1. Introduction
Tooth extraction inevitably leads to dimensional changes in the alveolar ridge due to the remodeling of hard and soft tissues. Within the first 3 to 6 months, horizontal ridge width may decrease by 2.6–4.6 mm and vertical height by 0.4–3.9 mm, with up to 60% of the original volume lost in the first year [
1,
2]. These changes can compromise future implant placement, esthetic outcomes, or orthodontic planning.
While conventional socket preservation techniques using bone grafts or membranes are effective, they often involve additional cost, foreign materials, and extended healing times [
3]. Platelet-rich fibrin (PRF), a second-generation autologous platelet concentrate, has emerged as a promising alternative due to its ability to promote soft and hard tissue regeneration without additives or anticoagulants [
4,
5]. PRF provides a fibrin scaffold rich in leukocytes and platelets, supporting angiogenesis and osteogenesis through the gradual release of growth factors such as PDGF and TGF-β [
6,
7,
8].
Several studies have suggested that PRF may reduce vertical ridge resorption and improve bone healing compared to natural healing [
9,
10]. However, the evidence regarding its impact on horizontal dimensional changes and bone tissue quality remains limited and somewhat inconsistent [
11]. Moreover, most clinical trials have focused on implant-related extractions in older patients, whereas few studies have evaluated PRF in young adults undergoing premolar extractions for orthodontic purposes—a scenario that represents potentially optimal healing conditions.
In this context, the present study aimed to assess the potential clinical utility of PRF for alveolar ridge preservation, using high-resolution cone beam computed tomography (CBCT). This imaging modality allows for precise three-dimensional measurements of ridge dimensions and assessment of bone density through Hounsfield Units (HU), providing a non-invasive method to monitor regenerative outcomes [
12].
Recent literature has explored the application of PRF in periodontal and oral surgical procedures. Silva et al. conducted a systematic review and meta-analysis supporting its use in periodontal surgery, highlighting its ability to enhance healing and reduce alveolar bone loss [
13]. Pradeep et al. compared PRF and PRP in periodontal regeneration and found that PRF offered superior outcomes in both clinical and radiographic parameters [
14]. Borie et al. provided an overview of PRF applications across dentistry, including oral surgery and implantology, noting its low cost, ease of preparation, and biocompatibility [
15]. Furthermore, Del Corso et al. discussed its role in bone grafting and implant procedures, emphasizing its capacity to support bone regeneration through sustained release of growth factors and structural integration with host tissues [
16].
Therefore, the rationale for this study is to evaluate the potential role of PRF in preserving alveolar ridge dimensions post-extraction, focusing primarily on vertical height preservation, with secondary assessment of horizontal width and descriptive analysis of bone density changes using CBCT.
Objectives
Primary Objective:
To evaluate the radiographic effectiveness of platelet-rich fibrin (PRF) in preserving alveolar ridge height following premolar extractions for orthodontic indications.
Secondary Objectives:
To descriptively compare alveolar width changes (measured at 1 mm, 3 mm, and 5 mm from the crest) between PRF-treated and control sockets, acknowledging the lack of statistically significant differences.
To descriptively assess bone tissue density at the extraction sites based on Hounsfield Unit (HU) values obtained from CBCT imaging, without formal statistical comparison.
2. Materials and Methods
2.1. Trial Design
2.1.1. Study Design
This study was designed as a single-blind, two-arm, parallel-group randomized controlled clinical trial with a 1:1 allocation ratio. The objective was to evaluate, through cone beam computed tomography (CBCT), the effectiveness of platelet-rich fibrin (PRF) in the preservation of alveolar ridge dimensions following premolar extractions indicated for orthodontic purposes. A total of 36 alveoli were included, allocated to an experimental group (
n = 19) treated with PRF and a control group (
n = 17) left to heal physiologically. The trial was conducted in accordance with the ethical principles of the Declaration of Helsinki and adhered to the Consolidated Standards of Reporting Trials (CONSORT) statement for reporting health research [
17]. Prior to participation, all subjects were thoroughly informed about the study protocol, including potential risks and benefits, and provided written informed consent. Ethical approval was obtained from the Bioethics Committee of the Catholic University of Cuenca under protocol reference number CEISH-UCACUE-031. The study was prospectively registered on ClinicalTrials.gov (NCT: NCT06900036), available online:
https://clinicaltrials.gov/study/NCT06900036 (accessed on 14 May 2025).
2.1.2. Study Population
Inclusion Criteria:
Participants included systemically healthy individuals aged 17–38 years who required bilateral premolar extractions for orthodontic reasons. Additional inclusion criteria involved candidates for orthognathic surgery or third molar extraction with proximity to critical structures, justifying the use of CBCT.
Exclusion Criteria:
Participants with systemic diseases, immunosuppression, smoking, pregnancy or lactation, low platelet count (<200,000/mm3), or use of medications interfering with healing were excluded.
2.2. Sample Size and Randomization
Sample size was calculated using G*Power software (version 3.1.9.4, Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany) based on repeated measures ANOVA with an effect size of 0.25, an alpha level of 0.05, and a power of 95%, resulting in a required total of 36 alveoli (17 control, 19 PRF). No interim analyses or stopping rules were applied.
Randomization was executed using Epidat software Statistical analyses were also performed using Epidat software, version 4.2 (Dirección Xeral de Saúde Pública, Xunta de Galicia, Santiago de Compostela, Spain), generating a simple random sequence without blocking or stratification. The allocation list was concealed until patient consent and surgical planning were completed. A biostatistician independent of the clinical team generated the random sequence. Participants were enrolled by the lead investigator, and allocation to intervention was performed by a separate clinician according to the concealed list.
This was a single-blind study where the radiographic evaluator, a maxillofacial imaging specialist, was blinded to group assignment. Due to the nature of the intervention, neither patients nor surgeons were blinded. However, the use of contralateral sites within the same patient reduced inter-individual variability and strengthened internal validity.
2.3. Surgical Procedure
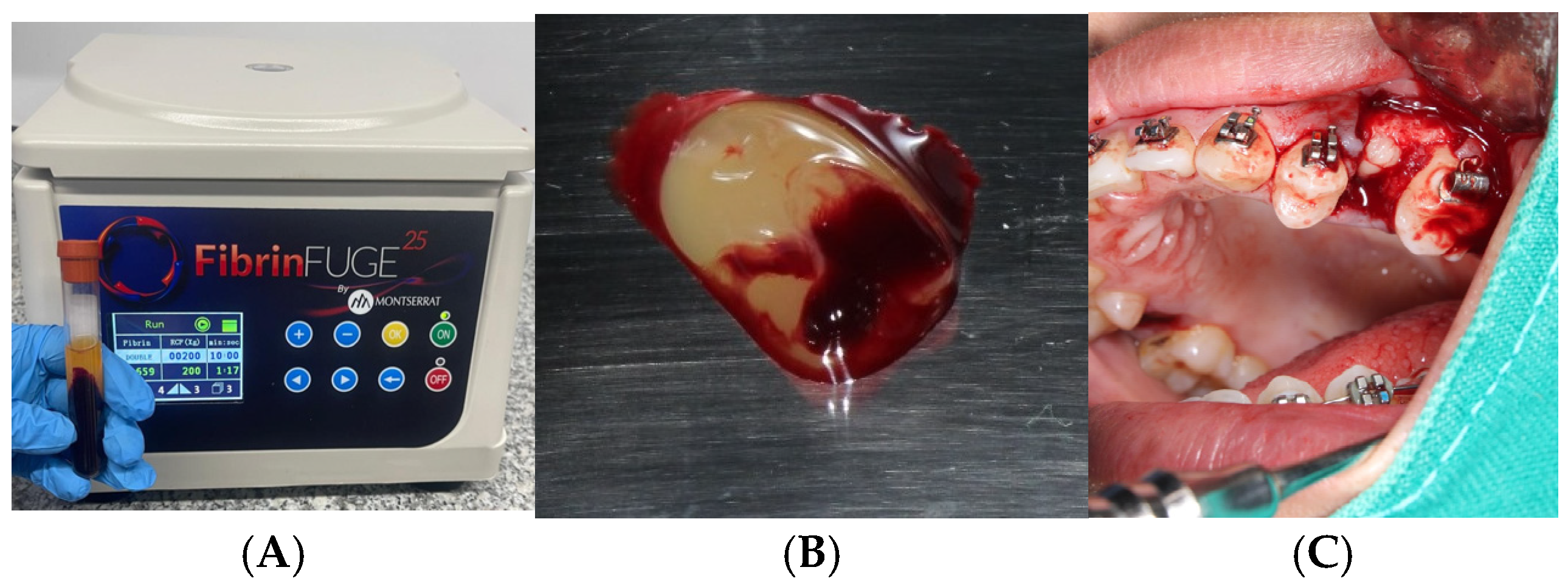
Experimental Group (PRF):
Following venipuncture and centrifugation using a PRF Duo system (Nice BD Vacutainer® Push Button Blood Collection Set, 23G x ¾″, 12″ tubing with Luer adapter (REF: 367342), Becton, Dickinson and Company, Franklin Lakes, NJ, USA), autologous platelet-rich fibrin (PRF) clots were obtained and applied to the extraction sockets. One PRF clot was used to fill the socket, while the second was flattened to serve as a guided tissue regeneration membrane. Both clots were stabilized with 3-0 catgut sutures.
Control Group:
Sockets were allowed to heal physiologically without the application of PRF or any additional biomaterial.
All surgical interventions were performed under local anesthesia using atraumatic extraction techniques. Test and control sites were treated simultaneously during the same surgical phase in each patient to minimize procedural variability.
PRF Preparation
PRF preparation was performed according to the protocol developed and patented by Choukroun et al. [
4], utilizing the PRF Duo centrifuge system and its corresponding surgical kit. No biochemical manipulation of the blood was performed.
For each alveolus to be treated with PRF, 10 mL of venous blood was collected from the antecubital vein into two sterile, additive-free glass tubes (10 mL each). Immediately after collection, the tubes were centrifuged at 2700 rpm for 12 min. The tubes were then left undisturbed for 5 min. The PRF clot located between the red blood cell layer and the acellular plasma was extracted.
One PRF clot was used to fill the extraction socket, and the second was compressed using the surgical box (Zimmer Biomet Dental, Palm Beach Gardens, FA, USA) to create a membrane for guided tissue regeneration. The fibrin matrix was gently inserted into the socket, and the membrane was positioned over the alveolar crest. Both were stabilized with 3-0 catgut sutures using a simple interrupted technique to ensure stability during early healing.
2.4. Surgical Protocol
Both experimental and control sites were treated in the same surgical session.
After achieving local anesthesia, an intrasulcular incision was made using a No. 15 scalpel (Bard-Parker®, Aspen Surgical Products, 6945 Southbelt Dr SE, Caledonia, MI 49316, USA) blade. Maxillary or mandibular premolars were extracted atraumatically according to orthodontic indications.
At control sites, extraction sockets were allowed to heal physiologically without intervention. At experimental sites, PRF clots were partially distributed within the socket, and the PRF membrane was placed over the socket opening as a barrier to promote guided tissue regeneration. Simple interrupted sutures were placed to secure the materials and promote primary closure.
The only difference between the surgical procedures at the test and control sites was the use of PRF in the experimental group.
All surgical interventions and clinical measurements were performed by a multidisciplinary team comprising a maxillofacial surgeon, periodontists, and a maxillofacial imaging specialist, following the standardized study protocol.
2.4.1. Postoperative Care
Sutures were removed 7 days after surgery. Clinical follow-up examinations were conducted at 3, 7, and 14 days postoperatively. No complications or adverse events were recorded during the follow-up period.
2.4.2. Postoperative Radiographic Evaluation
Immediately following tooth extractions, baseline CBCT scans were performed using the Axeos CBCT system (Dentsply Sirona, Bensheim, Germany). imaging equipment. Follow-up CBCTs were acquired at 30 and 120 days post-extraction, maintaining identical technical and radiation protection parameters for all imaging sessions.
All scans were obtained using a field of view (FOV) of 8 × 8 cm and a voxel size of 0.2 mm. Exposure parameters were set at 16 s, 30.89 mAs, and 120 kVp.
Image reconstruction, visualization, and measurements were performed using Sidexis 4.0 software. All analyses were conducted by a board-certified maxillofacial radiologist who was blinded to group allocation.
2.5. Measurement Techniques
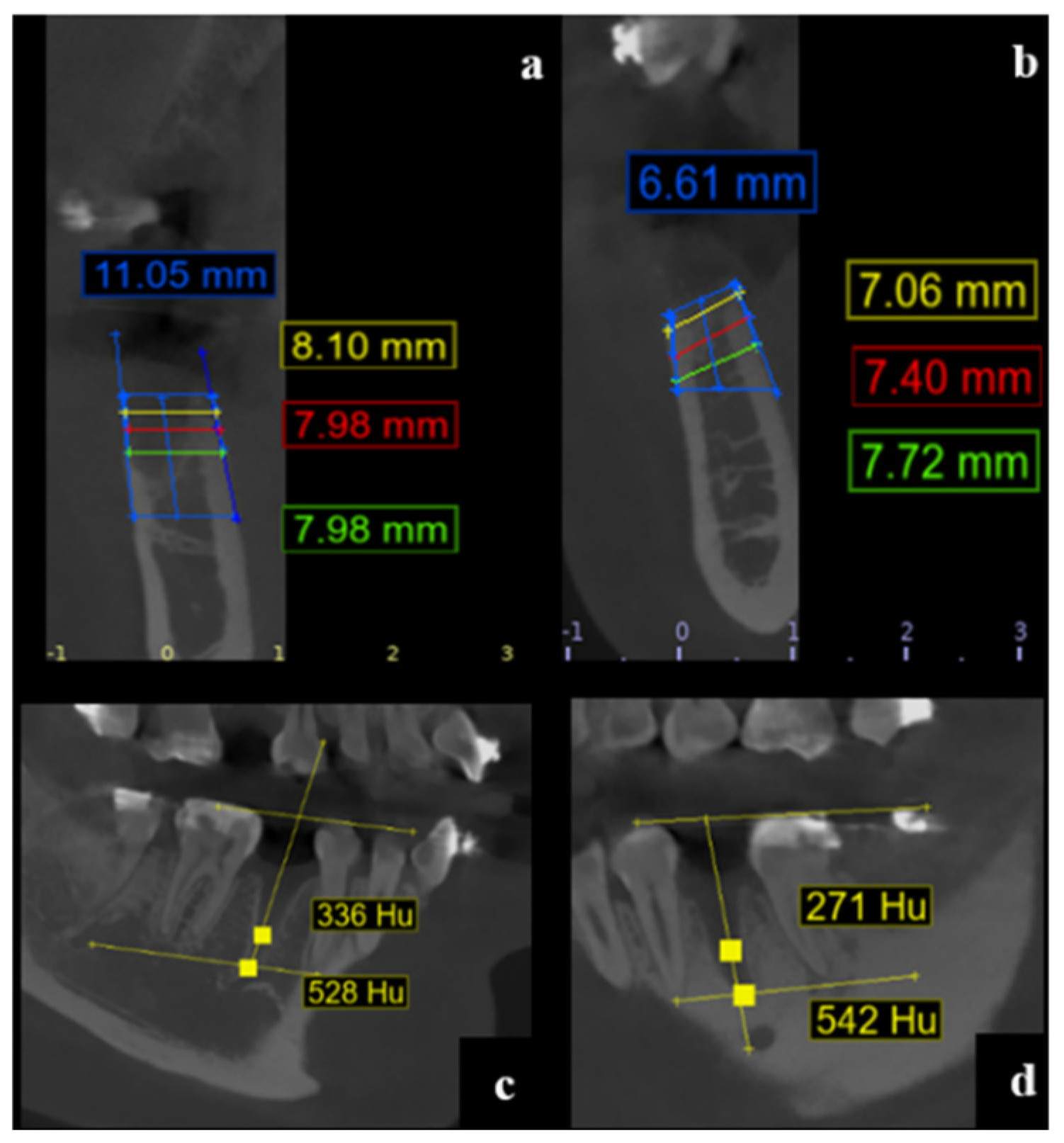
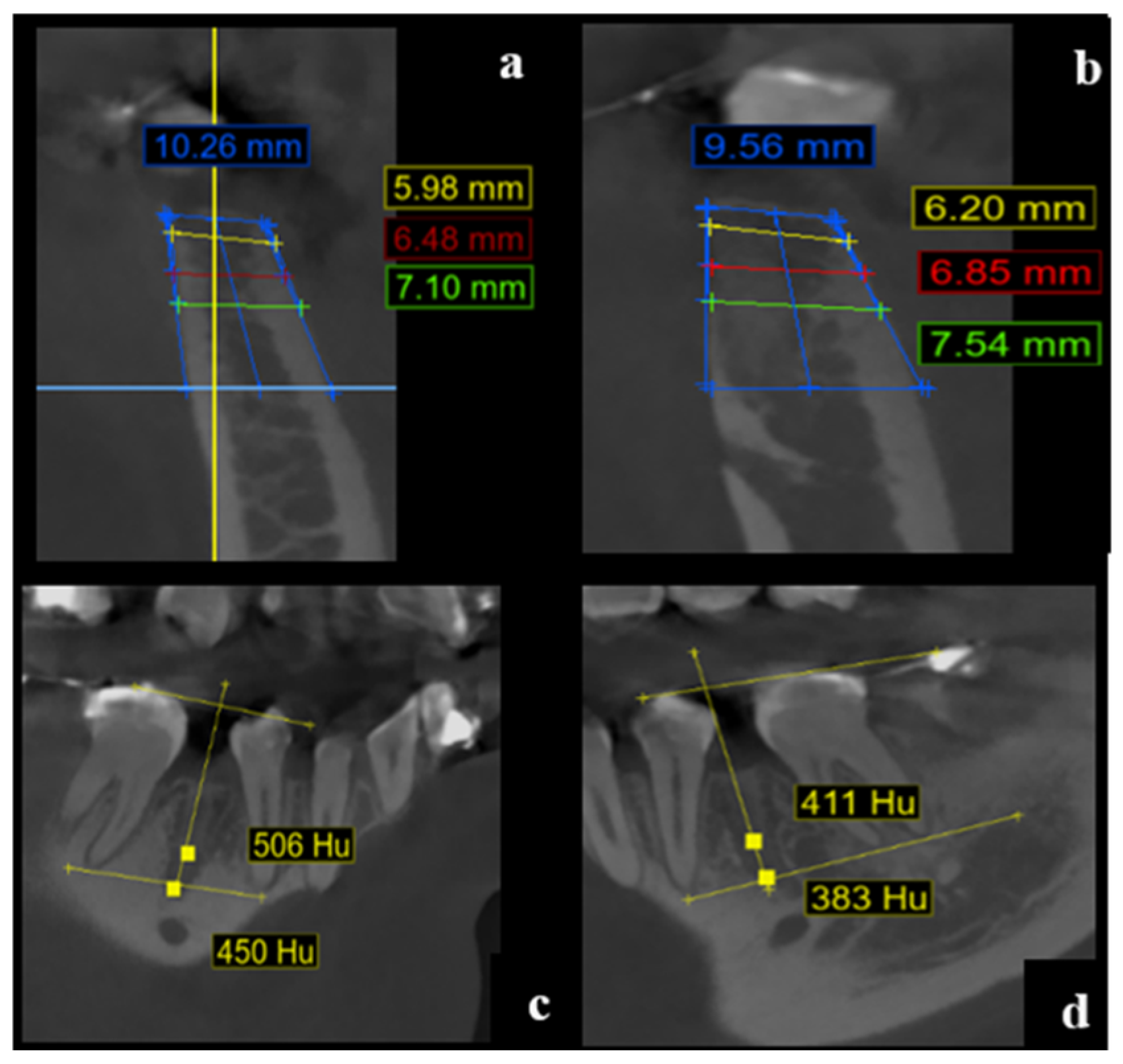
Alveolar Ridge Dimensions
Following the method proposed by [
18], measurements of alveolar dimensions were performed in both vertical and horizontal planes:
Depth Measurements (Vestibular–Palatal/Lingual):
The alveolar width was measured at three standardized depths: 1 mm, 3 mm, and 5 mm apical to the highest point of the vestibular cortical plate. Measurements were taken perpendicular to a vertical reference line drawn centrally within the alveolus from the apex to the alveolar crest, according to anatomical orientation (maxilla or mandible) (
Figure 1).
Width Measurement Landmarks:
Vestibular and palatal/lingual cortical plates were identified at each reference point (RW-1, RW-3, RW-5) for both baseline and follow-up CBCT images (
Figure 2).
Alveolar Height
To determine alveolar height, the following procedure was performed:
A horizontal line (B1) was drawn at the base of the alveolus (bottom cortical limit).
Two additional lines, parallel to the vestibular (BH) and palatal/lingual (LH) cortical plates, were delineated.
The vertical distance between these lines was measured to assess alveolar process height at baseline and follow-up.
Measurements were performed consistently at baseline, 30 days, and 120 days post-extraction.
Bone Tissue Density Assessment
The quality of the newly formed bone tissue was assessed using the gray value scale corresponding to Hounsfield Units (HU), a CBCT-derived measurement method. Although CBCT-based HU values have limitations compared to CT, they provide a descriptive estimation of bone mineralization.
Bone density was evaluated following the protocol adapted from [
15]:
Reference Lines
Line B1 was drawn between the apices of adjacent teeth. Line L1 was traced between the cusps of the same adjacent teeth.
Measurement Points
Point A was identified at the bottom of the alveolus.
Point B was located 4 mm apical to B1 along a vertical midline between B1 and L1.
HU values were recorded at 30 and 120 days post-extraction using the same technical acquisition parameters.
All measurements (height, depth at 1, 3, and 5 mm, and bone density in HU) were systematically registered in a standardized Excel 2018 database for statistical analysis.
2.6. Outcomes
The outcomes of this randomized controlled trial were assessed through a detailed radiographic analysis using cone beam computed tomography (CBCT), allowing precise three-dimensional evaluation of post-extraction alveolar changes in terms of dimensional stability and descriptive bone density parameters.
Primary Outcome:
The primary outcome was alveolar height preservation, defined as the cephalocaudal distance from the most apical point of the extraction socket to the crestal margin of the alveolar bone. This measurement reflects vertical bone loss or preservation following extraction.
Alveolar height was measured on cross-sectional CBCT views obtained immediately after extraction (baseline), and at 30 and 120 days postoperatively, using standardized anatomical landmarks and consistent imaging parameters (FOV 8 × 8 cm, voxel size 0.2 mm, 120 kVp, 30.89 mAs, exposure time 16 s).
Measurements were performed using Sidexis 4.0 software to ensure reproducibility and accuracy.
Secondary Outcomes:
Two secondary parameters were evaluated:
Alveolar Width:
Vestibulo-palatal (or vestibulo-lingual) width was assessed at three standardized depths: 1 mm, 3 mm, and 5 mm apical to the alveolar crest. Measurements were taken perpendicular to the long axis of the alveolus, from the buccal to the palatal or lingual cortical walls.
These measurements aimed to capture horizontal dimensional changes occurring progressively deeper within the socket, which are relevant for future implant site development.
Bone Tissue Density (Descriptive Analysis):
Bone density within the healing socket was descriptively evaluated through CBCT-derived Hounsfield Unit (HU) values, recognizing the limitations of CBCT for absolute mineral content quantification.
HU measurements were taken at standardized intra-alveolar locations based on anatomical references (e.g., lines B1 and L1), following validated radiographic protocols [
15]. Evaluations were conducted at 30 and 120 days post-extraction to monitor radiodensity changes over time, serving as an indirect indicator of bone maturation trends.
No formal statistical comparisons of HU values between groups were performed.
All outcome measurements were conducted by a calibrated, blinded maxillofacial radiologist using the same imaging equipment and software to minimize inter-observer and technical variability.
Statistical Analysis
All statistical analyses were performed using JASP software (version 0.18.1.0), a validated open-source platform designed for robust comparison of clinical datasets in biomedical research.
Descriptive statistics, including means and standard deviations (SD), were calculated for continuous variables to characterize baseline demographics and outcome measures. These descriptive metrics provided an overview of data distribution, central tendency, and variability.
Comparisons between the PRF and control groups at each time point (30 and 120 days) were conducted using independent-samples t-tests. This parametric test evaluates whether the means of two independent groups differ significantly, and was applied to outcome variables such as alveolar height, alveolar width at multiple depths, and bone tissue density (Hounsfield Units, HU).
Within-group changes over time (baseline vs. 30 days, and baseline vs. 120 days) were assessed using paired-samples t-tests to determine significant differences in each group independently.
To explore the interaction between time and treatment group, a repeated measures analysis of variance (ANOVA RM) was performed. This model assesses whether patterns of change over time differ significantly between groups, incorporating time × group interaction effects to capture the dynamics of regenerative behavior associated with PRF.
Data normality was verified using the Shapiro–Wilk test prior to the application of parametric tests. When assumptions of normality were violated, non-parametric alternatives were considered where appropriate.
The threshold for statistical significance was set at p < 0.05. All p-values reported are two-tailed.
No subgroup analyses (e.g., stratification by sex or jaw location) or multivariate adjustments were performed, given the small sample size and the homogeneity of the study sample with respect to clinical indication and procedural technique.
3. Results
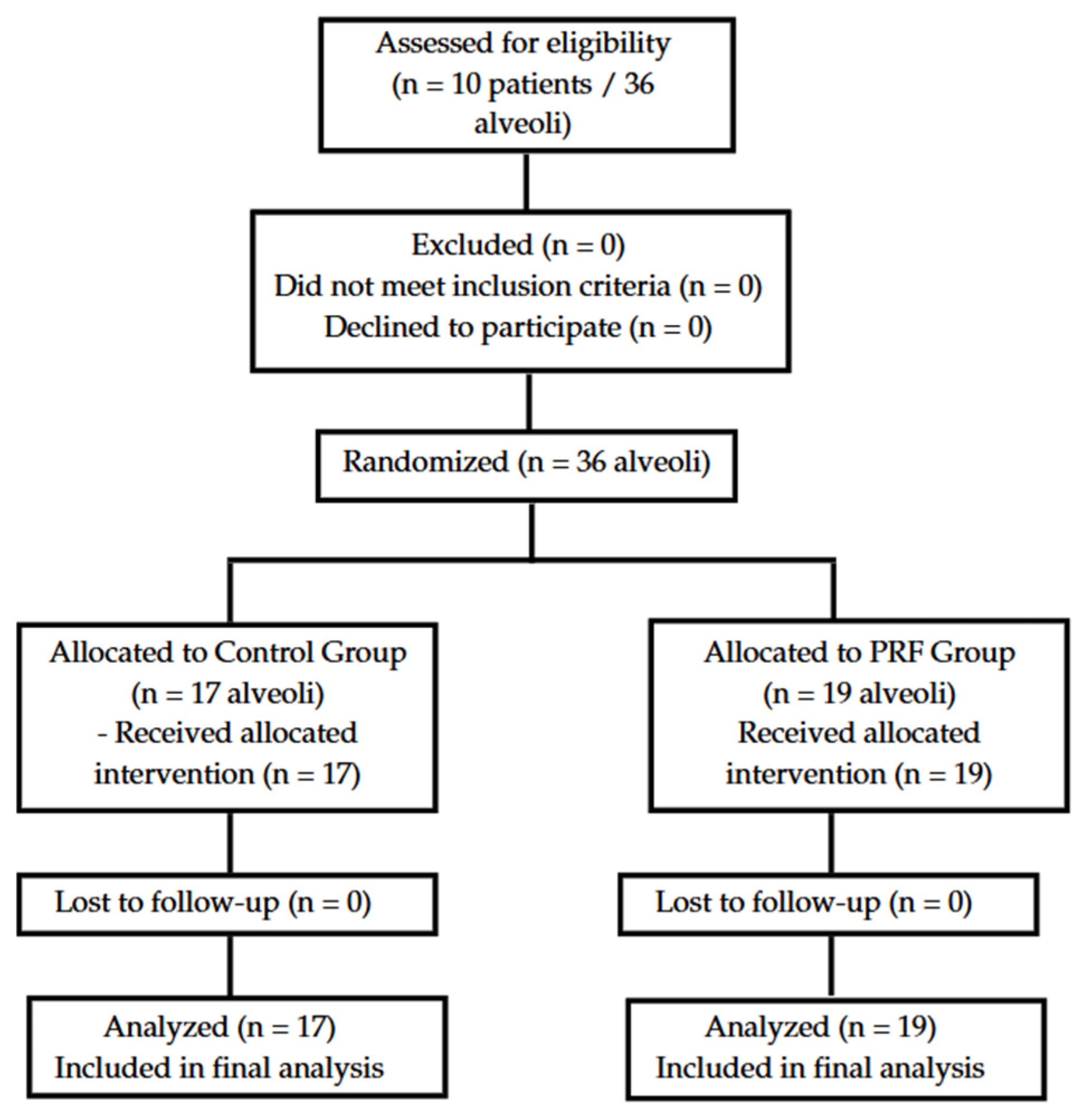
3.1. CONSORT Flow Diagram
A total of 10 systemically healthy patients were assessed for eligibility between January and May 2024. All participants met the inclusion criteria and were randomized, resulting in 36 alveoli: 17 allocated to the control group (Group I) and 19 to the PRF group (Group II). All participants received the assigned intervention, completed follow-up, and were included in the final analysis. No losses or exclusions occurred post-randomization. The CONSORT flow diagram illustrating patient progression is shown in
Figure 1.
3.2. Baseline Characteristics
The initial mean cephalocaudal alveolar height was comparable between groups:
There were no statistically significant differences (
t = −0.853;
p = 0.399), confirming baseline homogeneity between groups. Complete demographic and clinical characteristics are presented in
Table 1.
3.3. Primary Outcome–Alveolar Height
At 30 days post-extraction:
Group II (PRF) showed a significantly higher mean alveolar height (10.61 mm, SD = 0.85) compared to Group I (8.82 mm, SD = 1.68).
The mean difference was 1.79 mm (p < 0.001; 95% CI: −2.674 to −0.901).
These outcomes are illustrated in
Figure 3.
At 120 days post-extraction:
Intragroup analysis revealed:
Group I showed a marked decrease in height from baseline to 30 days, with partial recovery at 120 days.
Group II exhibited minimal variation across time points, indicating superior dimensional stability.
3.4. Secondary Outcomes–Alveolar Width and Bone Quality
Alveolar width measurements at 1 mm, 3 mm, and 5 mm depths showed no statistically significant differences between groups at either 30 or 120 days (p > 0.05).
At 120 days:
1 mm depth: Group II = 8.06 mm (SD = 1.78); Group I = 8.40 mm (SD = 1.92); p = 0.583
3 mm depth: Group II = 8.45 mm (SD = 1.67); Group I = 9.39 mm (SD = 1.69); p = 0.101
5 mm depth: Group II = 9.04 mm (SD = 1.65); Group I = 9.99 mm (SD = 1.93); p = 0.122
Bone tissue quality, descriptively assessed by Hounsfield Units (HU), ranged from 190 to 282 HU in the PRF group, suggesting denser bone compared to the control group.
However, no formal statistical comparison was performed for HU values.
Full alveolar width and bone quality data are provided in
Table 1.
3.5. Statistical and Additional Analyses
Repeated measures ANOVA revealed:
- ▪
A significant time × group interaction for alveolar height (p = 0.010; η2 = 0.046).
- ▪
A similarly significant interaction at 1 mm depth (p = 0.035; η2 = 0.020).
- ▪
These results are detailed in
Table 2
Intergroup analysis indicated:
- ▪
Group assignment explained 12.5% of the variance in alveolar height (
p = 0.004), detailed in
Table 3.
Post hoc tests showed:
- ▪
Significant reduction in alveolar height in Group I between baseline and 30 days (−0.630 mm; p = 0.005).
- ▪
Significant reduction in alveolar width at 3 mm (0.800 mm; p = 0.027) and 5 mm (p = 0.002) depths for Group I.
- ▪
Group II exhibited a significant decrease in width at 1 mm (−0.968 mm;
p = 0.004) over time. These results are detailed in
Table 4.
3.6. Adverse Events
No complications, adverse effects, or unexpected events were reported during the follow-up period. All patients healed uneventfully.
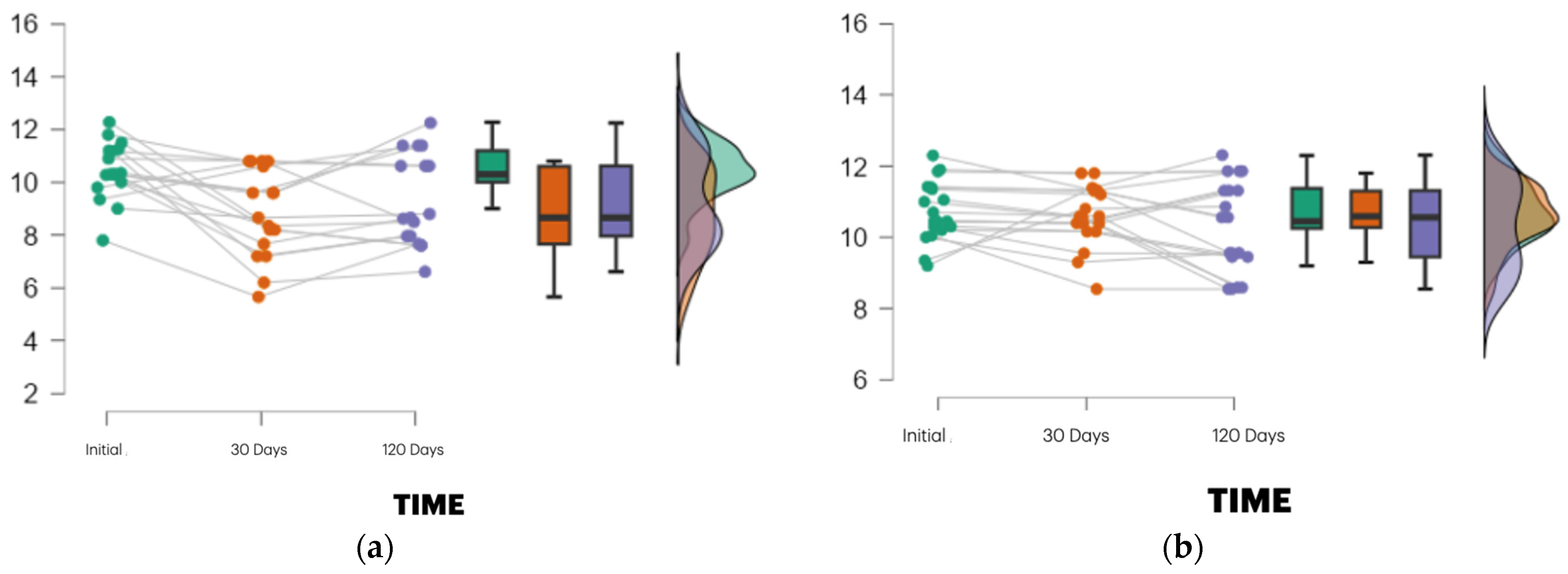
Each panel shows individual patient trajectories (grey lines) along with box plots and vio-lin plots at baseline, 30 days, and 120 days post-extraction. Alveolar height was measured using CBCT based on standardized anatomical landmarks. The PRF group demonstrated a higher median height and reduced variability, particularly at 30 days, compared to the control group, which showed a more pronounced height reduction followed by partial re-covery. The violin plots depict the data density and distribution. These results align with the significant time × group interaction found in the repeated measures ANOVA (p = 0.010; η2 = 0.046), suggesting enhanced early dimensional stability with PRF.
4. Discussion
This randomized controlled clinical trial aimed to evaluate the impact platelet-rich fibrin (PRF) on alveolar preservation following orthodontic premolar extractions. The findings reinforce the concept that PRF can serve as a biologically active scaffold that promotes early tissue regeneration and bone healing, particularly within the first four to six weeks post-extraction. At 30 days, the PRF group demonstrated a statistically significant improvement in vertical bone height preservation (Δ = 0.92 mm), which aligns with previous reports suggesting that PRF plays a regenerative role by enhancing neovascularization, osteoblastic activity, and early tissue integration [
13,
14,
15,
16,
19]. This early benefit, attributed to the sustained release of growth factors such The findings reinforce the concept that PRF can serve as a biologically active scaffold that promotes early tissue regeneration and bone healing, particularly within the first four to six weeks post-extraction. At 30 days, the PRF group demonstrated a statistically significant improvement in vertical bone height preservation (Δ = 0.92 mm), which aligns with previous reports suggesting that PRF plays a regenerative role by enhancing neovascularization, osteoblastic activity, and early tissue integration as PDGF, VEGF, and TGF-β, has been corroborated by multiple experimental and clinical studies, highlighting the material’s capacity to modulate both soft and hard tissue healing [
5,
7,
8].
By day 120, although the PRF group continued to show better vertical height maintenance (Δ = 0.84 mm), the difference was no longer statistically significant. This shift suggests that PRF’s regenerative potential may be time-sensitive, with its strongest effects concentrated in the early healing window. This interpretation is supported by Wang et al. [
12], who observed that PRF’s influence diminishes over time as the extraction socket progresses into the remodeling phase. Such a pattern implies that while PRF does not prevent long-term bone resorption entirely, it may provide a protective buffer during the critical initial stages when alveolar collapse is most rapid.
Regarding horizontal ridge width, no statistically significant differences were found at 1 mm, 3 mm, or 5 mm depths; however, a consistent, albeit modest, trend favoring the PRF group was noted. These findings resonate with previous literature, including the systematic review by Castro et al. [
18] and our own earlier work [
20], both of which reported more pronounced effects of PRF in preserving vertical bone dimension and soft tissue contours rather than buccolingual ridge width. This may reflect the biological limitations of PRF in resisting mechanical collapse of thin buccal plates, particularly in sites with pre-existing alveolar fragility or fenestration.
On a biological level, the observed outcomes are mechanistically supported by PRF’s unique structure: a dense fibrin matrix interlaced with platelets, leukocytes, and cytokines that foster cellular migration, angiogenesis, and differentiation. As reported by Ghanaati et al. [
21], advanced PRF (A-PRF) and injectable PRF (i-PRF) configurations further enhance this potential by altering the cellular composition and scaffold density, suggesting a modifiable regenerative profile depending on clinical needs. Importantly, PRF differs from traditional bone grafts or membranes in that it relies solely on autologous components, eliminating the risk of immune reactions or foreign body responses while also reducing costs.
CBCT imaging provided additional insight into the regenerative process. Sites treated with PRF exhibited higher mean Hounsfield Unit (HU) values (190–282 HU), potentially reflecting greater bone density and earlier mineralization. Although these radiographic findings were not statistically analyzed due to sample constraints, they align with prior reports by Molina-Barahona et al. [
20] and Cardaropoli et al. [
22], who identified similar HU increases in PRF-treated sockets. Nevertheless, caution must be exercised when interpreting HU values, as they are influenced by multiple variables, including voxel resolution, scanner calibration, and bone density thresholds [
11]. Therefore, while CBCT offers a non-invasive proxy for bone quality, it cannot replace histologic evaluation in confirming true osseous regeneration.
From a clinical perspective, the potential benefits of PRF extend beyond quantitative bone preservation. Studies by Silva et al. [
13] and Pradeep [
14] have demonstrated that PRF contributes to improved periodontal healing and may even surpass PRP in radiographic bone fill and soft tissue closure. Borie et al. [
15] emphasize its versatility in both surgical and non-surgical settings, and Del Corso et al. [
16] highlight its application in implantology and maxillofacial bone grafting. Collectively, these findings support the idea that PRF is not merely a filler material, but a bioactive tool that can meaningfully modulate the healing cascade.
Nevertheless, this study is not without limitations. The sample size was relatively small and restricted to young, systemically healthy individuals undergoing planned orthodontic extractions, which limits generalizability to more complex clinical scenarios. Additionally, we did not stratify patients by key anatomical variables such as buccal plate thickness, socket morphology, or baseline bone quality—factors that could influence the effectiveness of PRF. Furthermore, while CBCT was useful in tracking structural changes, histological validation remains essential for accurately assessing true bone regeneration and cellular architecture. Future trials should consider incorporating histomorphometric outcomes, longer follow-up intervals (≥12 months), and diverse patient populations including medically compromised individuals or those with pre-existing alveolar defects.
In summary, PRF emerges from this study as a biologically grounded and user-friendly adjunct for early alveolar preservation. Its ease of preparation, autologous nature, and ability to enhance tissue regeneration without added biomaterials make it an appealing option in both orthodontic and surgical contexts. Although its effects appear to be most impactful during the initial healing period, this early stabilization can have downstream benefits for future implant planning or prosthetic rehabilitation. As regenerative dentistry continues to evolve, PRF holds promise not only as a supportive material but as a key component in personalized, minimally invasive treatment strategies aimed at preserving native bone architecture and optimizing long-term outcomes.