Abstract
Background/Objectives: Orthognathic surgery has evolved with digital advancements, improving precision and predictability. Traditional free-hand techniques rely on surgeon expertise, often leading to variable outcomes. Fully guided approaches integrate computer-assisted surgery, including virtual surgical planning (VSP), CAD/CAM, and dynamic navigation, enhancing accuracy and efficiency. This review compares these approaches, assessing their impact on surgical accuracy, efficiency, and patient outcomes. Methods: A scoping review was conducted across PubMed, MEDLINE, Scopus, Cochrane Library, and Embase databases, focusing on clinical trials and cohort studies. Key parameters analyzed include surgical precision, operative efficiency, complication rates, and functional/aesthetic results. Results: Fully guided techniques achieve sub-millimetric accuracy with mean length deviations ranging from 1.3 mm to 2.4 mm and mean angular deviations between 2.29° and 3.51°. Moreover, these approaches markedly reduce operative time, averaging between 34 min and 1.7 h, and postoperative complications. Digital tools streamline workflow, improving reproducibility and aesthetic outcomes. Free-hand methods remain cost-effective but require greater surgical expertise, often resulting in longer recovery periods and higher variability. Conclusions: Computer-assisted orthognathic surgery enhances precision and efficiency, outperforming free-hand techniques in accuracy and predictability. While free-hand methods remain viable for simpler cases, fully guided approaches optimize surgical execution. Future research should explore hybrid strategies combining digital precision with manual adaptability to further refine surgical techniques.
1. Introduction
Orthognathic surgery has undergone a transformative evolution with the integration of digital technologies, significantly enhancing precision, predictability, and patient outcomes. Orthognathic procedures have been historically characterized by the use of free-hand surgical techniques that rely on the surgeon’s expertise, manual adjustments, and conventional occlusal wafers to achieve skeletal repositioning. However, these methodologies are inherently constrained by the variability of both intra- and inter-operators, which may result in postoperative discrepancies and prolonged hospital stays [1,2].
The invention of computer-assisted surgery has revolutionized the field even further by, incorporating artificial intelligence (AI), three-dimensional imaging, dynamic navigation, augmented reality, and real-time imaging to optimize surgical accuracy. These technologies facilitate comprehensive preoperative planning, allowing surgeons to analyze anatomical variations and mitigate risks associated with tissue damage. Virtual surgical planning (VSP), combined with computer-aided design/computer-aided manufacturing (CAD/CAM), enables the creation of patient-specific surgical guides and pre-bent osteosynthesis plates, streamlining intraoperative execution and improving functional and aesthetic outcomes [3,4,5].
Recent studies have demonstrated that intraoperative navigation significantly enhances surgical precision, particularly in vertical positioning, which remains a challenge with traditional techniques. Navigation systems consistently achieve accuracy within 2 mm, offering superior control in the cranial-caudal dimension compared to conventional occlusal wafers [6]. Additionally, augmented reality-assisted free-hand orthognathic surgery has emerged as a promising approach, utilizing electromagnetic tracking and skin-attached dynamic reference tools to improve intraoperative visualization and accuracy [2].
Regardless of the advantages provided via computer-assisted surgery, free-hand techniques remain relevant, particularly for simpler maxillofacial cases. While free-hand procedures are more cost-effective, they often result in longer hospital stays and less predictable postoperative outcomes. The hierarchy of surgical stability in orthognathic surgery suggests that procedures involving maxillary expansion and mandibular rotation present higher instability risks, reinforcing the need for advanced digital planning [7].
Given these innovative capabilities, the present scoping review aims to systematically explore and critically appraise the application of fully guided techniques compared to conventional free-hand methods in orthognathic surgery, evaluating their respective advantages, limitations, and clinical implications. By synthesizing current research on digital surgical planning, intraoperative navigation, and custom guide fabrication, this review seeks to highlight the transformative impact of digital technologies on surgical precision and patient outcomes.
2. Materials and Methods
A scoping review approach was adopted for this manuscript in lieu of a formal systematic review due to the heterogeneous nature of study designs, planning methodologies, execution strategies, and outcome measures encountered in the literature addressing fully guided versus free-hand techniques in orthognathic surgery. This methodological choice was made to comprehensively map the current body of research, capturing the variability in digital (fully guided) and conventional free-hand approaches without forcing disparate data into a single quantitative synthesis. By using a scoping review framework, the study aimed to delineate critical gaps in the literature regarding the comparative effectiveness, reproducibility, operative efficiency, and clinical outcomes between these two surgical methods. Furthermore, this approach facilitates a foundational understanding of implementation strategies across diverse clinical contexts and elucidates the practical challenges associated with integrating advanced digital technologies into routine orthognathic surgical practice. Ultimately, the findings are intended to inform future systematic reviews and guide translational research efforts in the evolving field of craniofacial surgery. A detailed protocol for this scoping review was systematically developed, and all results were documented following the PRISMA for Scoping Reviews (PRISMA-ScR) checklist adapted for scoping reviews, as detailed in Supplementary Table S1 [8].
2.1. Identifying Research Questions
To clarify the research query, a PICO (population, intervention, comparator, outcome) framework was employed, as detailed in Table 1. Accordingly, the following review question was developed:

Table 1.
Population, intervention, comparator, outcome table (PICO).
“Among adult (≥18 years) patients undergoing orthognathic surgery, what is the evidence regarding the efficacy of fully guided (digital, computer-assisted) surgical approaches, incorporating virtual surgical planning, CAD/CAM, and 3D imaging, in improving surgical accuracy, operative efficiency, functional and aesthetic outcomes, and reducing complication rates, compared with conventional free-hand techniques, over the past twenty years?”.
2.2. Identifying Relevant Studies
A comprehensive search was conducted on 1 May 2025 across multiple biomedical databases, including PubMed, MEDLINE, Scopus, Cochrane Library and Embase, using the following query strings: ((“orthognathic surgical procedures”[MeSH Terms] OR (“orthognathic”[All Fields] AND “surgical”[All Fields] AND “procedures”[All Fields]) OR “orthognathic surgical procedures”[All Fields] OR (“orthognathic”[All Fields] AND “surgery”[All Fields]) OR “orthognathic surgery”[All Fields] OR “orthognathic surgery”[MeSH Terms] OR (“orthognathic surgical procedures”[MeSH Terms] OR (“orthognathic”[All Fields] AND “surgical”[All Fields] AND “procedures”[All Fields]) OR “orthognathic surgical procedures”[All Fields] OR (“jaw”[All Fields] AND “surgery”[All Fields]) OR “jaw surgery”[All Fields])) AND ((“free”[All Fields] AND (“hand”[MeSH Terms] OR “hand”[All Fields])) OR (“full”[All Fields] AND (“guide”[All Fields] OR “guided”[All Fields] OR “guides”[All Fields] OR “guiding”[All Fields])))) for PubMed, MEDLINE, Cochrane, and Embase and (TITLE-ABS-KEY (orthognathic AND surgery) OR TITLE-ABS-KEY (jaw AND surgery)) AND (TITLE-ABS-KEY (free AND hand) OR TITLE-ABS-KEY (full AND guided)) for Scopus. References were reviewed for identification of any additional relevant articles.
2.3. Study Selection—Eligibility and Screening
The review was restricted to full-text research studies published in peer-reviewed journals in the English language. Eligible studies had to meet the following criteria: the study must include adult (≥18 years) patients undergoing orthognathic surgery; the study design must be a clinical trial, prospective or retrospective cohort study, or case report; the study must primarily focus on comparing the effectiveness of fully guided (digital, computer-assisted) approaches versus conventional free-hand techniques in orthognathic surgery, assessing outcomes such as surgical accuracy, operative efficiency, functional and aesthetic results, and complication rates; and the study must be published from 2001 until 2025. Duplicate records, review articles, systematic reviews and meta-analyses, protocols and guidelines, animal studies, conference abstracts and presentations, preprints, clinical trials under patient recruitment or without published results, ongoing clinical trials, and studies deemed irrelevant were excluded.
After excluding articles according to the above criteria using automated tools and researcher screening, the final set of articles was retrieved. To ensure accuracy and objectivity, two independent reviewers (I.K. and S.T.) initially screened titles and abstracts in a double-blinded process. For studies that passed this initial screening, full texts were obtained and further evaluated to determine final eligibility. Any discrepancies during the screening process were resolved by a third reviewer (T.P.).
2.4. Data Charting
Key variables were systematically extracted from all included studies by the primary researchers (I.K. and S.T.). The extracted variables included the following: first author, publication year, study design, sample size, and patient demographic characteristics (mean age). In addition, specific details pertaining to the surgical intervention were charted, such as the type of orthognathic procedure performed (e.g., mandibular, maxillary, or bimaxillary surgery), the intervention type (fully guided digital approach employing virtual surgical planning [VSP], CAD/CAM, and 3D imaging versus conventional free-hand techniques), and details of the intraoperative and preoperative planning protocols used. Outcome measures were also recorded, including metrics of surgical accuracy (e.g., linear and angular deviations, occlusion alignment, and condyle positioning), operative parameters (total operative time, ischemia time, and preoperative planning time), complication rates, and functional and aesthetic outcomes (including patient-reported satisfaction where available). Finally, the key conclusions of each study were documented to capture insights regarding the comparative effectiveness of fully guided versus free-hand approaches.
2.5. Collating, Summarizing, and Reporting Results
Extracted data were synthesized into results tables to facilitate a descriptive analysis of the key findings. Given the scoping nature of this review, a meta-analysis was not performed. Instead, a qualitative synthesis of the principal outcomes was conducted to explore and evaluate the comparative effectiveness of fully guided versus conventional free-hand orthognathic surgery techniques. This synthesis focused on delineating differences in surgical accuracy, operative efficiency, functional and aesthetic outcomes, and complication profiles, thereby mapping the current state of evidence and identifying gaps to guide future research in this evolving field.
3. Results
The PRISMA flow diagram (Figure 1) outlines the review selection and exclusion process. Initially, a total of 427 records were retrieved from the aforementioned databases (PubMed and MEDLINE, n = 208; Scopus, n = 183; Cochrane and Embase, n = 36). Automated screening excluded 319 records, leaving 108 records for further consideration. Of these, 24 duplicate records were removed manually. Subsequently, 73 studies were excluded based on ineligible study design, as determined through title and abstract screening. After a full-text review of the remaining 11 articles, all the studies met the inclusion criteria and, thus, were included in the review. From the review of references for identification of any additional relevant articles, 14 additional studies were included. Thus, a total of 25 studies were included in this scoping review.
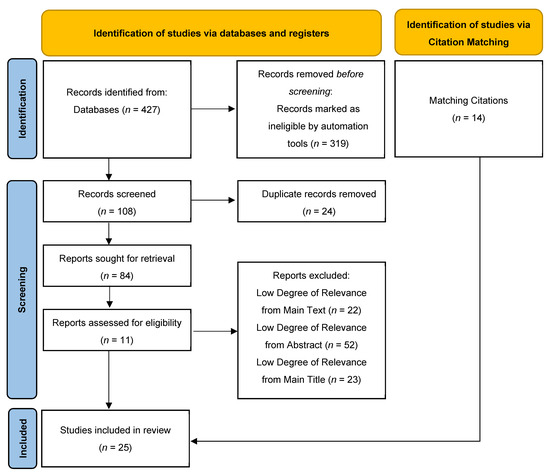
Figure 1.
PRISMA flow diagram.
Included studies were published between 2013 and 2022 (Table 2). Across multiple studies, fully guided surgery demonstrated superior accuracy, with intraoperative navigation achieving length and angular deviations between 1.34 mm and 2.4 mm and 2.29° and 3.51°, respectively, particularly in vertical positioning. Digital planning methods such as virtual surgical planning (VSP) and computer-aided design/computer-aided manufacturing (CAD/CAM) allowed for precise skeletal realignment, reducing intraoperative adjustments and improving postoperative symmetry.

Table 2.
Table of results from the included studies.
When it comes to operative efficiency, computer-assisted approaches significantly reduced surgical duration compared to free-hand methods, ranging from 34 min to 1.7 h. The use of pre-bent osteosynthesis plates and patient-specific guides streamlined workflows, with studies reporting a reduction in total operative time. Additionally, improved preoperative simulations facilitated faster execution and reduced intraoperative revisions.
Postoperative outcomes indicate that patients undergoing fully guided surgery experienced shorter hospital stays and fewer complications. The enhanced precision of digital planning resulted in higher patient satisfaction scores, with improved functional and aesthetic outcomes compared to free-hand techniques.
The comparative effectiveness of both approaches is outlined in Table 2, providing a detailed analysis of surgical accuracy, efficiency, postoperative results, and complication rates across various studies.
4. Discussion
Orthognathic surgery demands precise skeletal repositioning to optimize both functional outcomes and facial aesthetics. A considerable body of literature has addressed the comparative merits of fully guided, computer-assisted approaches and conventional free-hand techniques. The former utilizes VSP, three-dimensional (3D) modeling, CAD/CAM, and rapid prototyping to generate patient-specific cutting guides, pre-bent osteosynthesis plates, and splints, thereby creating a workflow that facilitates accurate preoperative simulation, replicable intraoperative execution, and improved predictability of outcomes. Conversely, traditional free-hand methods rely on conventional cephalometric analyses, stone model surgery, and manual fabrication processes that are inherently dependent on the surgeon’s experience and can be prone to inter- and intra-operator variability.
Fully guided approaches have revolutionized preoperative planning by incorporating three-dimensional imaging, virtual surgical planning (VSP), CAD/CAM, and rapid prototyping to generate patient-specific guides, cutting templates, and splints. The digital methods enable surgeons to simulate skeletal movements with submillimetric precision, effectively “translating” a meticulously designed virtual plan into the operating suite [9,13,30]. On the contrary, conventional free-hand techniques rely on two-dimensional cephalometric tracings, stone model surgery, and manual splint fabrication—a process that inherently involves multiple laboratory steps [23,24]. Although such traditional methods can achieve acceptable outcomes when performed by experienced surgeons, they are more susceptible to errors due to human handling and the limitations of 2D data when representing complex 3D anatomy [12,25].
Studies consistently report that the fully guided approach yields superior accuracy and reproducibility. For instance, Zhang L et al., 2016, found that digitally planned osteotomies produced mean linear deviations of approximately 1.34 mm and angular deviations near 2.29°, results that are less variable than those obtained with free-hand surgery [14]. Similarly, multiple articles [11,15,20] indicate that guided methods offer improved replication of the preoperative design, with enhanced control over fibular segment positioning and mandibular arc restoration. Even though De Maesschalck T et al., 2017, acknowledged that highly experienced free-hand surgeons can achieve comparable outcomes, the digital approach reduces inter-operator variability and provides standardized, measurable endpoints, thus fostering consistency across a wider range of clinical scenarios [15].
A prominent advantage of fully guided techniques is the reduction in operative time and associated ischemia. The utilization of patient-specific guides and prebent plates has been documented in numerous reports [9,10,17,18] to show a significant reduction in surgical durations. The guided approach eliminates time-consuming intraoperative adjustments, such as manual plate bending and repetitive trial fragment positioning, which streamlines the operating room workflow. This results in measurable reductions in both overall surgery time and flap ischemia periods. Furthermore, research conducted during the preoperative phase [24,27,29] has demonstrated that digital planning significantly reduces laboratory work, thereby reducing the overall planning time and alleviating the stress on resident training schedules.
The improved aesthetic and functional outcomes are a clear indication of the precision that computer-assisted planning provides. It is well-established that fully guided techniques result in superior mandibular symmetry, improved occlusal relationships, and improved condyle positioning, all of which contribute to satisfactory masticatory function and facial aesthetics [13,21,31,32]. The guided method is frequently preferred by objective measures such as soft tissue prediction and landmark accuracy [22,25] in contexts where subjective aesthetic preferences differ, even though certain free-hand cases exhibit high patient satisfaction [21]. These enhancements are indispensable in complex orthognathic cases, as even minor discrepancies can lead to long-term functional impairment or facial asymmetry.
Although fully guided techniques require a higher upfront investment, due to the cost of specialized equipment, software, and custom fabrication [16,26,30] several studies argue that these initial expenses are offset by subsequent savings. Particularly in high-volume centers, an overall cost-effective profile is facilitated by reduced operative time, shorter ischemia durations, and a decrease in corrective revision surgeries [17,26]. The economic analysis emphasizes that digital planning can result in substantial annual savings and improved departmental efficiency when indirect costs (such as operating room time and surgeon labor) are considered.
A further consideration is the impact on surgical training and the flexibility of the technique. Fully guided approaches can shorten the learning curve for less experienced surgeons by providing a clear, reproducible digital plan [11,27]. However, concerns have been raised regarding a potential overreliance on digital workflows at the expense of traditional tactile skills—a particularly important factor when considering unusual or unanticipated intraoperative scenarios [12,18]. In contrast, the free-hand approach offers greater adaptability during surgery, enabling real-time adjustments in response to intraoperative findings, although this flexibility may come at the cost of increased variability and prolonged operative duration [9,19].
In maxillary repositioning and bimaxillary surgeries, both digital and conventional techniques produce clinically acceptable outcomes. For instance, Kwon TG et al., 2014, and Ritto FG et al., 2018 [23,28] demonstrated that the error margins for maxillary repositioning using digital methods are within 1–2 mm, with some studies reporting better alignment and occlusion. Additionally, Schwartz HC, 2014, and Van Hemelen G et al., 2015 [24,25] further support that the 3D guided approach enhances soft tissue prediction and overall symmetry, factors that are critical in achieving optimal facial balance in bimaxillary orthognathic surgeries.
Prospectively, the integration of emerging digital technologies, such as direct digital intraoral scanning and in-house desktop 3D printing, promises to further streamline the fully guided approach, potentially reducing both cost and planning time even further [26,30]. Prospective, large-scale studies are warranted to validate these early findings and to determine the long-term impacts on functional outcomes, patient satisfaction, and training paradigms.
Despite the promising outcomes associated with fully guided orthognathic surgery, several limitations deserve consideration. In particular, the heterogeneity in study designs, the variability of patient populations, and the use of disparate outcome measures across the literature pose significant challenges to drawing generalized conclusions and directly comparing digital and free-hand techniques. In addition, the practical implications of implementing fully guided approaches extend beyond clinical accuracy: the high initial investment for digital tools, software, and custom fabrication, coupled with the need for specialized surgeon training, may restrict broader adoption—especially in resource-constrained settings. These fiscal and infrastructural constraints underscore the necessity for standardized protocols and cost-effective strategies, as well as enhanced training curricula that integrate both digital and traditional surgical skills. Future investigations should prioritize robust, multicenter studies that not only harmonize methodological approaches but also explore scalable implementation models to bridge the gap between technological advancements and real-world surgical practice.
5. Conclusions
Fully guided techniques in orthognathic surgery substantially improve surgical precision, reproducibility, and workflow efficiency in comparison to traditional free-hand methods. The consistent replication of preoperative simulations and submillimetric accuracy is made possible by digital planning and patient-specific guides, which leads to enhanced functional and aesthetic outcomes with a reduced operative time. The overall benefits of these computer-assisted approaches, which include reduced variability and potential cost-effectiveness in high-volume settings, emphasize the transformative potential, despite the initial investment and potential loss of intraoperative flexibility. The validation of long-term results and the investigation of hybrid strategies that integrate the adaptability of traditional techniques with digital precision should be the primary focus of future research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/dj13060260/s1, Table S1: Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist.
Author Contributions
Conceptualization, T.P. and I.C.; methodology, S.T. and I.K.; software, S.T.; validation, S.T., I.K. and S.K.; formal analysis, S.T., I.K. and A.K.; investigation, S.T. and I.K.; resources, S.T. and I.K.; data curation, S.T. and I.K.; writing—original draft preparation, S.T., I.K., A.K., G.K., K.S., D.A., G.-N.K. and S.K.; writing—review and editing, S.T. and I.K.; visualization, S.T.; supervision, T.P. and I.C.; project administration, T.P. and I.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We would like to thank Manolis Heliotis for advice given for this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lee, Y.-J.; Kim, S.-G. Custom surgical guide for orthognathic surgery. Oral Biol. Res. 2025, 49, 1. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.J.; Choi, M.H.; Yang, H.J.; Kim, J.E.; Huh, K.H.; Lee, S.S.; Heo, M.S.; Hwang, S.J.; Yi, W.J. Quantitative Augmented Reality-Assisted Free-Hand Orthognathic Surgery Using Electromagnetic Tracking and Skin-Attached Dynamic Reference. J. Craniofacial Surg. 2020, 31, 2175–2181. [Google Scholar] [CrossRef]
- Kwon, T.-G. Application of 3D Technology for Orthognathic Surgery. In Handbook of Oral and Maxillofacial Surgery and Implantology; Springer International Publishing: Cham, Switzerland, 2024; pp. 1–15. [Google Scholar]
- Shetty, S.K.; Kasrija, R. Analog to Digital Diagnosis and Planning in Orthognathic Surgery: A Narrative Review. Cureus 2025, 17, e80858. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.-H.; Youn, S.M.; Kim, C.Y.; Jeong, C.-G.; Choi, J.-Y. Surgical Accuracy of 3D Virtual Surgery and CAD/CAM-Assisted Orthognathic Surgery for Skeletal Class III Patients. J. Craniofacial Surg. 2023, 34, 96–102. [Google Scholar] [CrossRef]
- Kang, D.H. Intraoperative navigation in craniofacial surgery. Arch. Craniofacial Surg. 2024, 25, 209–216. [Google Scholar] [CrossRef]
- Junior, O.H.; Guijarro-Martínez, R.; de Sousa Gil, A.P.; da Silva Meirelles, L.; Scolari, N.; Muñoz-Pereira, M.E.; Hernández-Alfaro, F.; De Oliveira, R.B. Hierarchy of surgical stability in orthognathic surgery: Overview of systematic reviews. Int. J. Oral Maxillofac. Surg. 2019, 48, 1415–1433. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMAScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Hanasono, M.M.; Skoracki, R.J. Computer-assisted design and rapid prototype modeling in microvascular mandible reconstruction. Laryngoscope 2013, 123, 597–604. [Google Scholar] [CrossRef]
- Ma, H.; Shujaat, S.; Bila, M.; Sun, Y.; Vranckx, J.; Politis, C.; Jacobs, R. Computer-assisted versus traditional freehand technique for mandibular reconstruction with free vascularized fibular flap: A matched-pair study. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 3031–3039. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Zhu, H.; Liu, S.S.-Y. Technical procedures for template-guided surgery for mandibular reconstruction based on digital design and manufacturing. Biomed. Eng. Online 2014, 13, 63. [Google Scholar] [CrossRef]
- Ciocca, L.; Marchetti, C.; Mazzoni, S.; Baldissara, P.; Gatto, M.R.; Cipriani, R.; Scotti, R.; Tarsitano, A. Accuracy of fibular sectioning and insertion into a rapid-prototyped bone plate, for mandibular reconstruction using CAD-CAM technology. J. Cranio-Maxillofac. Surg. 2015, 43, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.; Bauer, F.J.M.; Hapfelmeier, A.; Rohleder, N.H.; Wolff, K.-D.; Kesting, M.R. Accuracy of mandibular reconstruction by three-dimensional guided vascularised fibular free flap after segmental mandibulectomy. Br. J. Oral Maxillofac. Surg. 2016, 54, 506–510. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Li, B.; Yu, H.; Shen, S.G.; Wang, X. Evaluation of computer-assisted mandibular reconstruction with vascularized fibular flap compared to conventional surgery. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 121, 139–148. [Google Scholar] [CrossRef] [PubMed]
- De Maesschalck, T.; Courvoisier, D.S.; Scolozzi, P. Computer-assisted versus traditional freehand technique in fibular free flap mandibular reconstruction: A morphological comparative study. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Gil, R.S.; Roig, A.M.; Obispo, C.A.; Morla, A.; Pagès, C.M.; Perez, J.L. Surgical planning and microvascular reconstruction of the mandible with a fibular flap using computer-aided design, rapid prototype modelling, and precontoured titanium reconstruction plates: A prospective study. Br. J. Oral Maxillofac. Surg. 2015, 53, 49–53. [Google Scholar] [CrossRef]
- Zweifel, D.F.; Simon, C.; Hoarau, R.; Pasche, P.; Broome, M. Are Virtual Planning and Guided Surgery for Head and Neck Reconstruction Economically Viable? J. Oral Maxillofac. Surg. 2015, 73, 170–175. [Google Scholar] [CrossRef]
- Tarsitano, A.; Battaglia, S.; Ciocca, L.; Scotti, R.; Cipriani, R.; Marchetti, C. Surgical reconstruction of maxillary defects using a computer-assisted design/computer-assisted manufacturing-produced titanium mesh supporting a free flap. J. Cranio-Maxillofac. Surg. 2016, 44, 1320–1326. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhang, H.Q.; Fan, S.; Zhang, D.M.; Huang, Z.Q.; Chen, W.L.; Ye, J.T.; Li, J.S. Mandibular reconstruction with the vascularized fibula flap: Comparison of virtual planning surgery and conventional surgery. Int. J. Oral Maxillofac. Surg. 2016, 45, 1400–1405. [Google Scholar] [CrossRef]
- Culié, D.; Dassonville, O.; Poissonnet, G.; Riss, J.-C.; Fernandez, J.; Bozec, A. Virtual planning and guided surgery in fibular free-flap mandibular reconstruction: A 29-case series. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2016, 133, 175–178. [Google Scholar] [CrossRef]
- Bouchet, B.; Raoul, G.; Julieron, B.; Wojcik, T. Functional and morphologic outcomes of CAD/CAM-assisted versus conventional microvascular fibular free flap reconstruction of the mandible: A retrospective study of 25 cases. J. Stomatol. Oral Maxillofac. Surg. 2018, 119, 455–460. [Google Scholar] [CrossRef]
- Bartier, S.; Mazzaschi, O.; Benichou, L.; Sauvaget, E. Computer-assisted versus traditional technique in fibular free-flap mandibular reconstruction: A CT symmetry study. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2021, 138, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Lee, C.; Park, J.; Choi, S.; Rijal, G.; Shin, H. Osteonecrosis associated with dental implants in patients undergoing bisphosphonate treatment. Clin. Oral Implant. Res. 2014, 25, 632–640. [Google Scholar] [CrossRef]
- Schwartz, H.C. Does computer-aided surgical simulation improve efficiency in bimaxillary orthognathic surgery? Int. J. Oral Maxillofac. Surg. 2014, 43, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Van Hemelen, G.; Van Genechten, M.; Renier, L.; Desmedt, M.; Verbruggen, E.; Nadjmi, N. Three-dimensional virtual planning in orthognathic surgery enhances the accuracy of soft tissue prediction. J. Cranio-Maxillofac. Surg. 2015, 43, 918–925. [Google Scholar] [CrossRef]
- Resnick, C.M.; Inverso, G.; Wrzosek, M.; Padwa, B.L.; Kaban, L.B.; Peacock, Z.S. Is There a Difference in Cost Between Standard and Virtual Surgical Planning for Orthognathic Surgery? J. Oral Maxillofac. Surg. 2016, 74, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Wrzosek, M.K.; Peacock, Z.S.; Laviv, A.; Goldwaser, B.R.; Ortiz, R.; Resnick, C.M.; Troulis, M.J.; Kaban, L.B. Comparison of time required for traditional versus virtual orthognathic surgery treatment planning. Int. J. Oral Maxillofac. Surg. 2016, 45, 1065–1069. [Google Scholar] [CrossRef]
- Ritto, F.G.; Schmitt, A.R.M.; Pimentel, T.; Canellas, J.V.; Medeiros, P.J. Comparison of the accuracy of maxillary position between conventional model surgery and virtual surgical planning. Int. J. Oral Maxillofac. Surg. 2018, 47, 160–166. [Google Scholar] [CrossRef]
- Steinhuber, T.; Brunold, S.; Gärtner, C.; Offermanns, V.; Ulmer, H.; Ploder, O. Is Virtual Surgical Planning in Orthognathic Surgery Faster Than Conventional Planning? A Time and Workflow Analysis of an Office-Based Workflow for Single- and Double-Jaw Surgery. J. Oral Maxillofac. Surg. 2018, 76, 397–407. [Google Scholar] [CrossRef]
- Schneider, D.; Kämmerer, P.W.; Hennig, M.; Schön, G.; Thiem, D.G.E.; Bschorer, R. Customized virtual surgical planning in bimaxillary orthognathic surgery: A prospective randomized trial. Clin. Oral Investig. 2019, 23, 3115–3122. [Google Scholar] [CrossRef]
- Al-Sabahi, M.E.; Jamali, O.M.; Shindy, M.I.; Moussa, B.G.; Amin, A.A.-W.; Zedan, M.H. Aesthetic Reconstruction of Onco-surgical Mandibular Defects Using Free Fibular Flap with and without CAD/CAM Customized Osteotomy Guide: A Randomized Controlled Clinical Trial. BMC Cancer 2022, 22, 1252. [Google Scholar] [CrossRef]
- Bao, T.; He, J.; Yu, C.; Zhao, W.; Lin, Y.; Wang, H.; Liu, J.; Zhu, H. Utilization of a pre-bent plate-positioning surgical guide system in precise mandibular reconstruction with a free fibula flap. Oral Oncol. 2017, 75, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Ritschl, L.M.; Mücke, T.; Fichter, A.; Güll, F.D.; Schmid, C.; Duc, J.M.; Kesting, M.R.; Wolff, K.D.; Loeffelbein, D.J. Functional Outcome of CAD/CAM-Assisted versus Conventional Microvascular, Fibular Free Flap Reconstruction of the Mandible: A Retrospective Study of 30 Cases. J. Reconstr. Microsurg. 2017, 33, 281–291. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).