Impact of Clinician Experience on Intraosseous Anesthesia Outcomes in Endodontic Treatment: Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
2.2. Clinical Settings
2.3. Sample Size Determination
2.4. Participants
2.5. Pre-Anesthetic Assessment
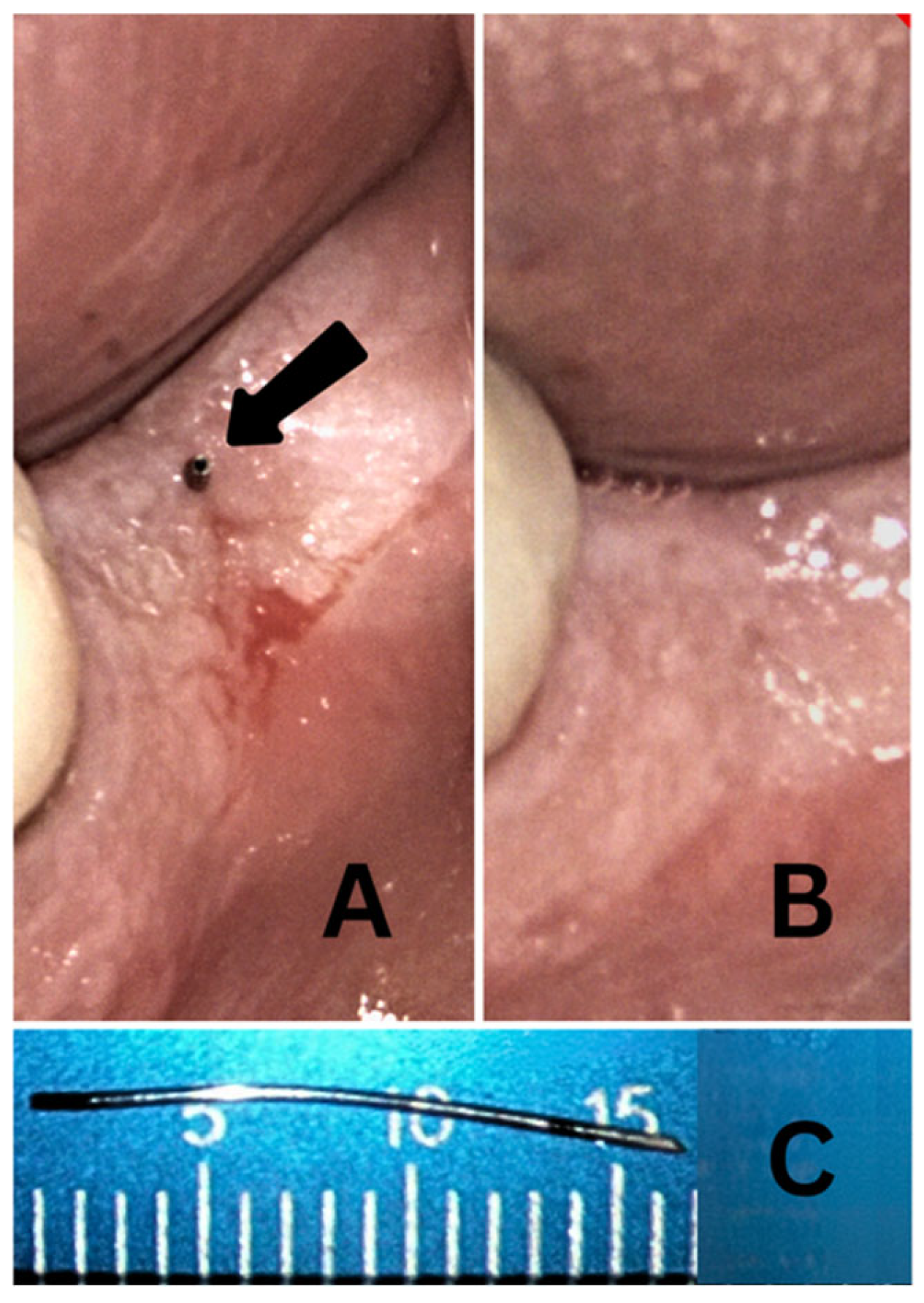
2.6. IOA Administration
2.7. Endodontic Treatment and Pain Assessment
2.8. Statistical Analysis
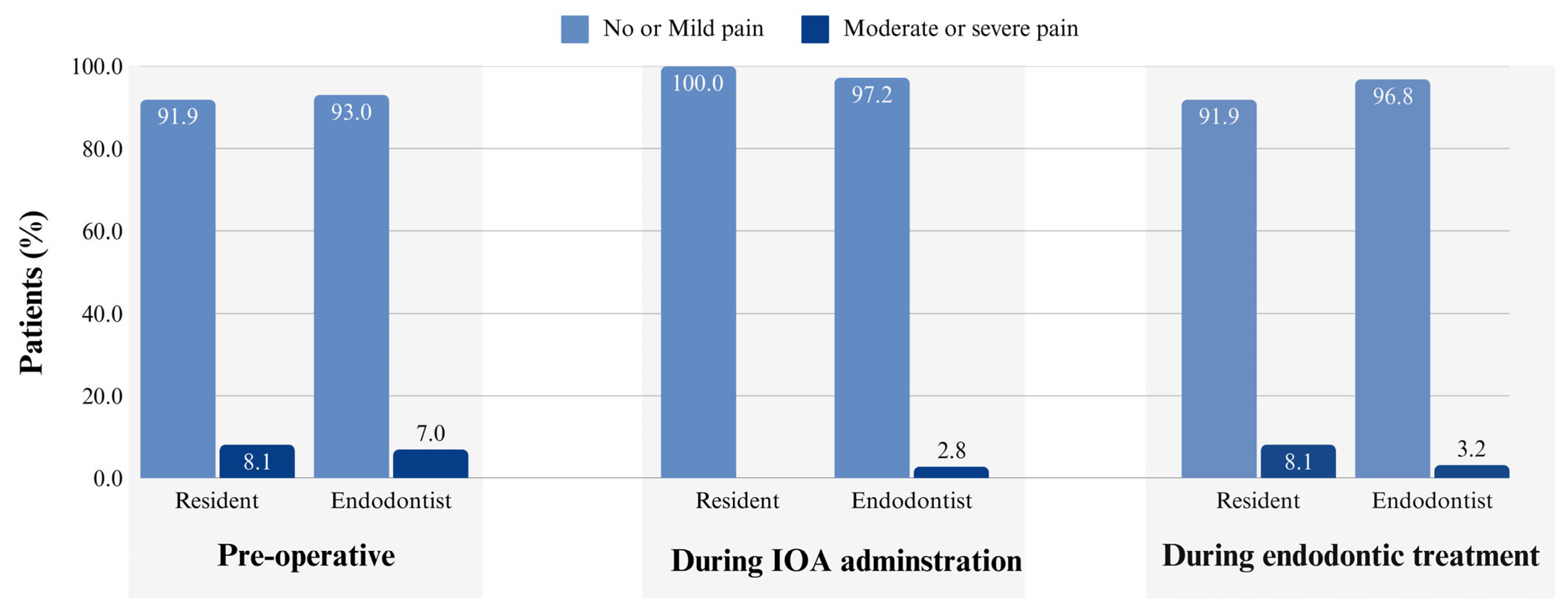
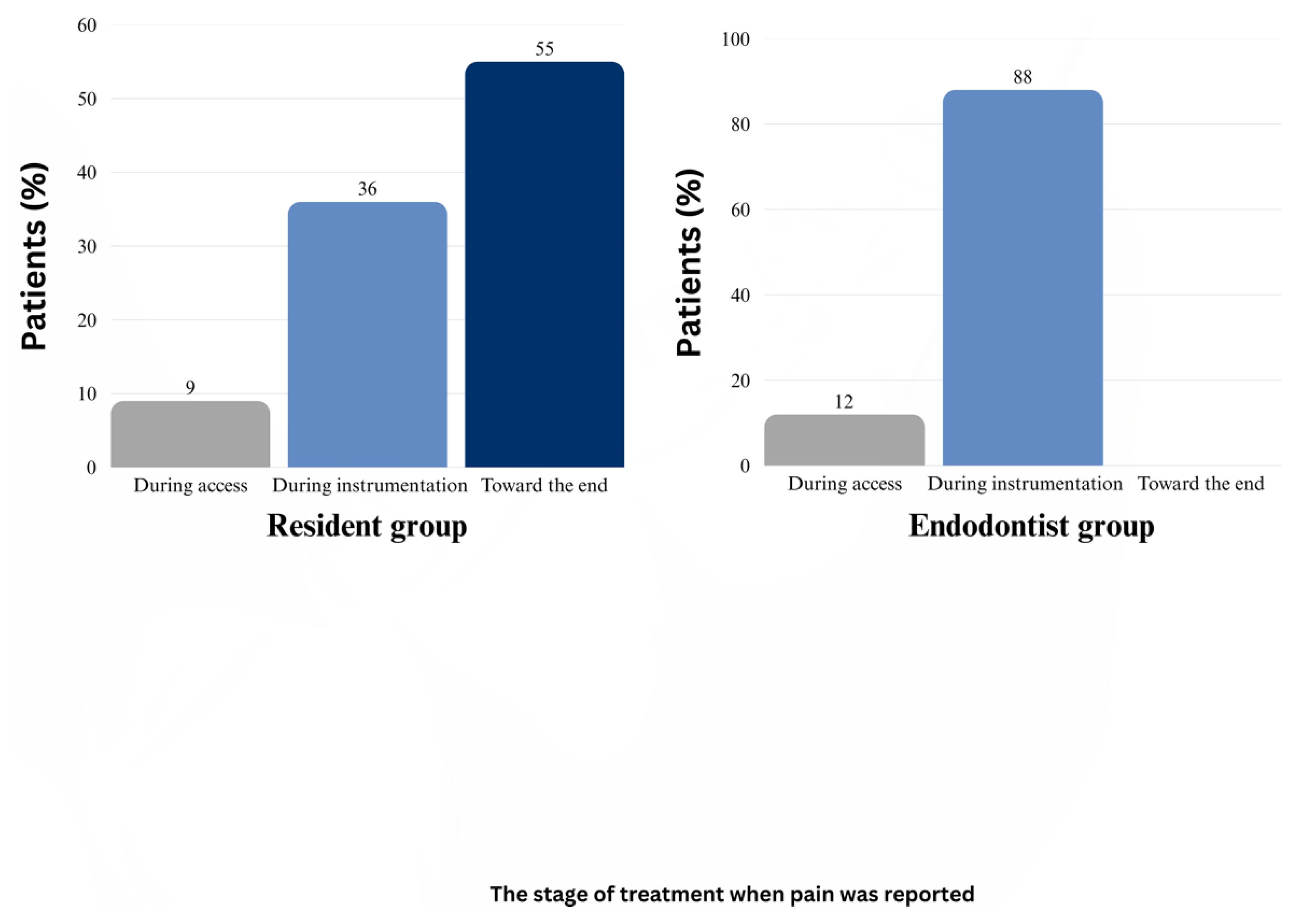
3. Results
4. Discussion
4.1. Clinical Significance of This Study
4.2. Methodology Considerations
4.3. Impact of Clinician Experience on the IOA Outcomes
4.4. Clinical Applications
4.5. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olch, P.D.; William, S. Halsted and local anesthesia: Contributions and complications. Anesthesiology 1975, 43, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Claffey, E.; Reader, A.; Nusstein, J.; Beck, M.; Weaver, J. Anesthetic efficacy of articaine for inferior alveolar nerve blocks in patients with irreversible pulpitis. J. Endod. 2004, 30, 568–571. [Google Scholar] [CrossRef]
- Zanjir, M.; Lighvan, N.L.; Yarascavitch, C.; Beyene, J.; Shah, P.S.; Azarpazhooh, A. Efficacy and safety of pulpal anesthesia strategies during endodontic treatment of permanent mandibular molars with symptomatic irreversible pulpitis: A systematic review and network meta-analysis. J. Endod. 2019, 45, 1435–1464.e1410. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, D.; Reader, A.; Nist, R.; Beck, M.; Meyers, W.J. Anesthetic efficacy of the intraosseous injection after an inferior alveolar nerve block. J. Endod. 1996, 22, 481–486. [Google Scholar] [CrossRef]
- Childers, M.; Reader, A.; Nist, R.; Beck, M.; Meyers, W.J. Anesthetic efficacy of the periodontal ligament injection after an inferior alveolar nerve block. J. Endod. 1996, 22, 317–320. [Google Scholar] [CrossRef]
- Nusstein, J.; Reader, A.; Nist, R.; Beck, M.; Meyers, W.J. Anesthetic efficacy of the supplemental intraosseous injection of 2% lidocaine with 1:100,000 epinephrine in irreversible pulpitis. J. Endod. 1998, 24, 487–491. [Google Scholar] [CrossRef]
- Reisman, D.; Reader, A.; Nist, R.; Beck, M.; Weaver, J. Anesthetic efficacy of the supplemental intraosseous injection of 3% mepivacaine in irreversible pulpitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 84, 676–682. [Google Scholar] [CrossRef]
- Cohen, H.P.; Cha, B.Y.; Spångberg, L.S. Endodontic anesthesia in mandibular molars: A clinical study. J. Endod. 1993, 19, 370–373. [Google Scholar] [CrossRef]
- Nusstein, J.; Claffey, E.; Reader, A.; Beck, M.; Weaver, J. Anesthetic effectiveness of the supplemental intraligamentary injection, administered with a computer-controlled local anesthetic delivery system, in patients with irreversible pulpitis. J. Endod. 2005, 31, 354–358. [Google Scholar] [CrossRef]
- Fowler, S.; Drum, M.; Reader, A.; Beck, M. Anesthetic success of an inferior alveolar nerve block and supplemental articaine buccal infiltration for molars and premolars in patients with symptomatic irreversible pulpitis. J. Endod. 2016, 42, 390–392. [Google Scholar] [CrossRef]
- Matthews, R.; Drum, M.; Reader, A.; Nusstein, J.; Beck, M. Articaine for supplemental buccal mandibular infiltration anesthesia in patients with irreversible pulpitis when the inferior alveolar nerve block fails. J. Endod. 2009, 35, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Gow-Gates, G.A. Mandibular conduction anesthesia: A new technique using extraoral landmarks. Oral Surg. Oral Med. Oral Pathol. 1973, 36, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Akinosi, J.O. A new approach to the mandibular nerve block. Br. J. Oral Surg. 1977, 15, 83–87. [Google Scholar] [CrossRef]
- Click, V.; Drum, M.; Reader, A.; Nusstein, J.; Beck, M. Evaluation of the Gow-Gates and Vazirani-Akinosi techniques in patients with symptomatic irreversible pulpitis: A prospective randomized study. J. Endod. 2015, 41, 16–21. [Google Scholar] [CrossRef]
- Goldberg, S.; Reader, A.; Drum, M.; Nusstein, J.; Beck, M. Comparison of the anesthetic efficacy of the conventional inferior alveolar, Gow-Gates, and Vazirani-Akinosi techniques. J. Endod. 2008, 34, 1306–1311. [Google Scholar] [CrossRef]
- Masselink, B.H. The advent of painless dentistry. Dent. Cosm. 1910, 52, 868–872. [Google Scholar]
- Lilienthal, B. A clinical appraisal of intraosseous dental anesthesia. Oral Surg. Oral Med. Oral Pathol. 1975, 39, 692–697. [Google Scholar] [CrossRef]
- Parente, S.A.; Anderson, R.W.; Herman, W.W.; Kimbrough, W.F.; Weller, R.N. Anesthetic efficacy of the supplemental intraosseous injection for teeth with irreversible pulpitis. J. Endod. 1998, 24, 826–828. [Google Scholar] [CrossRef]
- Reitz, J.; Reader, A.; Nist, R.; Beck, M.; Meyers, W.J. Anesthetic efficacy of the intraosseous injection of 0.9 mL of 2% lidocaine (1:100,000 epinephrine) to augment an inferior alveolar nerve block. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 1998, 86, 516–523. [Google Scholar] [CrossRef]
- Gaudin, A.; Clouet, R.; Boëffard, C.; Laham, A.; Martin, H.; Amador Del Valle, G.; Enkel, B.; Prud’homme, T. Comparing intraosseous computerized anaesthesia with inferior alveolar nerve block in the treatment of symptomatic irreversible pulpitis: A randomized controlled trial. Int. Endod. J. 2023, 56, 922–931. [Google Scholar] [CrossRef]
- Simeonova, E.; Tsanova, S.; Zagorchev, P. Effectiveness of primary intraosseous anesthesia in the endodontic treatment of mandibular molars with irreversible pulpitis. J. IMAB 2020, 26, 3063–3067. [Google Scholar] [CrossRef]
- Farhad, A.; Razavian, H.; Shafiee, M. Effect of intraosseous injection versus inferior alveolar nerve block as primary pulpal anaesthesia of mandibular posterior teeth with symptomatic irreversible pulpitis: A prospective randomized clinical trial. Acta Odontol. Scand. 2018, 76, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Demir, E.; Ataoglu, H. Clinical evaluation of efficacy of transcortical anesthesia for the extraction of impacted mandibular third molars: A randomized controlled trial. J. Dent. Anesth. Pain Med. 2020, 20, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.D.; Carrico, C.K.; Bermanis, I.; Rehil, S. Intraosseous anesthesia using dynamic navigation technology. J. Endod. 2020, 46, 1894–1900. [Google Scholar] [CrossRef]
- Sovatdy, S.; Vorakulpipat, C.; Kiattavorncharoen, S.; Saengsirinavin, C.; Wongsirichat, N. Inferior alveolar nerve block by intraosseous injection with Quicksleeper® at the retromolar area in mandibular third molar surgery. J. Dent. Anesth. Pain Med. 2018, 18, 339–347. [Google Scholar] [CrossRef]
- Vatankhah, M.; Dianat, O.; Zargar, N.; Bejestani, S.G.; Baghban, A.A.; Shojaeian, S. Efficacy of QuickSleeper Intraosseous Injection of 4% Articaine in Mandibular First Molars With Symptomatic Irreversible Pulpitis: A Randomized Controlled Trial. Anesth. Prog. 2024, 71, 123–130. [Google Scholar] [CrossRef]
- Pereira, L.A.; Groppo, F.C.; Bergamaschi Cde, C.; Meechan, J.G.; Ramacciato, J.C.; Motta, R.H.; Ranali, J. Articaine (4%) with epinephrine (1:100,000 or 1:200,000) in intraosseous injections in symptomatic irreversible pulpitis of mandibular molars: Anesthetic efficacy and cardiovascular effects. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, e85–e91. [Google Scholar] [CrossRef]
- Guglielmo, A.; Reader, A.; Nist, R.; Beck, M.; Weaver, J. Anesthetic efficacy and heart rate effects of the supplemental intraosseous injection of 2% mepivacaine with 1:20,000 levonordefrin. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1999, 87, 284–293. [Google Scholar] [CrossRef]
- Replogle, K.; Reader, A.; Nist, R.; Beck, M.; Weaver, J.; Meyers, W.J. Cardiovascular effects of intraosseous injections of 2 percent lidocaine with 1:100,000 epinephrine and 3 percent mepivacaine. J. Am. Dent. Assoc. 1999, 130, 649–657. [Google Scholar] [CrossRef]
- Nusstein, J.; Kennedy, S.; Reader, A.; Beck, M.; Weaver, J. Anesthetic efficacy of the supplemental X-tip intraosseous injection in patients with irreversible pulpitis. J. Endod. 2003, 29, 724–728. [Google Scholar] [CrossRef]
- Pol, R.; Ruggiero, T.; Bezzi, M.; Camisassa, D.; Carossa, S. Programmed-release intraosseous anesthesia as an alternative to lower alveolar nerve block in lower third molar extraction: A randomized clinical trial. J. Dent. Anesth. Pain. Med. 2022, 22, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Saber, S.; Hashem, A.A.; Khalil, D.M.; Pirani, C.; Ordinola-Zapata, R. Efficacy of four local anaesthesia protocols for mandibular first molars with symptomatic irreversible pulpitis: A randomized clinical trial. Int. Endod. J. 2021, 55, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Reitz, J.; Reader, A.; Nist, R.; Beck, M.; Meyers, W.J. Anesthetic efficacy of a repeated intraosseous injection given 30 min following an inferior alveolar nerve block/intraosseous injection. Anesth. Prog. 1998, 45, 143–149. [Google Scholar]
- Gallatin, J.; Reader, A.; Nusstein, J.; Beck, M.; Weaver, J. A comparison of two intraosseous anesthetic techniques in mandibular posterior teeth. J. Am. Dent. Assoc. 2003, 134, 1476–1484. [Google Scholar] [CrossRef]
- Coggins, R.; Reader, A.; Nist, R.; Beck, M.; Meyers, W.J. Anesthetic efficacy of the intraosseous injection in maxillary and mandibular teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1996, 81, 634–641. [Google Scholar] [CrossRef]
- Woodmansey, K.F.; White, R.K.; He, J. Osteonecrosis related to intraosseous anesthesia: Report of a case. J. Endod. 2009, 35, 288–291. [Google Scholar] [CrossRef]
- Bangerter, C.; Mines, P.; Sweet, M. The use of intraosseous anesthesia among endodontists: Results of a questionnaire. J. Endod. 2009, 35, 15–18. [Google Scholar] [CrossRef]
- Heft, M.W.; Parker, S.R. An experimental basis for revising the graphic rating scale for pain. Pain 1984, 19, 153–161. [Google Scholar] [CrossRef]
- Dental Hi Tec. Available online: https://www.dentalhitec.com (accessed on 5 May 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Bigby, J.; Reader, A.; Nusstein, J.; Beck, M.; Weaver, J. Articaine for supplemental intraosseous anesthesia in patients with irreversible pulpitis. J. Endod. 2006, 32, 1044–1047. [Google Scholar] [CrossRef]
- Bhuyan, A.C.; Latha, S.S.; Jain, S.; Kataki, R. Anesthetic efficacy of the supplemental X-tip intraosseous injection using 4% articaine with 1:100,000 adrenaline in patients with irreversible pulpitis: An in vivo study. J. Conserv. Dent. 2014, 17, 522–525. [Google Scholar] [CrossRef]
- Idris, M.; Sakkir, N.; Naik, K.G.; Jayaram, N.K. Intraosseous injection as an adjunct to conventional local anesthetic techniques: A clinical study. J. Conserv. Dent. 2014, 17, 432–435. [Google Scholar] [PubMed]
- Replogle, K.; Reader, A.; Nist, R.; Beck, M.; Weaver, J.; Meyers, W.J. Anesthetic efficacy of the intraosseous injection of 2% lidocaine (1:100,000 epinephrine) and 3% mepivacaine in mandibular first molars. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 83, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, K.M.; Keiser, K. Local anesthetic failure in endodontics: Mechanisms and management. Endod. Top. 2002, 1, 26–39. [Google Scholar] [CrossRef]
- Puleio, F.; Tosco, V.; Monterubbianesi, R.; Pirri, R.; Alibrandi, A.; Pulvirenti, D.; Simeone, M. Comparison of Four Ni-Ti Rotary Systems: Dental Students’ Perceptions in a Multi-Center Simulated Study. Dent. J. 2025, 13, 97. [Google Scholar] [CrossRef]
- Jensen, J.; Nusstein, J.; Drum, M.; Reader, A.; Beck, M. Anesthetic efficacy of a repeated intraosseous injection following a primary intraosseous injection. J. Endod. 2008, 34, 126–130. [Google Scholar] [CrossRef]
| Variable | Category | Resident (n = 37) | Expert (n = 35) | p-Value |
|---|---|---|---|---|
| Sex | Female | 21 (56.8%) | 14 (40.0%) | 0.236 |
| Male | 16 (43.2%) | 21 (60.0%) | ||
| Tooth Type | Mandibular 1st molar | 29 (78.4%) | 16 (45.7%) | 0.009 |
| Mandibular 2nd molar | 8 (21.6%) | 19 (54.3%) | ||
| Pulpal Diagnosis | Irreversible Pulpitis | 12 (32.4%) | 8 (22.9%) | 0.014 |
| Necrosis | 7 (18.9%) | 18 (51.4%) | ||
| Previously treated/initiated | 18 (48.6%) | 9 (25.7%) | ||
| Preoperative Pain | None | 27 (73.0%) | 20 (69.0%) | 0.938 |
| Mild | 7 (18.9%) | 7 (24.1%) | ||
| Moderate | 2 (5.4%) | 1 (3.4%) | ||
| Severe | 1 (2.7%) | 1 (3.8%) | ||
| Location of IOA | Distal | 32 (86.5%) | 21 (63.6%) | 0.045 |
| Mesial | 5 (13.5%) | 9 (27.3%) | ||
| Mesial + Distal | 0 (0.0%) | 3 (9.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alali, A.I.; Lopez Gross, J.; Al-Sumaidaee, M.A.; Wong, M.; Feng, S.X.; Cherkas, P.S.; Basrani, B.R. Impact of Clinician Experience on Intraosseous Anesthesia Outcomes in Endodontic Treatment: Retrospective Cohort Study. Dent. J. 2025, 13, 238. https://doi.org/10.3390/dj13060238
Alali AI, Lopez Gross J, Al-Sumaidaee MA, Wong M, Feng SX, Cherkas PS, Basrani BR. Impact of Clinician Experience on Intraosseous Anesthesia Outcomes in Endodontic Treatment: Retrospective Cohort Study. Dentistry Journal. 2025; 13(6):238. https://doi.org/10.3390/dj13060238
Chicago/Turabian StyleAlali, Ahmed I., Jacqueline Lopez Gross, Mustafa Ayad Al-Sumaidaee, Michelle Wong, Sean Xinyang Feng, Pavel S. Cherkas, and Bettina R. Basrani. 2025. "Impact of Clinician Experience on Intraosseous Anesthesia Outcomes in Endodontic Treatment: Retrospective Cohort Study" Dentistry Journal 13, no. 6: 238. https://doi.org/10.3390/dj13060238
APA StyleAlali, A. I., Lopez Gross, J., Al-Sumaidaee, M. A., Wong, M., Feng, S. X., Cherkas, P. S., & Basrani, B. R. (2025). Impact of Clinician Experience on Intraosseous Anesthesia Outcomes in Endodontic Treatment: Retrospective Cohort Study. Dentistry Journal, 13(6), 238. https://doi.org/10.3390/dj13060238







