CBCT Evaluation of Maxillary Incisive Canal Characteristics Among Population in Regard to Possibility of Implant Cortical Anchorage—A Multicenter Study
Abstract
1. Introduction
2. Material and Methods
3. Results
4. Discussion
4.1. Limitations
4.2. Future Scope
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- King, E.M.; Schofield, J. Restoratively driven planning for implants in the posterior maxilla—Part 2: Implant planning, biomechanics and prosthodontic planning a proposed prosthodontic complexity index. Br. Dent. J. 2023, 235, 695–706. [Google Scholar] [CrossRef]
- Sorní, M.; Guarinos, J.; Peñarrocha, M. Implants in anatomical buttresses of the upper jaw. Med. Oral Patol. Oral Cir. Bucal. 2005, 10, 163–168. [Google Scholar]
- Krekmanov, L. Placement of posterior mandibular and maxillary implants in patients with severe bone deficiency: A clinical report of procedure. Int. J. Oral Maxillofac. Implant. 2000, 15, 722–730. [Google Scholar]
- de Oliveira Nicolau Mantovani, A.K.; de Mattias Sartori, I.A.; Azevedo-Alanis, L.R.; Tiossi, R.; Fontão, F.N.G.K. Influence of cortical bone anchorage on the primary stability of dental implants. Oral Maxillofac. Surg. 2018, 22, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Galárraga-Vinueza, M.E.; Tangl, S.; Bianchini, M.; Magini, R.; Obreja, K.; Gruber, R.; Schwarz, F. Histological characteristics of advanced peri-implantitis bone defects in humans. Int. J. Implant. Dent. 2020, 6, 12. [Google Scholar] [CrossRef]
- Durrani, F.; Nahid, R.; Pandey, S.; Singh, P.; Pandey, A. One-piece implants: Careful approach for complex rehabilitation. Natl. J. Maxillofac. Surg. 2021, 12, 266–270. [Google Scholar] [CrossRef]
- Wagner, G.; Hartung, D. One-Piece Titanium Implants: Retrospective Case Series. Case Rep. Dent. 2021, 2021, 6688355. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Skorupska, M.; Wach, T. What Does Bone Corticalization around Dental Implants Mean in Light of Ten Years of Follow-Up? J. Clin. Med. 2022, 11, 3545. [Google Scholar] [CrossRef]
- Albrektsson, T.; Chrcanovic, B.; Jacobsson, M.; Wennerberg, A. Osseointegration of implants- a biological and clinical overview. JSM Dent. Surg. 2017, 2, 1022–1028. [Google Scholar]
- Ihde, S. Indications and Treatment Modalities with Corticobasal Jaw Implants. Ann. Maxillofac. Surg. 2019, 9, 379–386. [Google Scholar] [CrossRef]
- Katranji, A.; Misch, K.; Wang, H.L. Cortical bone thickness in dentate and edentulous human cadavers. J. Periodontol. 2007, 78, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Vasiljevic, M.; Selakovic, D.; Rosic, G.; Stevanovic, M.; Milanovic, J.; Arnaut, A.; Milanovic, P. Anatomical Factors of the Anterior and Posterior Maxilla Affecting Immediate Implant Placement Based on Cone Beam Computed Tomography Analysis: A Narrative Review. Diagnostics 2024, 14, 1697. [Google Scholar] [CrossRef]
- Leighton, Y.; de Souza, R.F.; Borie, E. Using the Nasopalatine Canal for Enhanced Distribution in Severely Atrophic Maxilla with Immediate Loading of Zygomatic Implants: An 8-Year Retrospective Cohort Study. J. Craniofac. Surg. 2024; in press. [Google Scholar] [CrossRef]
- Rosenquist, J.B.; Nyström, E. Occlusion of the incisal canal with bone chips. A procedure to facilitate insertion of implants in the anterior maxilla. Int. J. Oral Maxillofac. Surg. 1992, 21, 210–211. [Google Scholar] [CrossRef]
- Scher, E.L. Use of the incisive canal as a recipient site for root form implants: Preliminary clinical reports. Implant. Dent. 1994, 3, 38–41. [Google Scholar] [CrossRef]
- Peñarrocha, D.; Candel, E.; Guirado, J.L.; Canullo, L.; Peñarrocha, M. Implants placed in the nasopalatine canal to rehabilitate severely atrophic maxillae: A retrospective study with long follow-up. J. Oral Implantol. 2014, 40, 699–706. [Google Scholar] [CrossRef]
- Urban, I.; Jovanovic, S.A.; Buser, D.; Bornstein, M.M. Partial lateralization of the nasopalatine nerve at the incisive foramen for ridge augmentation in the anterior maxilla prior to placement of dental implants: A retrospective case series evaluating self-reported data and neurosensory testing. Int. J. Periodontics Restor. Dent. 2015, 35, 169–177. [Google Scholar] [CrossRef]
- Kraut, R.A.; Boyden, D.K. Location of incisive canal in relation to central incisor implants. Implant. Dent. 1998, 7, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Peña-Cardelles, J.F.; Markovic, J.; De Souza, A.; Hamilton, A.; Lanis, A.; Gallucci, G.O. Survival, Success and Neuropathic Alterations Related to Implant Placement Procedures in The Nasopalatine Canal. A Systematic Review and Meta-Analysis. Int. J. Periodontics Restorative Dent. ahead of print. 2024. [Google Scholar] [CrossRef]
- Jacob, S.; Zelano, B.; Gungor, A.; Abbott, D.; Naclerio, R.; McClintock, M.K. Location and gross morphology of the nasopalatine duct in human adults. Arch. Otolaryngol. Head Neck Surg. 2000, 126, 741–748. [Google Scholar] [CrossRef]
- Rodrigues, M.T.; Munhoz, E.A.; Cardoso, C.L.; Junior, O.F.; Damante, J.H. Unilateral patent nasopalatine duct: A case report and review of the literature. Am. J. Otolaryngol. 2009, 30, 137–140. [Google Scholar] [CrossRef]
- Falci, S.G.; Verli, F.D.; Consolaro, A.; Santos, C.R. Morphological characterization of the nasopalatine region in human fetuses and its association to pathologies. J. Appl. Oral Sci. 2013, 21, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Mraiwa, N.; Jacobs, R.; Van Cleynenbreugel, J.; Sanderink, G.; Schutyser, F.; Suetens, P.; van Steenberghe, D.; Quirynen, M. The nasopalatine canal revisited using 2D and 3D CT imaging. Dentomaxillofac. Radiol. 2004, 33, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Güncü, G.N.; Yıldırım, Y.D.; Yılmaz, H.G.; Galindo-Moreno, P.; Velasco-Torres, M.; Al-Hezaimi, K.; Al-Shawaf, R.; Karabulut, E.; Wang, H.-L.; Tözüm, T.F. Is there a gender difference in anatomic features of incisive canal and maxillary environmental bone? Clin. Oral Implant. Res. 2013, 24, 1023–1026. [Google Scholar] [CrossRef]
- Sarna, K.; Estreed, M.A.; Sonigra, K.J.; Amuti, T.; Opondo, F.; Kamau, M.; Ngeow, W.C. Anatomical Patterns of the Nasopalatine Canal and Incisive Foramen in an African Setting: A Cross-Sectional Study. Craniomaxillofac. Trauma Reconstr. 2023, 16, 222–233. [Google Scholar] [CrossRef]
- Alhumaidi, A.M.; Okshah, A.; Al Moaleem, M.M.; Alsabi, M.A.A.; Muharraq, I.H.A.; Asiri, A.Y.; Wasli, Y.A.M.; Alhijji, H.H.S.; Namazi, M.A.; Alfaqih, A.I.I.; et al. Sex and Population Variations in Nasopalatine Canal Dimensions: A CBCT-Based Systematic Review. Med. Sci. Monit. 2024, 30, e945949. [Google Scholar] [CrossRef]
- Radlanski, R.J.; Emmerich, S.; Renz, H. Prenatal morphogenesis of the human incisive canal. Anat. Embryol. 2004, 208, 265–271. [Google Scholar] [CrossRef]
- von Arx, T.; Lozanoff, S. Nasopalatine Canal. In Clinical Oral Anatomy; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Akkoca, F.; Yeşilova, E. Assessment of the Naso-Maxilla and Related Dentoalveolar Region with Cone Beam Computed Tomography. Int. J. Oral Maxillofac. Implants. 2023, 38, 885–896. [Google Scholar] [CrossRef]
- Allaberdiyev, M.; Avsever, İ.H.; Akyol, M.; Ayran, Ş.; Öztürk, H.P.; Özarslantürk, S. Retrospective evaluation and descriptive analysis of the prevalence of anatomical structures and variations in CBCT images before dental implant planning in a group Turkish population, part I. Surg. Radiol. Anat. 2024, 46, 1081–1091. [Google Scholar] [CrossRef]
- Pierrisnard, L.; Renouard, F.; Renault, P.; Barquins, M. Influence of implant length and bicortical anchorage on implant stress distribution. Clin. Implant Dent. Relat. Res. 2003, 5, 254–262. [Google Scholar] [CrossRef]
- Bidez, M.W.; Misch, C.E. Issues in bone mechanics related to oral implants. Implant. Dent. 1992, 1, 289–294. [Google Scholar] [CrossRef]
- Mardinger, O.; Namani-Sadan, N.; Chaushu, G.; Schwartz-Arad, D. Morphologic changes of the nasopalatine canal related to dental implantation: A radiologic study in different degrees of absorbed maxillae. J. Periodontol. 2008, 79, 1659–1662. [Google Scholar] [CrossRef] [PubMed]
- Milanovic, P.; Vasiljevic, M. Gender Differences in the Morphological Characteristics of the Nasopalatine Canal and the Anterior Maxillary Bone—CBCT Study. Experimental and Applied Biomedical Research (EABR). Sciendo 2024, 25, 145–156. [Google Scholar] [CrossRef]
- Liang, X.; Jacobs, R.; Martens, W.; Hu, Y.; Adriaensens, P.; Quirynen, M.; Lambrichts, I. Macro- and micro-anatomical, histological and computed tomography scan characterization of the nasopalatine canal. J. Clin. Periodontol. 2009, 36, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Etoz, M.; Sisman, Y. Evaluation of the nasopalatine canal and variations with cone-beam computed tomography. Surg. Radiol. Anat. 2014, 36, 805–812. [Google Scholar] [CrossRef]
- Bornstein, M.M.; Balsiger, R.; Sendi, P.; von Arx, T. Morphology of the nasopalatine canal and dental implant surgery: A radiographic analysis of 100 consecutive patients using limited cone-beam computed tomography. Clin. Oral Implant. Res. 2011, 22, 295–301. [Google Scholar] [CrossRef]
- Acar, B.; Kamburoğlu, K. Use of cone beam computed tomography in periodontology. World J. Radiol. 2014, 6, 139–147. [Google Scholar] [CrossRef]
- Cavallaro, J.; Tsuji, S.; Chiu, T.S.; Greenstein, G. Management of the Nasopalatine Canal and Foramen Associated with Dental Implant Therapy. Compend. Contin. Educ. Dent. 2016, 38, 367–374. [Google Scholar]
- Romanos, G.E.; Greenstein, G. The incisive canal. Considerations during implant placement: Case report and literature review. Int. J. Oral Maxillofac. Implant. 2009, 24, 740–745. [Google Scholar]
- Panjnoush, M.; Norouzi, H.; Kheirandish, Y.; Shamshiri, A.R.; Mofidi, N. Evaluation of Morphology and Anatomical Measurement of Nasopalatine Canal Using Cone Beam Computed Tomography. J. Dent. 2016, 13, 287–294. [Google Scholar]
- Raghoebar, G.M.; den Hartog, L.; Vissink, A. Augmentation in proximity to the incisive foramen to allow placement of endosseous implants: A case series. J. Oral Maxillofac. Surg. 2010, 68, 2267–2271. [Google Scholar] [CrossRef]
- Peñarrocha, M.; Carrillo, C.; Uribe, R.; Garcia, B. The nasopalatine canal as an anatomic buttress for implant placement in the severely atrophic maxillae: A pilot study. Int. J. Oral Maxillofac. Implants. 2009, 24, 936–942. [Google Scholar] [PubMed]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Reasons for failures of oral implants. J. Oral Rehabil. 2014, 41, 443–476. [Google Scholar] [CrossRef] [PubMed]
- de Mello, J.S.; Faot, F.; Correa, G.; Chagas Júnior, O.L. Success rate and complications associated with dental implants in the incisive canal region: A systematic review. Int. J. Oral Maxillofac. Surg. 2017, 46, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.R.; Burde, K.; Guttal, K.; Naikmasur, V.G. Anatomy and morphology of the nasopalatine canal using cone-beam computed tomography. Imaging Sci. Dent. 2013, 43, 273–281. [Google Scholar] [CrossRef]
- Fernández Bodereau, E.; Flores, V.Y.; Naldini, P.; Torassa, D.; Tortolini, P. Clinical Evaluation of the Nasopalatine Canal in Implant-Prosthetic Treatment: A Pilot Study. Dent. J. 2020, 8, 30. [Google Scholar] [CrossRef]
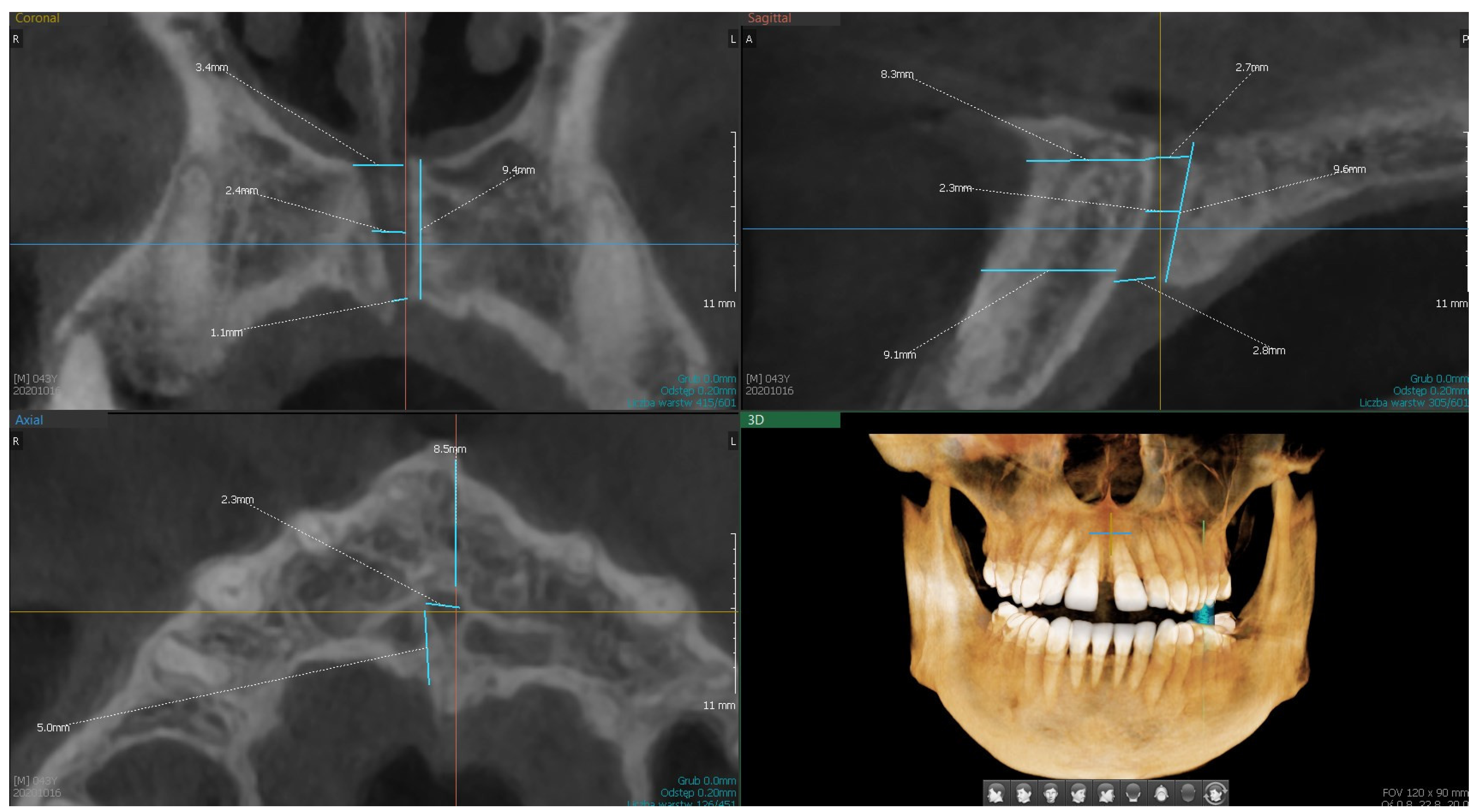
| Variable | Descriptive Statistics | |||||||
|---|---|---|---|---|---|---|---|---|
| Valid N | Mean | Median | Min | Max | Q1 | Q3 | SD | |
| S1.—length of the canal from the base of the nose to the palate | 150 | 9.67 | 9.60 | 4.00 | 16.60 | 8.00 | 11,20 | 2.47 |
| S2.—width of the canal at the base of the palate | 150 | 3.71 | 3.55 | 1.10 | 7.60 | 2.90 | 4.50 | 1.28 |
| S3.—width of the canal at the base of the nose | 149 | 3.30 | 3.00 | 0.90 | 6.90 | 2.30 | 4.20 | 1.29 |
| S4.—width of the canal in half-length | 150 | 2.62 | 2.50 | 0.80 | 6.70 | 1.60 | 3.30 | 1.14 |
| S5.—distance to the labial plate at the level of the mouth opening of the palatine canal | 150 | 6.66 | 6.75 | 2.00 | 11.30 | 5.70 | 7.70 | 1.73 |
| S6.—distance to the labial plate at the level of the nasal outlet | 149 | 9.31 | 9.10 | 4.80 | 14.40 | 8.00 | 10.90 | 2.15 |
| C1.—width of the canal at the base of the nose | 150 | 4.06 | 4.15 | 1.20 | 9.90 | 3.00 | 5.00 | 1.38 |
| C2.—width of the canal in half | 150 | 3.32 | 3.25 | 1.20 | 6.30 | 2.40 | 4.00 | 1.16 |
| C3.—width of the canal at the base of the palate | 150 | 3.87 | 3.70 | 1.10 | 9.20 | 2.60 | 5.00 | 1.54 |
| C4.—canal length | 150 | 10.27 | 10.10 | 1.00 | 21.10 | 7.70 | 12.70 | 3.52 |
| C5.—number of canals | 150 | 1.15 | 1.00 | 1.00 | 2.00 | 1.00 | 1.00 | 0.36 |
| A1.—number of openings | 150 | 1.09 | 1.00 | 1.00 | 3.00 | 1.00 | 1.00 | 0.31 |
| A2.—diameter of the main canal | 149 | 3.55 | 3.40 | 1.00 | 9.00 | 2.50 | 4.30 | 1.41 |
| A3.—distance to the palatal plate (bone thickness) | 150 | 3.57 | 3.30 | 0.60 | 7.80 | 2.40 | 4.70 | 1.55 |
| A4.—distance to the labial plate | 150 | 6.49 | 6.45 | 1.40 | 10.40 | 5.40 | 7.50 | 1.78 |
| age | 150 | 51.65 | 52.00 | 18.00 | 81.00 | 43.00 | 62.00 | 14.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calin, F.; Dalewski, B.; Ellmann, M.; Kiczmer, P.; Ihde, S.; Bieńkowska, M.; Kotuła, J.; Pałka, Ł. CBCT Evaluation of Maxillary Incisive Canal Characteristics Among Population in Regard to Possibility of Implant Cortical Anchorage—A Multicenter Study. Dent. J. 2025, 13, 211. https://doi.org/10.3390/dj13050211
Calin F, Dalewski B, Ellmann M, Kiczmer P, Ihde S, Bieńkowska M, Kotuła J, Pałka Ł. CBCT Evaluation of Maxillary Incisive Canal Characteristics Among Population in Regard to Possibility of Implant Cortical Anchorage—A Multicenter Study. Dentistry Journal. 2025; 13(5):211. https://doi.org/10.3390/dj13050211
Chicago/Turabian StyleCalin, Fodor, Bartosz Dalewski, Maciej Ellmann, Paweł Kiczmer, Stefan Ihde, Marta Bieńkowska, Jacek Kotuła, and Łukasz Pałka. 2025. "CBCT Evaluation of Maxillary Incisive Canal Characteristics Among Population in Regard to Possibility of Implant Cortical Anchorage—A Multicenter Study" Dentistry Journal 13, no. 5: 211. https://doi.org/10.3390/dj13050211
APA StyleCalin, F., Dalewski, B., Ellmann, M., Kiczmer, P., Ihde, S., Bieńkowska, M., Kotuła, J., & Pałka, Ł. (2025). CBCT Evaluation of Maxillary Incisive Canal Characteristics Among Population in Regard to Possibility of Implant Cortical Anchorage—A Multicenter Study. Dentistry Journal, 13(5), 211. https://doi.org/10.3390/dj13050211






