yoshida, S.; Tomokiyo, A.; Hasegawa, D.; Hamano, S.; Sugii, H.; Maeda, H. Insight into the Role of Dental Pulp Stem Cells in Regenerative Therapy. Biology 2020, 9, 160. [Google Scholar] [CrossRef]
- Akbar, M.; Essa, M.M.; Daradkeh, G.; Abdelmegeed, M.A.; Choi, Y.; Mahmood, L.; Song, B.-J. Mitochondrial Dysfunction and Cell Death in Neurodegenerative Diseases through Nitroxidative Stress. Brain Res. 2016, 1637, 34–55. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Li, K.; Li, B.; Gao, L.-N.; Chen, F.-M.; Jin, Y. Mesenchymal Stem Cell Characteristics of Dental Pulp and Periodontal Ligament Stem Cells after in Vivo Transplantation. Biomaterials 2014, 35, 6332–6343. [Google Scholar] [CrossRef] [PubMed]
- Alothman, F.A.; Hakami, L.S.; Alnasser, A.; AlGhamdi, F.M.; Alamri, A.A.; Almutairii, B.M.; Alothman, F.A.; Hakami, L.S.; Alnasser, A.; AlGhamdi, F.; et al. Recent Advances in Regenerative Endodontics: A Review of Current Techniques and Future Directions. Cureus 2024, 16, e74121. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, F.; Sun, Y.; Jiang, B.; Zhang, W.; Yang, J.; Xu, G.-T.; Liang, A.; Liu, S. Concise Reviews: Characteristics and Potential Applications of Human Dental Tissue-Derived Mesenchymal Stem Cells. Stem Cells 2015, 33, 627–638. [Google Scholar] [CrossRef]
- Sugiaman, V.K.; Djuanda, R.; Pranata, N.; Naliani, S.; Demolsky, W.L.; Jeffrey. Tissue Engineering with Stem Cell from Human Exfoliated Deciduous Teeth (SHED) and Collagen Matrix, Regulated by Growth Factor in Regenerating the Dental Pulp. Polymers 2022, 14, 3712. [Google Scholar] [CrossRef]
- Mattei, V.; Delle Monache, S. Dental Pulp Stem Cells (DPSCs) and Tissue Regeneration: Mechanisms Mediated by Direct, Paracrine, or Autocrine Effects. Biomedicines 2023, 11, 386. [Google Scholar] [CrossRef]
- Xian, X.; Gong, Q.; Li, C.; Guo, B.; Jiang, H. Exosomes with Highly Angiogenic Potential for Possible Use in Pulp Regeneration. J. Endod. 2018, 44, 751–758. [Google Scholar] [CrossRef]
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L.; et al. Deciduous Autologous Tooth Stem Cells Regenerate Dental Pulp after Implantation into Injured Teeth. Sci. Transl. Med. 2018, 10, eaaf3227. [Google Scholar] [CrossRef]
- Tomokiyo, A.; Wada, N.; Maeda, H. Periodontal Ligament Stem Cells: Regenerative Potency in Periodontium. Stem Cells Dev. 2019, 28, 974–985. [Google Scholar] [CrossRef]
- Iwasaki, K.; Komaki, M.; Yokoyama, N.; Tanaka, Y.; Taki, A.; Honda, I.; Kimura, Y.; Takeda, M.; Akazawa, K.; Oda, S.; et al. Periodontal Regeneration Using Periodontal Ligament Stem Cell-Transferred Amnion. Tissue Eng. Part A 2014, 20, 693–704. [Google Scholar] [CrossRef]
- Sanz, A.R.; Carrión, F.S.; Chaparro, A.P. Mesenchymal Stem Cells from the Oral Cavity and Their Potential Value in Tissue Engineering. Periodontol. 2000 2015, 67, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Wang, K.; Zhang, L.; Bai, L. Stem Cell Therapy for Alzheimer’s Disease: An Overview of Experimental Models and Reality. Anim. Models Exp. Med. 2022, 5, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Thorne, R.G.; Pronk, G.J.; Padmanabhan, V.; Frey, W.H. Delivery of Insulin-like Growth Factor-I to the Rat Brain and Spinal Cord along Olfactory and Trigeminal Pathways Following Intranasal Administration. Neuroscience 2004, 127, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.-H.; Ji, W.-L.; Chen, H.; Sun, Y.-Y.; Zhao, X.-Y.; Wang, F.; Shi, Y.; Hu, Y.-N.; Liu, B.-X.; Wu, J.; et al. Intranasal Transplantation of Human Neural Stem Cells Ameliorates Alzheimer’s Disease-Like Pathology in a Mouse Model. Front. Aging Neurosci. 2021, 13, 650103. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Jang, J.-H.; Lee, Y.-B. Drug Delivery to the Brain via the Nasal Route of Administration: Exploration of Key Targets and Major Consideration Factors. J. Pharm. Investig. 2023, 53, 119–152. [Google Scholar] [CrossRef]
- Mastrolia, I.; Foppiani, E.M.; Murgia, A.; Candini, O.; Samarelli, A.V.; Grisendi, G.; Veronesi, E.; Horwitz, E.M.; Dominici, M. Challenges in Clinical Development of Mesenchymal Stromal/Stem Cells: Concise Review. Stem Cells Transl. Med. 2019, 8, 1135–1148. [Google Scholar] [CrossRef]
- Chan, H.J.; Yanshree; Roy, J.; Tipoe, G.L.; Fung, M.-L.; Lim, L.W. Therapeutic Potential of Human Stem Cell Implantation in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 10151. [Google Scholar] [CrossRef]
- Lalu, M.M.; Montroy, J.; Begley, C.G.; Bubela, T.; Hunniford, V.; Ripsman, D.; Wesch, N.; Kimmelman, J.; Macleod, M.; Moher, D.; et al. Identifying and Understanding Factors That Affect the Translation of Therapies from the Laboratory to Patients: A Study Protocol. F1000Research 2020, 9, 485. [Google Scholar] [CrossRef]
- Polis, B.; Samson, A.O. Addressing the Discrepancies Between Animal Models and Human Alzheimer’s Disease Pathology: Implications for Translational Research. J. Alzheimers Dis. JAD 2024, 98, 1199–1218. [Google Scholar] [CrossRef]


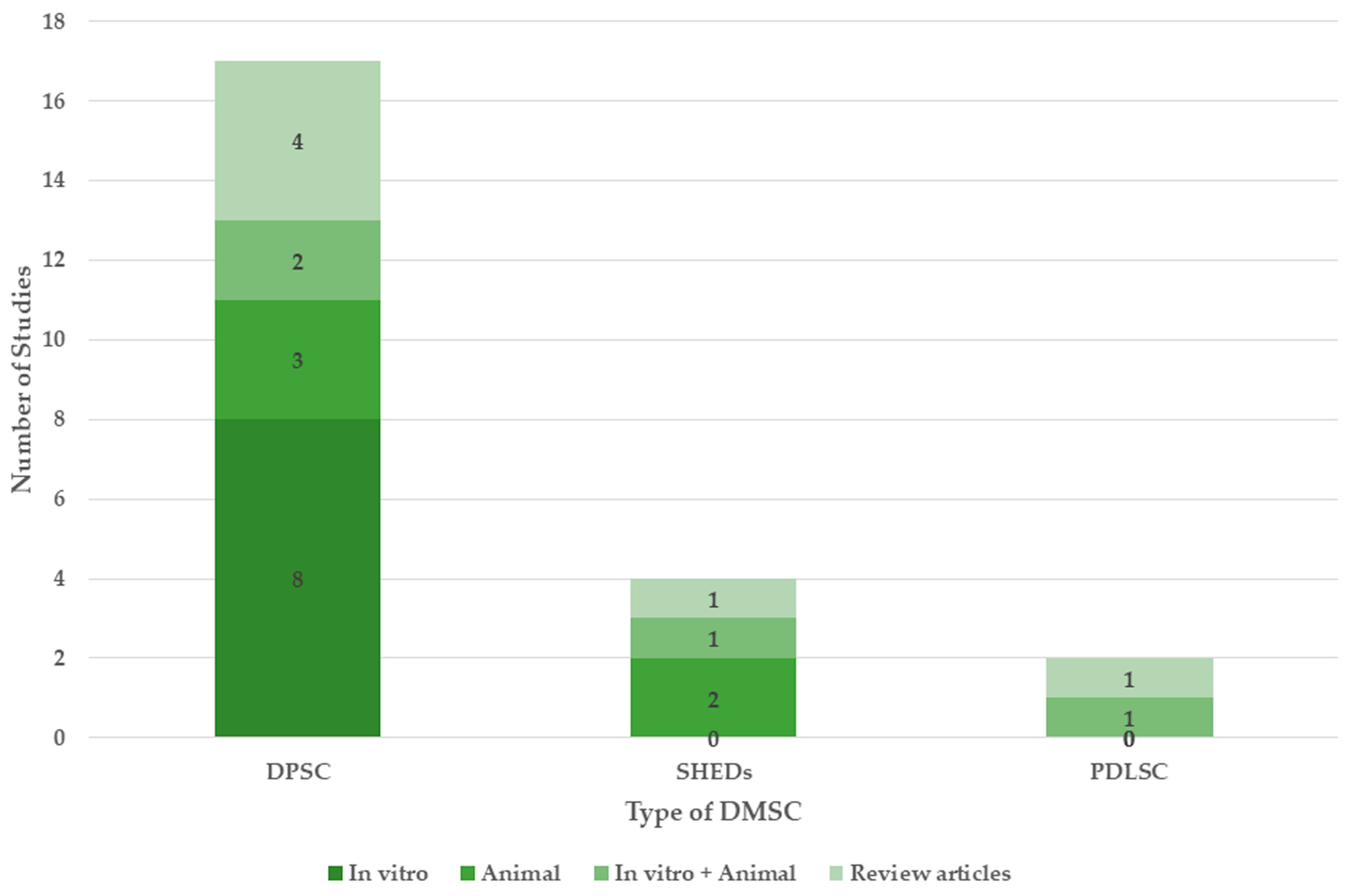


| Author | Study Type | Type of DMSC | Component of DMSC Applied and Dosage | Therapeutic Property of DMSC Applied | Summary and Conclusion |
|---|---|---|---|---|---|
| Apel C. et al., 2009 [15] | In vitro | DPSC | Cells 5 × 104 cells per insert | Neuroprotection: Increased neuron number and vitality | DPSCs mitigated the impact of neurotoxins, exhibited a neuronal phenotype, generated neurotrophic effects, and safeguarded primary neurons in in vitro models of Alzheimer’s disease and Parkinson’s disease. |
| Yalvac M.E. et al., 2013 [24] | In vitro | DPSC | Secretome 1:5 ratio of Secretome SH-SY5Y cells | Neuroprotection
| DPSCs increased antioxidant enzyme activity and reduced neuronal apoptosis. |
| Mita T. et al., 2015 [32] | Animal model | SHED | Conditioned medium 50 μL | Neuroprotection
| Intranasal administration of SHEDs substantially improved cognitive function and their conditioned medium produced a tissue-regenerating environment. |
| Ahmed N.E. -M.B. et al., 2016 [26] | In vitro | DPSC | Secretome 5 μg/mL | Neuroprotection
| The DPSC secretome had the highest concentrations of growth factors, decreased the apoptotic regulator Bax, enhanced neuronal cell viability, raised the endogenous survival factor Bcl-2, and substantially lowered the cytotoxicity of Aβ peptide. Within 12 h, the neprilysin enzyme in DPSC secretome degraded Aβ1-42. |
| Wang F. et al., 2017 [27] | In vitro | DPSC | Cells 6 × 104 cells | Neuroprotection
| DPSCs facilitated neuroregeneration, as seen by elongated dendrites, densely organized microfilaments, thickened microtubular fibrils, enhanced cell survival, reduced apoptosis, and tau phosphorylation. |
| Kang Y.H. et al., 2019 [28] | In vitro | DPSC | Cells | Neuro differentiation | DPSCs effectively trans-differentiated across all treatments and displayed neuron-like morphologies with elevated cholinergic neuron-specific markers. |
| Man R.C. et al., 2019 [36] | Review | DPSC SHED | Secretome | Neuroprotection
| Alpha 2 macroglobulin derived from DSCs secretome bound the β-amyloid plaque and promoted its clearance while fractalkine promoted phagocytic functions. Siglec-9 and MCP-1 promoted nerve regeneration by converting M1 to M2 phenotype. |
| Goudarzi G. et al., 2020 [29] | In vitro | DPSC | Neurospheres | Neuro differentiation | Human hDPSCs treated with 5% embryonic cerebrospinal fluid and cultured in media containing DMEM, retinoic acid, glial-derived neurotrophic factor and brain-derived neurotrophic factor showed neuron like features. |
| Zhang X.M. et al., 2021 [16] | Animal Model | DPSC | Cells 5 × 106 cells | Neuroprotection
| DPSCs enhanced cognitive and behavioral functions by upregulating the expression of neuron-related doublecortin, NeuN, and neurofilament 200 while downregulating amyloid-β. |
| Bar J.K. et al., 2021 [37] | Review | DPSC | Secretome | Neuroprotective Angiogenesis Neuronal growth | DPSC secretome through paracrine mechanism enhances neuronal survival and reduces apoptosis through RANTES, FGF2, and Fractalkine, supports neuronal growth through BDNF and NGF and angiogenesis through VEGF. |
| Malekzadeh S. et al., 2022 [25] | Animal Model | DPSC | Cells 1 × 106 cells/mL | Neuroprotection
| Transplanting DPSCs enhanced learning and memory while lowering the percentage of injured pyramidal neurons and the NF-Kβ serum level. |
| Venugopal C. et al., 2022 [33] | Animal Model | DPSC | Cells/ Secretome 4 μL of cells or Secretome | Neuroprotection
| In addition to preventing neurodegeneration and neuroinflammation, DPSCs also improved neurogenesis, spatial learning, and memory, decreased pro-apoptotic factors, increased anti-apoptotic factors, and raised the expression of endogenous neural survival factors. Results were better with DPSCs and their secretome than with bone marrow-derived stem cells and their secretome. |
| Zhang X. et al., 2022 [17] | Animal Model | SHED | Cells 5 × 105 cells | Neuroprotection
| Transplanted SHED entered the brain and improved the glucose metabolism in AD mice by upregulating the PPARγ signaling pathway |
| Guo W et al., 2022 [18] | In vitro Animal Model | SHED | Cells 2 × 106 cells | Neuroprotection
| Treatment with SHED reduced AD symptoms, enhanced cognitive performance, and restored memory loss in SAMP8 mice, potentially by restoring damaged mitochondria through the mitochondrial pathway, Hook3, Mic13, and MIF. |
| Mohebichamkhorami F. et al., 2022 [19] | In vitro Animal Model | PDLSC | Secretome Spheroids 20 mg/mL |
Neuroprotection
| Modified secretome of 3D cultured spheroids of PDLSCs treated with BH-AD was a reservoir of regenerating neural factors useful in AD treatment. |
| Dong Z. et al., 2022 [38] | Review | Oral MSC (DPSC) | - |
Neural differentiation Neuroprotection
| Reduced symptoms of AD and improved cognitive function. |
| Mohebichamkhorami F. et al., 2022 [39] | Review | PDLSC | - | Neural differentiation | After a phase of dedifferentiation, PDLSCs undergo a differentiation process without cell division to become neural-like cells. |
| Xiong W. et al., 2022 [20] | Review | DPSC | - | Neural differentiation Neuroprotection
| DPSCs are an important source of stem cells for the regeneration of neurons or protection of existing neurons in the neurodegenerative diseases like AD |
| Mishra M. et al., 2023 [30] | In vitro | DPSC | Mitochondria 40 g/mL | Neuroprotection
| Internalization of DPSC-derived mitochondria produced significant neuroprotection in the cellular AD. |
| Howlader M.S.I. et al., 2024 [31] | In vitro | DPSC | Secretome 2 mL | Neuroprotection
| DPSC secretome decreased inflammatory markers, induced anti-inflammatory molecules in microglial cells, and decreased mitochondrial membrane potential in microglial cells. Subsequently, microglial cell proliferation was inhibited, the MAPK P38 pathway and downstream signaling of inflammation were inhibited, intracellular ROS and their production from mitochondria were decreased. |
| Xiong W. et al., 2024 [34] | In vitro Animal model | DPSC | Cells | Neuroprotection
| In in vitro AD models, human DPSCs regulated the polarization of hyperactive microglia cells, decreased oxidative stress, and encouraged neuronal repair. Nrf2 nuclear accumulation and the production of downstream antioxidant enzymes via the AKT-GSK3β-Nrf2 signaling pathway were the mechanisms underlying these effects. |
| Xiong W. et al., 2025 [35] |
In vitro Animal model | DPSC | Cells |
Neuronal differentiation Improved Cognition and memory | Human DPSCs activated the Wnt/β-catenin pathway which stabilized the hippocampal neural network and reversed memory deficits and promoted neural regeneration. |
| DPSCs | SHEDs | |
|---|---|---|
| Origin | Adult human dental pulp of impacted third molars, orthodontic teeth and supernumerary teeth | Exfoliated human deciduous teeth |
| Characteristics | Increased clonogenicity, proliferation and ability to form mineralized nodules | Increased proliferation |
| Differentiation potential | Potential for multilineage differentiation; ability to differentiate into neural cells, endothelial cells, myocytes, hepatocytes, adipocytes, chondrocytes, osteoblasts, odontoblasts, and pancreatic cells. Additional differentiation into cardiomyocytes and corneal epithelial cells | Differentiate into osteoblasts, odontoblasts, adipocytes, chondrocytes, neural cells, endothelial cells, myocytes, hepatocytes, and pancreatic cells |
| Proliferation rate | Lower proliferation rate than SHEDs | Exhibits a superior proliferation rate compared to DPSCs and BMSCs, attributed to enhanced expression of genes associated with cell proliferation, such as fibroblast growth factor-2 and transforming growth factor-β, and elevated expression of stemness markers. |
| Stem cell Markers | MSC markers: CD29, CD44, CD90, CD166, STRO-1, and CD146 | MSC markers: CD73, CD13, CD90, CD105, CD166, and STRO-1 Embryonic stemness markers: OCT-4 SOX-2 and NANOG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agnihotri, R.; Gaur, S. Therapeutic Applications of Dental Mesenchymal Stem Cells in Alzheimer’s Disease—A Scoping Review. Dent. J. 2025, 13, 288. https://doi.org/10.3390/dj13070288
Agnihotri R, Gaur S. Therapeutic Applications of Dental Mesenchymal Stem Cells in Alzheimer’s Disease—A Scoping Review. Dentistry Journal. 2025; 13(7):288. https://doi.org/10.3390/dj13070288
Chicago/Turabian StyleAgnihotri, Rupali, and Sumit Gaur. 2025. "Therapeutic Applications of Dental Mesenchymal Stem Cells in Alzheimer’s Disease—A Scoping Review" Dentistry Journal 13, no. 7: 288. https://doi.org/10.3390/dj13070288
APA StyleAgnihotri, R., & Gaur, S. (2025). Therapeutic Applications of Dental Mesenchymal Stem Cells in Alzheimer’s Disease—A Scoping Review. Dentistry Journal, 13(7), 288. https://doi.org/10.3390/dj13070288






