Unveiling Drug-Drug Interactions in Dental Patients: A Retrospective Real-World Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
2.2. Testing the Severity Level of Drug-Drug Interactions
2.3. Statistical Analysis
3. Results
3.1. Patient Demographics and Preexisting Clinical Profile
3.2. Dental Patient Profile
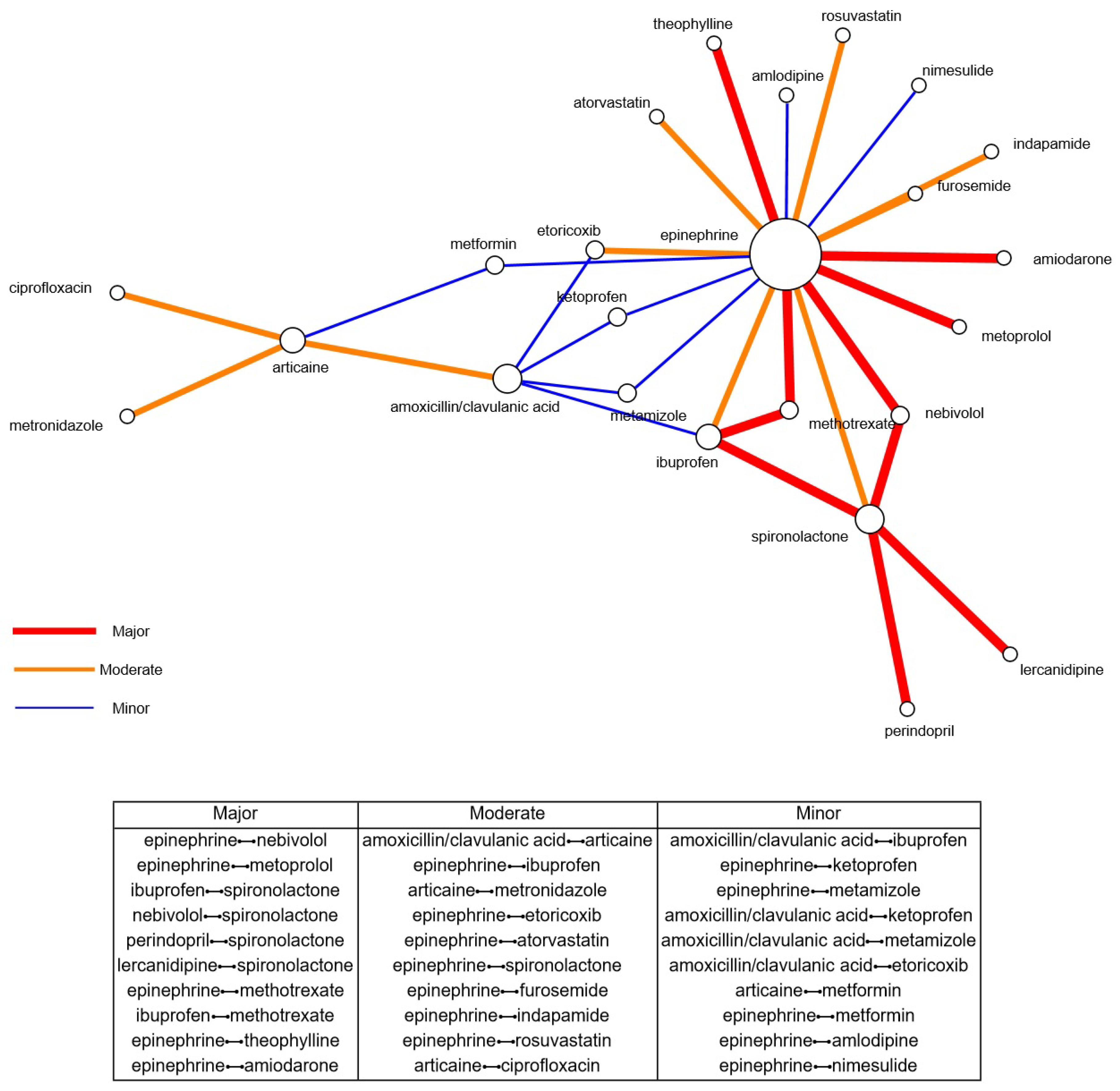
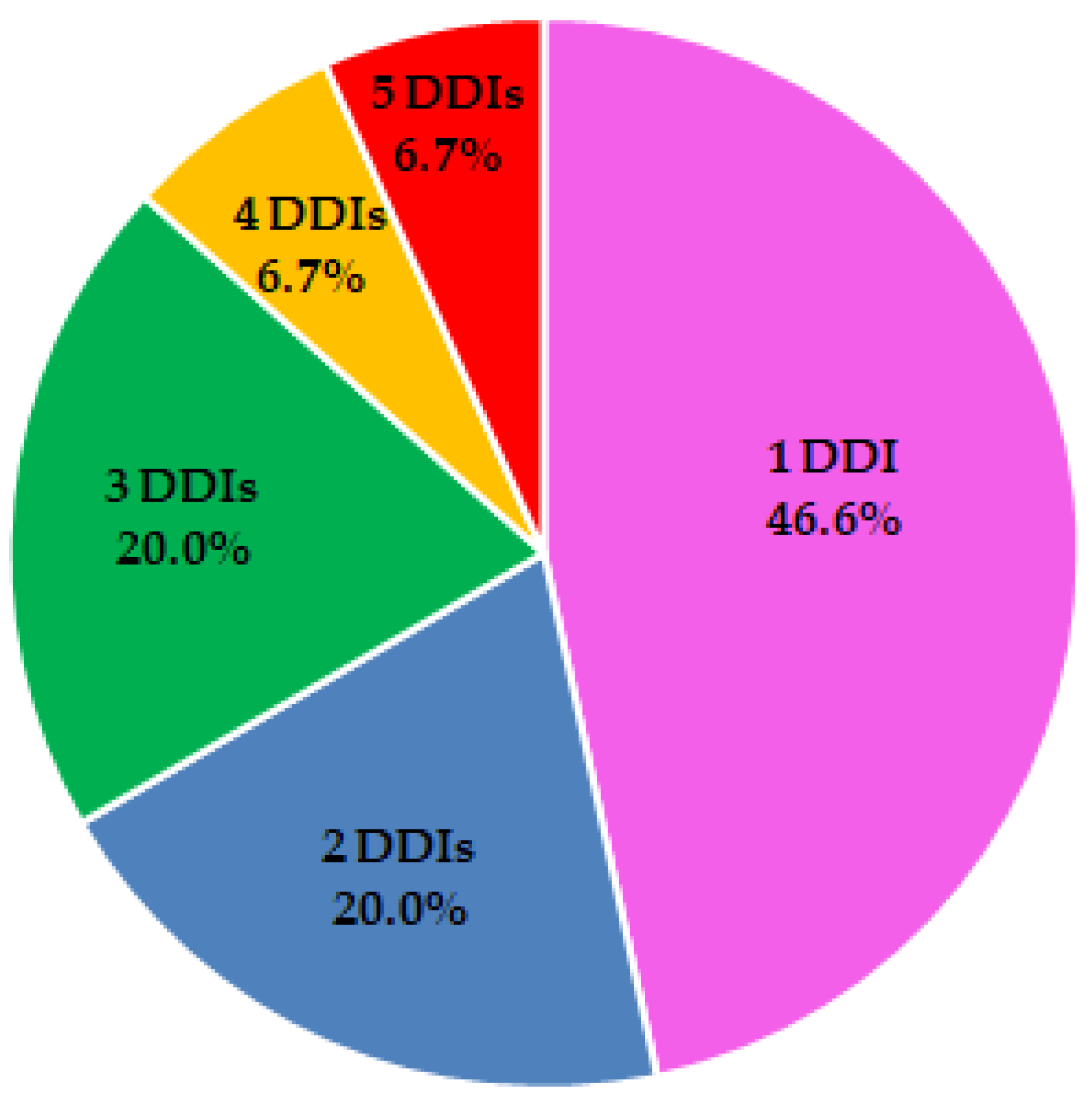
3.3. Drug-Drug Interaction Analysis
4. Discussion
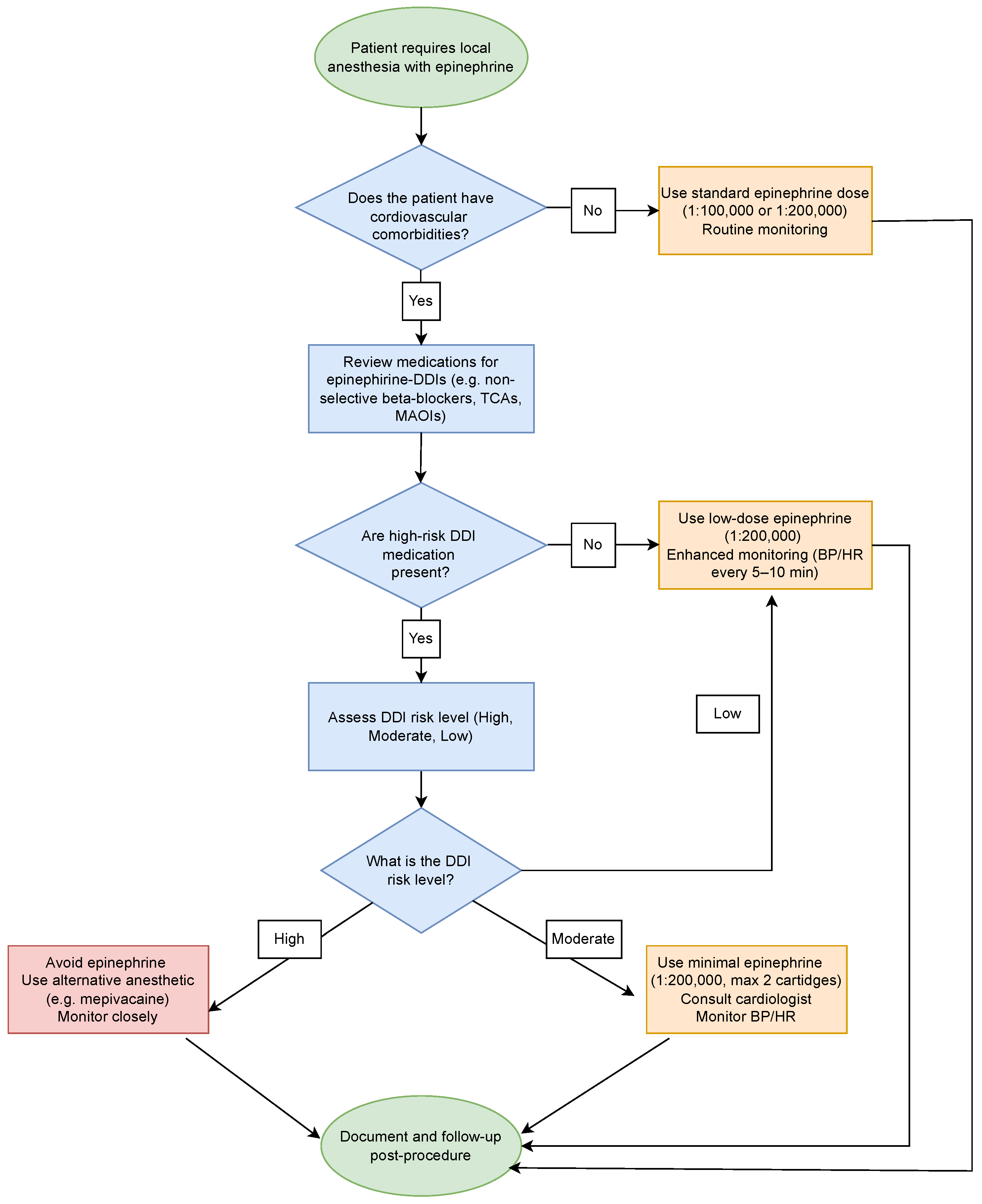
4.1. Clinical Management Strategies
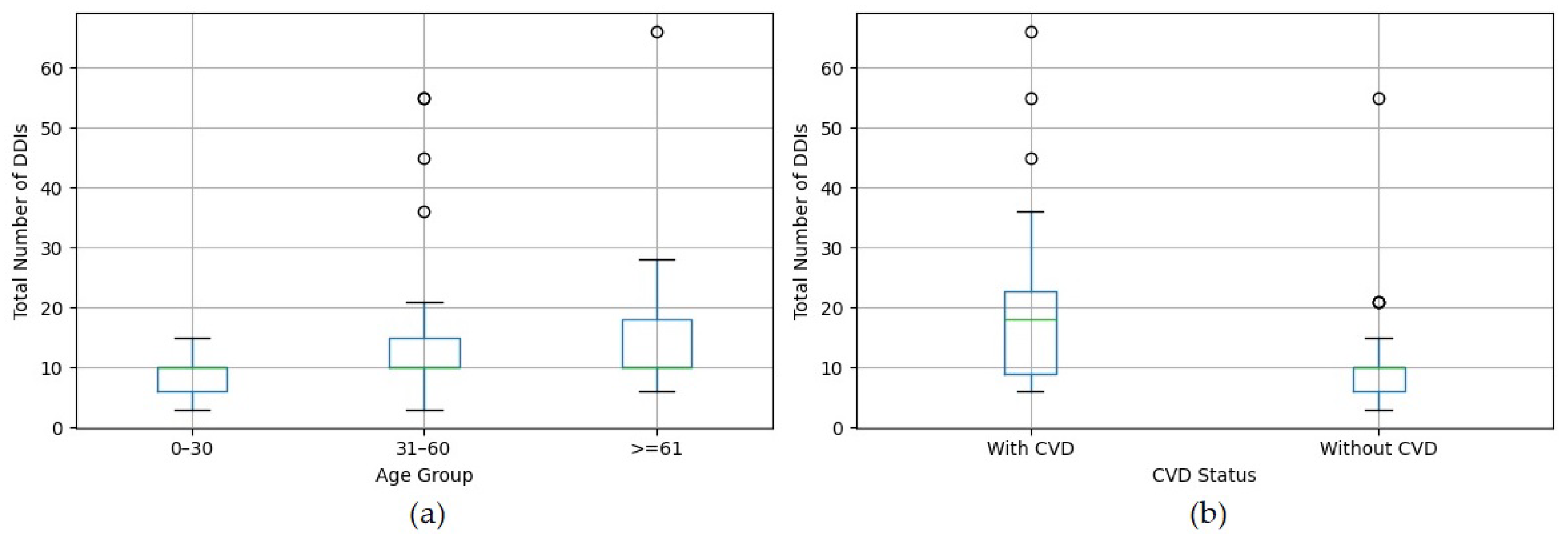
4.2. DDI Prevalence by Age and CVD
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE | Angiotensin converting enzyme |
| CVD | Cardiovascular diseases |
| DDIs | Drug-drug interactions |
| NSAIDs | Non-steroidal anti-inflammatories |
| RWD | Real-world data |
References
- Seymour, R.A. Drug interactions in dentistry. Dent. Update 2009, 36, 458–470. [Google Scholar] [CrossRef] [PubMed]
- da Cruz Furini, A.A.; de Almeida Malagoli, J.M.; Dias, N.; Lima, B.M.; Bonjardin, M.M.; Machado, P.S.; de Lima, T.A.M. Analysis of drug interactions in dental prescriptions. Rev. Bras. Odontol. 2018, 75, e1021. [Google Scholar]
- Ouanounou, A.; Ng, K.; Chaban, P. Adverse drug reactions in dentistry. Int. Dent. J. 2020, 70, 79–84. [Google Scholar] [CrossRef]
- Dawoud, B.; Roberts, A.; Yates, J. Drug interactions in general dental practice–considerations for the dental practitioner. Br. Dent. J. 2014, 216, 15–23. [Google Scholar] [CrossRef]
- Decloux, D.; Ouanounou, A. Local anaesthesia in dentistry: A review. Int. Dent. J. 2021, 71, 87–95. [Google Scholar] [CrossRef]
- Knox, C.; Wilson, M.; Klinger, C.M.; Franklin, M.; Oler, E.; Wilson, A.; Pon, A.; Cox, J.; Chin, N.E.; Strawbridge, S.A.; et al. DrugBank 6.0: The DrugBank knowledgebase for 2024. Nucleic Acids Res. 2024, 52, D1265–D1275. [Google Scholar] [CrossRef] [PubMed]
- Seymour, R.A. Antibiotics in dentistry—An update. Dent. Update 2013, 40, 319–322. [Google Scholar] [CrossRef]
- Salgado-Peralvo, A.O.; Mateos-Moreno, M.V.; Velasco-Ortega, E.; Peña-Cardelles, J.F.; Kewalramani, N. Preventive antibiotic therapy in bone augmentation procedures in oral implantology: A systematic review. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, 74–80. [Google Scholar] [CrossRef]
- Luchian, I.; Goriuc, A.; Martu, M.A.; Covasa, M. Clindamycin as an alternative option in optimizing periodontal therapy. Antibiotics 2021, 10, 814. [Google Scholar] [CrossRef]
- Nagaja, A.S.; John, R.S.; Rubin, S.; Murugesan, K. Comparison of Normal Saline and Metronidazole as Irrigating Solutions in Surgical Extraction of Third Molars. Libr.-Prog.-Libr. Sci. Inf. Technol. Comput. 2024, 44, 2703. [Google Scholar]
- Becker, D.E. Pain management: Part 1: Managing acute and postoperative dental pain. Anesth. Prog. 2010, 57, 67. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.A.; Ziegler, K.M.; Lipman, R.D.; Aminoshariae, A.; Carrasco-Labra, A.; Mariotti, A. Benefits and harms associated with analgesic medications used in the management of acute dental pain: An overview of systematic reviews. J. Am. Dent. Assoc. 2018, 149, 256–265. [Google Scholar] [CrossRef]
- Sanghavi, J.; Aditya, A. Applications of corticosteroids in dentistry. J. Dent. Allied Sci. 2015, 4, 19–24. [Google Scholar]
- Pimenta, R.P.; Takahashi, C.M.; Barberato-Filho, S.; McClung, D.C.F.; Moraes, F.d.S.; de Souza, I.M.; Bergamaschi, C.d.C. Preemptive use of anti-inflammatories and analgesics in oral surgery: A review of systematic reviews. Front. Pharmacol. 2024, 14, 1303382. [Google Scholar] [CrossRef]
- Hargreaves, K.; Abbott, P. Drugs for pain management in dentistry. Aust. Dent. J. 2005, 50, S14–S22. [Google Scholar] [CrossRef]
- Appukuttan, D.P. Strategies to manage patients with dental anxiety and dental phobia: Literature review. Clin. Cosmet. Investig. Dent. 2016, 8, 35–50. [Google Scholar] [CrossRef]
- Weissheimert, T.; da Silveira Gerzson, A.; Schwengber, H.E.; Neto, A.M. Benzodiazepines for conscious sedation in the dental office. Stomatos 2016, 22, 42–53. [Google Scholar]
- Ogle, O.E.; Hertz, M.B. Anxiety control in the dental patient. Dent. Clin. 2012, 56, 1–16. [Google Scholar] [CrossRef]
- Masuda, R.; Nonaka, M.; Nishimura, A.; Gotoh, K.; Oka, S.; Iijima, T. Optimal and safe standard doses of midazolam and propofol to achieve patient and doctor satisfaction with dental treatment: A prospective cohort study. PLoS ONE 2017, 12, e0171627. [Google Scholar] [CrossRef]
- Khinda, V.; Rao, D.; Sodhi, S.P.S. Nitrous oxide inhalation sedation rapid analgesia in dentistry: An overview of technique, objectives, indications, advantages, monitoring, and safety profile. Int. J. Clin. Pediatr. Dent. 2023, 16, 131. [Google Scholar] [CrossRef]
- Inoue, T.; Yamamoto, T.; Kishimoto, N.; Seo, K. Comparison of Anesthetic Features in Diazepam and Midazolam for Sedation Dentistry: A Scoping Review. Cureus 2025, 17, e79079. [Google Scholar] [CrossRef] [PubMed]
- Hersh, E.V.; Moore, P.A. Three serious drug interactions that every dentist should know about. Compendium 2015, 36, 739–744. [Google Scholar]
- Weinstock, R.J.; Johnson, M.P. Review of top 10 prescribed drugs and their interaction with dental treatment. Dent. Clin. 2016, 60, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Poornachitra, P.; Narayan, V.; Maragathavalli, G. An Update on Common Drug Interactions in Dental Practice. J. Indian Acad. Oral Med. Radiol. 2023, 35, 284–285. [Google Scholar]
- Becker, D.E. Antithrombotic drugs: Pharmacology and implications for dental practice. Anesth. Prog. 2013, 60, 72. [Google Scholar] [CrossRef]
- Paraschiv, C.; Esanu, I.; Ghiuru, R.; Manea, P.; Munteanu, D.; Gavrilescu, C.M. Dental implications of the new oral anticoagulants. Rom. J. Oral Rehabil. 2015, 7, 30–36. [Google Scholar]
- Muñoz-Martínez, C.; Segura-Puertas, M.; Gómez-Moreno, G. Disease-Modifying Antirheumatic Drugs (DMARDs) and drug interactions in dentistry. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2834–2842. [Google Scholar] [PubMed]
- Valtellini, R.; Ouanounou, A. Management of the Hypertensive Dental Patient. J. Can. Dent. Assoc. 2023, 89, 1–4. [Google Scholar]
- Gomez-Moreno, G. Remdesivir-COVID-19: Drug interactions in dentistry. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9739–9743. [Google Scholar]
- Mohan, S.; Govila, V.; Saini, A.; Verma, S.C. Prime drug interplay in dental practice. J. Clin. Diagn. Res. JCDR 2016, 10, ZE07. [Google Scholar] [CrossRef]
- Goh, W.; Tao, X.; Zhang, J.; Yong, J. A study of drug interaction for personalised decision support in dental clinics. In Proceedings of the 2015 IEEE/WIC/ACM International Conference on Web Intelligence and Intelligent Agent Technology (WI-IAT), Singapore, 6–9 December 2015; IEEE: Piscataway, NJ, USA, 2015; Volume 3, pp. 88–91. [Google Scholar]
- De Oliveira, M.L.R.; Nery, G.O.; Torresan, T.T.; Arcanjo, R.A.; Ferreira, M.B.C.; Montagner, F. Frequency and characterization of potential drug interactions in dentistry—A cross-sectional study. Clin. Oral Investig. 2022, 26, 6829–6837. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh, E.; Hakemi, N.G.; Rad, M.; Torabi, M. Drug-Drug Interactions in Elderly Adults in Dentistry Care: A Cross-Sectional Study. J. Dent. 2022, 23, 459. [Google Scholar]
- Abeleira-Pazos, M.T.; García-Mato, E.; Diniz-Freitas, M.; Muñoz-Navarro, C.; Lago-Méndez, L.; Vázquez-García, E.; Rivas-Mundiña, B. Discrepancy in medications reported by elderly patients in the dental office and in their electronic medical records: A pilot study. Spec. Care Dent. 2024, 44, 1162–1170. [Google Scholar] [CrossRef]
- DrugBank Website. Available online: https://go.drugbank.com/drug-interaction-checker (accessed on 30 January 2025).
- Suciu, L.; Ardelean, S.M.; Udrescu, M.; Goldiş, F.D.; Hânda, D.; Tuică, M.M.; Vasii, S.O.; Udrescu, L. Categorical Analysis of Database Consistency in Reporting Drug–Drug Interactions for Cardiovascular Diseases. Pharmaceutics 2024, 16, 339. [Google Scholar] [CrossRef] [PubMed]
- Udrescu, M.; Ardelean, S.M.; Udrescu, L. The curse and blessing of abundance—The evolution of drug interaction databases and their impact on drug network analysis. GigaScience 2023, 12, giad011. [Google Scholar] [CrossRef] [PubMed]
- Hersh, E.V.; Moore, P.A. Drug interactions in dentistry: The importance of knowing your CYPs. J. Am. Dent. Assoc. 2004, 135, 298–311. [Google Scholar] [CrossRef]
- Soto, A.P.; Meyer, S.L. Oral implications of polypharmacy in older adults. Dent. Clin. 2021, 65, 323–343. [Google Scholar] [CrossRef]
- Southerland, J.H.; Gill, D.G.; Gangula, P.R.; Halpern, L.R.; Cardona, C.Y.; Mouton, C.P. Dental management in patients with hypertension: Challenges and solutions. Clin. Cosmet. Investig. Dent. 2016, 8, 111–120. [Google Scholar] [CrossRef]
- Sankar, V.; Saaed, Y.; Joseph, R.M.; Azizi, H.; Mariyam Thomas, P. Serious drug-drug interactions in the prescriptions of diabetic patients. Med. Sci. 2015, 3, 93–103. [Google Scholar] [CrossRef]
- Tibúrcio-Machado, C.; Michelon, C.; Zanatta, F.; Gomes, M.S.; Marin, J.A.; Bier, C.A. The global prevalence of apical periodontitis: A systematic review and meta-analysis. Int. Endod. J. 2021, 54, 712–735. [Google Scholar] [CrossRef]
- Segura-Egea, J.; Gould, K.; Şen, B.H.; Jonasson, P.; Cotti, E.; Mazzoni, A.; Sunay, H.; Tjäderhane, L.; Dummer, P. Antibiotics in Endodontics: A review. Int. Endod. J. 2017, 50, 1169–1184. [Google Scholar] [CrossRef] [PubMed]
- Teoh, L.; Moses, G.; McCullough, M. A review of drugs that contribute to bleeding risk in general dental practice. Aust. Dent. J. 2020, 65, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, M.; Dayer, M.; Durkin, M.; Lockhart, P.; Baddour, L. Risk of adverse reactions to oral antibiotics prescribed by dentists. J. Dent. Res. 2019, 98, 1081–1087. [Google Scholar] [CrossRef]
- Gasner, N.; Ouanounou, A. Analgesics and pain management following root canal therapy. Essent. Dent. 2021, 1, 1–11. [Google Scholar] [CrossRef]
- Malamed, S.F. Pain management following dental trauma and surgical procedures. Dent. Traumatol. 2023, 39, 295–303. [Google Scholar] [CrossRef]
- Chappuis, V.; Avila-Ortiz, G.; Araújo, M.G.; Monje, A. Medication-related dental implant failure: Systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29, 55–68. [Google Scholar] [CrossRef]
- D’Ambrosio, F.; Amato, A.; Chiacchio, A.; Sisalli, L.; Giordano, F. Do systemic diseases and medications influence dental implant osseointegration and dental implant health? An umbrella review. Dent. J. 2023, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, C.C.; Lopes, L.C.; de Cássia Bergamaschi, C.; Ramacciato, J.C.; Silva, M.T.; de Oliveira Araújo, J.; de Andrade, N.K.; Motta, R.H.L. Local anaesthetics combined with vasoconstrictors in patients with cardiovascular disease undergoing dental procedures: Systematic review and meta-analysis. BMJ Open 2021, 11, e044357. [Google Scholar] [CrossRef]
- Pökel, C.; Schulze, A.; Busse, M. Cardiovascular and Vector-Cardiographic Effects of Articaine Anesthesia with Epinephrine. Int. J. Dent. 2024, 2024, 8610423. [Google Scholar] [CrossRef]
- Su, N.; Liu, Y.; Yang, X.; Shi, Z.; Huang, Y. Efficacy and safety of mepivacaine compared with lidocaine in local anaesthesia in dentistry: A meta-analysis of randomised controlled trials. Int. Dent. J. 2014, 64, 96–107. [Google Scholar] [CrossRef]
- Brockmann, W.G. Mepivacaine: A closer look at its properties and current utility. Gen. Dent. 2014, 62, 70–75. [Google Scholar] [PubMed]
- Deol, N.; Alvarez, G.; Elrabi, O.; Chen, G.; Ferraro, N. A comparative review of epinephrine and phenylephrine as vasoconstrictors in dental anesthesia: Exploring the factors behind epinephrine’s prevalence in the US. J. Dent. Anesth. Pain Med. 2023, 23, 293. [Google Scholar] [CrossRef] [PubMed]
- Zahno, A.; Brecht, K.; Morand, R.; Maseneni, S.; Török, M.; Lindinger, P.W.; Krähenbühl, S. The role of CYP3A4 in amiodarone-associated toxicity on HepG2 cells. Biochem. Pharmacol. 2011, 81, 432–441. [Google Scholar] [CrossRef]
- Tamargo, J.; Le Heuzey, J.Y.; Mabo, P. Narrow therapeutic index drugs: A clinical pharmacological consideration to flecainide. Eur. J. Clin. Pharmacol. 2015, 71, 549–567. [Google Scholar] [CrossRef]
- Harris-Vieyra, L.E.; Johnson, K.L.; Van der Werf, A.N. Prevalence of Medications that may Induce QT Interval Prolongation in a Dental Clinic Population. EC Dent. Sci. 2019, 18, 2507–2514. [Google Scholar]
- Guimaraes, C.C.; Motta, R.H.L.; de Cássia Bergamaschi, C.; de Oliveira Araújo, J.; de Andrade, N.K.; Figueiró, M.F.; Ramacciato, J.C.; Lopes, L.C. Local anaesthetics combined with vasoconstrictors in patients with cardiovascular disease undergoing dental procedures: Systematic review and meta-analysis protocol. BMJ Open 2017, 7, e014611. [Google Scholar] [CrossRef]
- Singgih, M.F.; Achmad, H.; Sukmana, B.I.; Carmelita, A.B.; Putra, A.P.; Ramadhany, S.; Putri, A.P. A review of nonsteroidal anti-inflammatory drugs (NSAIDs) medications in dentistry: Uses and side effects. Syst. Rev. Pharm. 2020, 11, 293–298. [Google Scholar]
- Hughes, J.E.; Russo, V.; Walsh, C.; Menditto, E.; Bennett, K.; Cahir, C. Prevalence and factors associated with potential drug-drug interactions in older community-dwelling adults: A prospective cohort study. Drugs Aging 2021, 38, 1025–1037. [Google Scholar] [CrossRef]
- Delara, M.; Murray, L.; Jafari, B.; Bahji, A.; Goodarzi, Z.; Kirkham, J.; Chowdhury, M.; Seitz, D.P. Prevalence and factors associated with polypharmacy: A systematic review and meta-analysis. BMC Geriatr. 2022, 22, 601. [Google Scholar]
- Rochon, P.A.; Petrovic, M.; Cherubini, A.; Onder, G.; O’Mahony, D.; Sternberg, S.A.; Stall, N.M.; Gurwitz, J.H. Polypharmacy, inappropriate prescribing, and deprescribing in older people: Through a sex and gender lens. Lancet Healthy Longev. 2021, 2, e290–e300. [Google Scholar] [CrossRef]
- Davies, L.E.; Spiers, G.; Kingston, A.; Todd, A.; Adamson, J.; Hanratty, B. Adverse outcomes of polypharmacy in older people: Systematic review of reviews. J. Am. Med. Dir. Assoc. 2020, 21, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Harris, E. Study: Polypharmacy Nearly Doubled in 20 Years Among Older Adults in US. JAMA 2024, 332, 524. [Google Scholar] [CrossRef] [PubMed]
- Malekzadeh, M.; Khadivi, Y.; Sohrevardi, S.M.; Afzal, G. Drug prescription patterns and compliance with WHO and beers criteria in older patients. BMC Geriatr. 2025, 25, 135. [Google Scholar] [CrossRef]
- Sipos, M.; Farcas, A.; Leucuta, D.C.; Bulik, N.B.; Huruba, M.; Dumitrascu, D.; Mogosan, C. Prevalence and Risk Factors Associated with Potentially Inappropriate Prescribing According to STOPP-2 Criteria among Discharged Older Patients—An Observational Retrospective Study. Pharmaceuticals 2023, 16, 852. [Google Scholar] [CrossRef]
- Błeszyńska, E.; Wierucki, Ł.; Zdrojewski, T.; Renke, M. Pharmacological interactions in the elderly. Medicina 2020, 56, 320. [Google Scholar] [CrossRef]
- Ngcobo, N.N. Influence of Ageing on the Pharmacodynamics and Pharmacokinetics of Chronically Administered Medicines in Geriatric Patients: A Review. Clin. Pharmacokinet. 2025, 64, 335–367. [Google Scholar] [CrossRef]
- Maher, D.; Ailabouni, N.; Mangoni, A.A.; Wiese, M.D.; Reeve, E. Alterations in drug disposition in older adults: A focus on geriatric syndromes. Expert Opin. Drug Metab. Toxicol. 2021, 17, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Drenth-van Maanen, A.C.; Wilting, I.; Jansen, P.A. Prescribing medicines to older people—How to consider the impact of ageing on human organ and body functions. Br. J. Clin. Pharmacol. 2020, 86, 1921–1930. [Google Scholar] [CrossRef]
- Drenth-van Maanen, A.C.; Spee, J.; van Hensbergen, L.; Jansen, P.; Egberts, T.; van Marum, R.J. Structured history taking of medication use reveals iatrogenic harm due to discrepancies in medication histories in hospital and pharmacy records. J. Am. Geriatr. Soc. 2011, 59, 1976–1977. [Google Scholar] [CrossRef]
- Bennie, M.; Santa-Ana-Tellez, Y.; Galistiani, G.F.; Trehony, J.; Despres, J.; Jouaville, L.S.; Poluzzi, E.; Morin, L.; Schubert, I.; MacBride-Stewart, S.; et al. The prevalence of polypharmacy in older Europeans: A multi-national database study of general practitioner prescribing. Br. J. Clin. Pharmacol. 2024, 90, 2124–2136. [Google Scholar] [CrossRef]
- Faber, J.; Fonseca, L.M. How sample size influences research outcomes. Dent. Press J. Orthod. 2014, 19, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Sechidis, K.; Sperrin, M.; Petherick, E.S.; Luján, M.; Brown, G. Dealing with under-reported variables: An information theoretic solution. Int. J. Approx. Reason. 2017, 85, 159–177. [Google Scholar] [CrossRef]
| Age (years), n (%) | 0–30 | 27 (25.7) |
| 31–60 | 59 (56.2) | |
| ≥61 | 19 (18.1) | |
| Gender, n (%) | Females | 65 (61.9) |
| Males | 40 (30.1) | |
| Preexistent diseases, n (%) | Cardiovascular | 20 (19.0) |
| Respiratory | 8 (7.6) | |
| Hematological | 7 (6.7) | |
| Psychiatric | 6 (5.7) | |
| Neurological | 6 (5.7) | |
| Diabetes mellitus | 5 (4.8) | |
| Gastrointestinal | 4 (3.8) | |
| Urological | 3 (2.8) | |
| Dermatological | 2 (1.9) | |
| Otolaryngological | 1 (0.9) | |
| Systemic infections | 1 (0.9) | |
| Allergic | 1 (0.9) |
| Dental Diagnosis | n (%) | Dental Procedure | n (%) |
|---|---|---|---|
| Apical lesion | 50 (47.6) | Tooth extraction | 56 (53.3) |
| Abscess | 19 (18.1) | Endodontic treatment | 20 (19.1) |
| Pulpitis | 16 (15.2) | Dental implant | 13 (12.4) |
| Edentulism | 13 (12.4) | Endodontic retreatment | 8 (7.6) |
| Dental caries | 7 (6.7) | Caries treatment | 4 (3.8) |
| Surgical tooth extraction | 2 (1.9) | ||
| Endodontic microsurgery | 2 (1.9) |
| Severity Level | n (%) |
|---|---|
| Major | 31 (2.3%) |
| Moderate | 333 (25.0%) |
| Minor | 178 (13.4%) |
| No interactions found | 790 (59.3%) |
| Age Group | Major, n (%) | Moderate, n (%) | Minor, n (%) |
|---|---|---|---|
| 0–30 | 0 | 54 (16.2) | 31 (17.4) |
| 31–60 | 19 (61.3) | 197 (59.2) | 115 (64.6) |
| ≥61 | 12 (38.7) | 82 (24.6) | 32 (18.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colibășanu, D.; Ardelean, S.M.; Goldiș, F.-D.; Drăgoi, M.-M.; Vasii, S.-O.; Maksimović, T.; Colibășanu, Ș.; Șoica, C.; Udrescu, L. Unveiling Drug-Drug Interactions in Dental Patients: A Retrospective Real-World Study. Dent. J. 2025, 13, 255. https://doi.org/10.3390/dj13060255
Colibășanu D, Ardelean SM, Goldiș F-D, Drăgoi M-M, Vasii S-O, Maksimović T, Colibășanu Ș, Șoica C, Udrescu L. Unveiling Drug-Drug Interactions in Dental Patients: A Retrospective Real-World Study. Dentistry Journal. 2025; 13(6):255. https://doi.org/10.3390/dj13060255
Chicago/Turabian StyleColibășanu, Daiana, Sebastian Mihai Ardelean, Florina-Diana Goldiș, Maria-Medana Drăgoi, Sabina-Oana Vasii, Tamara Maksimović, Șerban Colibășanu, Codruța Șoica, and Lucreția Udrescu. 2025. "Unveiling Drug-Drug Interactions in Dental Patients: A Retrospective Real-World Study" Dentistry Journal 13, no. 6: 255. https://doi.org/10.3390/dj13060255
APA StyleColibășanu, D., Ardelean, S. M., Goldiș, F.-D., Drăgoi, M.-M., Vasii, S.-O., Maksimović, T., Colibășanu, Ș., Șoica, C., & Udrescu, L. (2025). Unveiling Drug-Drug Interactions in Dental Patients: A Retrospective Real-World Study. Dentistry Journal, 13(6), 255. https://doi.org/10.3390/dj13060255









