Minimally Invasive Root Canal Cleaning: Evaluating Supplementary Irrigation Techniques
Abstract
1. Introduction
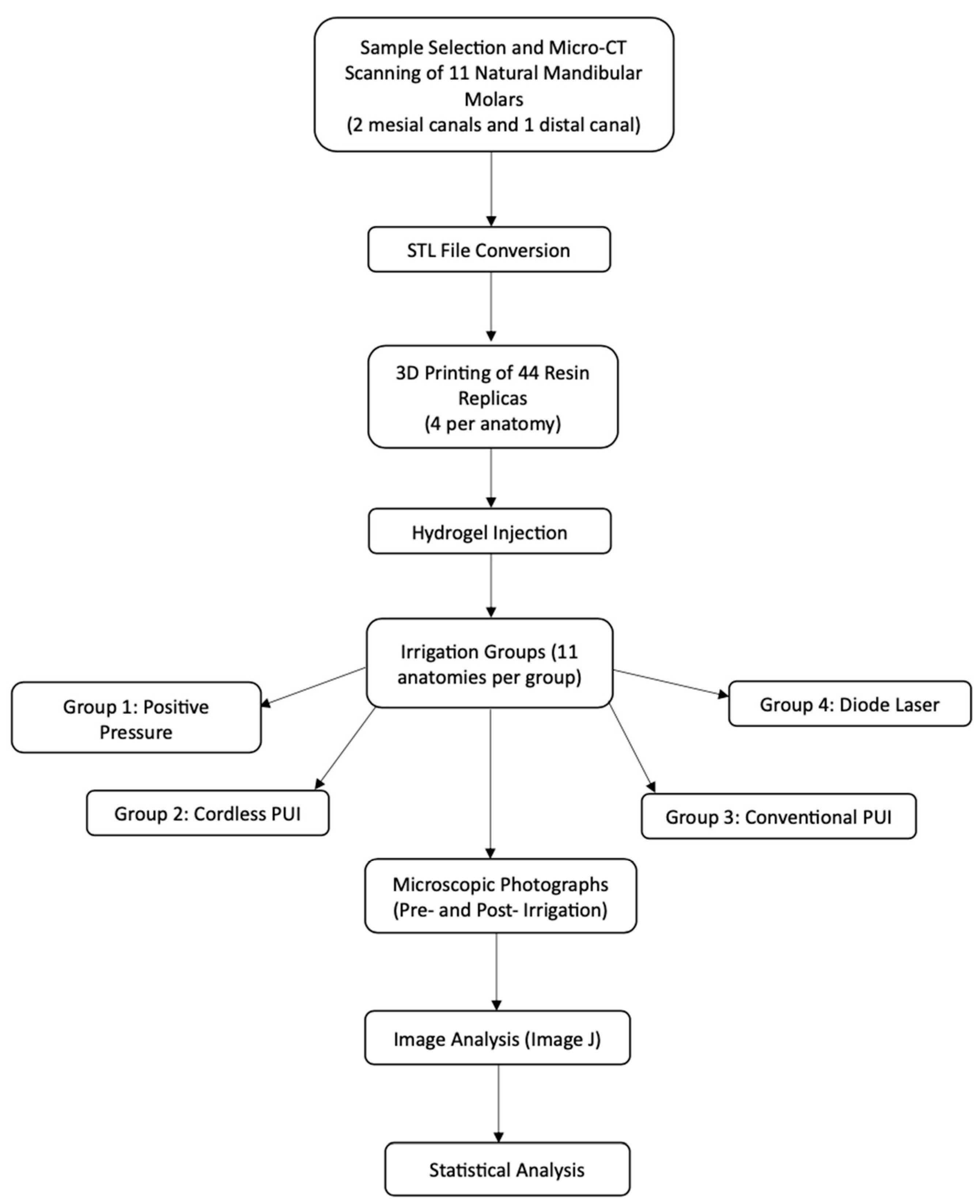
2. Materials and Methods
2.1. Sample Preparation
2.2. Micro-CT Scanning
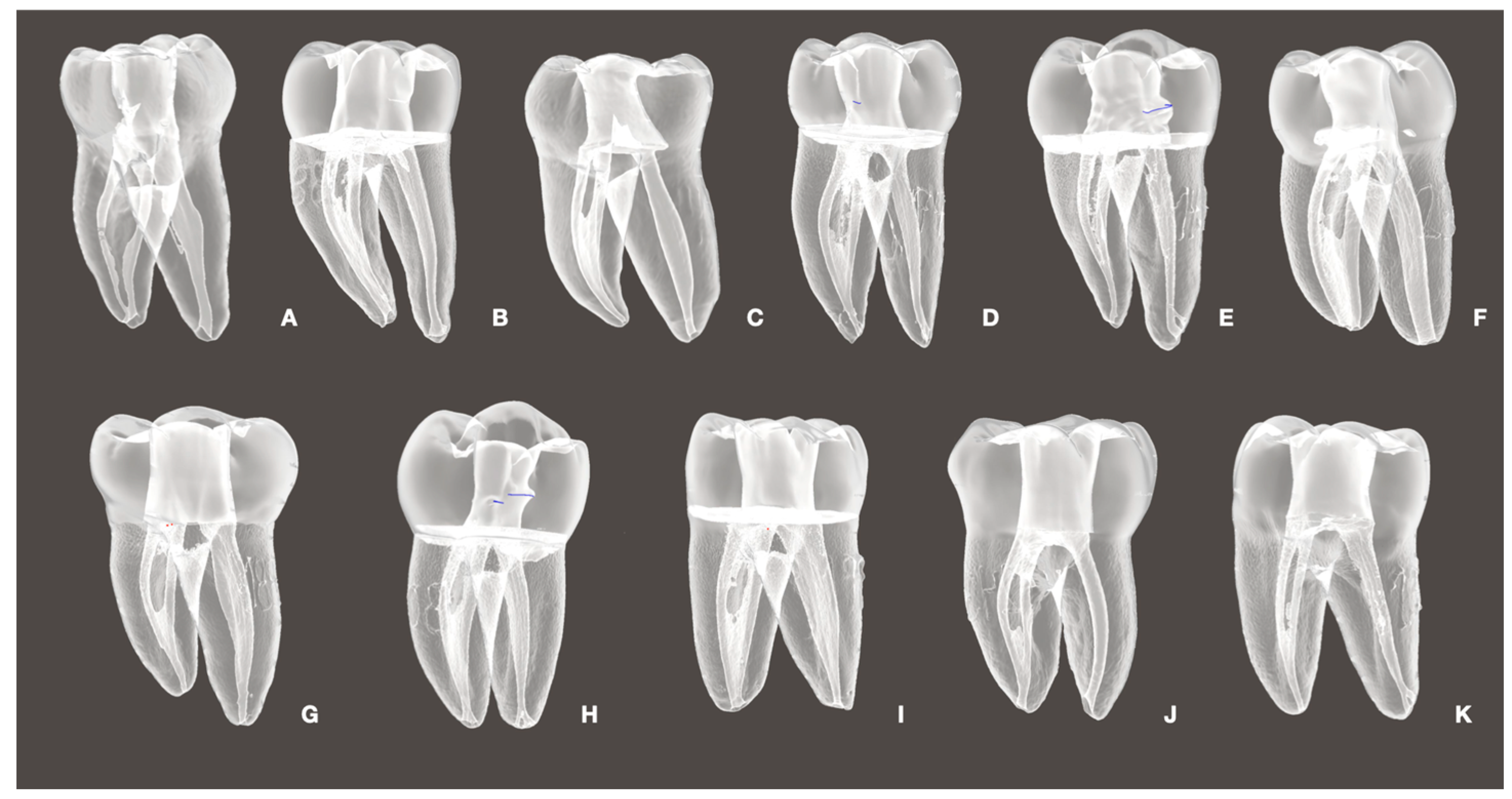
2.3. Manufacture of 3D Replicas in Resin
2.4. Tissue Simulation with Hydrogel Model
2.5. Experimental Groups
- Positive Pressure (Control) irrigation with an open-ended 30G needle positioned 3 mm up from the WL.
- Cordless PUI Ultra X (Eighteeth, Changzhou, Jiangsu, China), with two activation cycles of 30 seg using a silver tip size of 20/.02 positioned 2 mm up from the WL.
- Conventional PUI (Varios 370 Lux, NSK America Corp., Hoffman Estates, IL, USA) with two activation cycles of 30 seg using a Irrisafe 20/.02 tip (Acteon, Merignac, France) positioned 2 mm up from the WL.
- DL at 980 nm with a power of LX16 plus (Woodpecker Medical Instrument Co., LTD, Guangdong, China) and a 0.2 mm tip positioned 3 mm up from the WL. Two activation cycles of 30 s were performed during the procedure.
2.6. Tissue Cleaning Efficiency
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zandi, H.; Petronijevic, N.; Mdala, I.; Kristoffersen, A.K.; Enersen, M.; Rôças, I.N.; Siqueira, J.F., Jr.; Ørstavik, D. Outcome of Endodontic Retreatment Using 2 Root Canal Irrigants and Influence of Infection on Healing as Determined by a Molecular Method: A Randomized Clinical Trial. J. Endod. 2019, 45, 1089–1098.e5. [Google Scholar] [CrossRef]
- Pérez, A.R.; Ricucci, D.; Vieira, G.C.S.; Provenzano, J.C.; Alves, F.R.F.; Marceliano-Alves, M.F.; Rôças, I.N.; Siqueira, J.F., Jr. Cleaning, Shaping, and Disinfecting Abilities of 2 Instrument Systems as Evaluated by a Correlative Micro-computed Tomographic and Histobacteriologic Approach. J. Endod. 2020, 46, 846–857. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Pérez, A.R.; Marceliano-Alves, M.F.; Provenzano, J.C.; Silva, S.G.; Pires, F.R.; Vieira, G.C.S.; Rôças, I.N.; Alves, F.R.F. What happens to unprepared root canal walls: A correlative analysis using micro-computed tomography and histology/scanning electron microscopy. Int. Endod. J. 2018, 51, 501–508. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rôças, I.N.; Marceliano-Alves, M.F.; Perez, A.R.; Ricucci, D. Unprepared root canal surface areas: Causes, clinical implications, and therapeutic strategies. Braz. Oral Res. 2018, 32 (Suppl. 1), e65. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.R.; Andrade-Junior, C.V.; Marceliano-Alves, M.F.; Perez, A.R.; Rocas, I.N.; Versiani, M.A.; Sousa-Neto, M.D.; Provenzano, J.C.; Siqueira, J.F., Jr. Adjunctive Steps for Disinfection of the Mandibular Molar Root Canal System: A Correlative Bacteriologic, Micro-Computed Tomography, and Cryopulverization Approach. J. Endod. 2016, 42, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Paiva, S.S.M.; Siqueira, J.E.; Rocas, I.N.; Carmo, F.L.; Ferreira, D.C.; Curvelo, J.A.R.; Soares, R.M.A.; Rosado, A.S. Supplementing the Antimicrobial Effects of Chemomechanical Debridement with Either Passive Ultrasonic Irrigation or a Final Rinse with Chlorhexidine: A Clinical Study. J. Endod. 2012, 38, 1202–1206. [Google Scholar] [CrossRef] [PubMed]
- Vianna, M.E.; Horz, H.P.; Gomes, B.P.; Conrads, G. In vivo evaluation of microbial reduction after chemo-mechanical preparation of human root canals containing necrotic pulp tissue. Int. Endod. J. 2006, 39, 484–492. [Google Scholar] [CrossRef]
- Neves, M.A.; Provenzano, J.C.; Rôças, I.N.; Siqueira, J.F., Jr. Clinical antibacterial effectiveness of root canal preparation with reciprocating single-instrument or continuously rotating multi-instrument systems. J. Endod. 2016, 42, 25–29. [Google Scholar] [CrossRef]
- Sjögren, U.; Figdor, D.; Persson, S.; Sundqvist, G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int. Endod. J. 1997, 30, 297–306. [Google Scholar] [CrossRef]
- Lacerda, M.; Marceliano-Alves, M.F.; Pérez, A.R.; Provenzano, J.C.; Neves, M.A.S.; Pires, F.R.; Gonçalves, L.S.; Rôças, I.N.; Siqueira, J.F., Jr. Cleaning and shaping oval canals with 3 instrumentation systems: A correlative micro-computed tomographic and histologic study. J. Endod. 2017, 43, 1878–1884. [Google Scholar] [CrossRef]
- Weller, R.N.; Niemczyk, S.P.; Kim, S. Incidence and position of the canal isthmus. Part 1. Mesiobuccal root of the maxillary first molar. J. Endod. 1995, 21, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Mannocci, F.; Peru, M.; Sherriff, M.; Cook, R.; Pitt Ford, T.R. The isthmuses of the mesial root of mandibular molars: A micro-computed tomographic study. Int. Endod. J. 2005, 38, 558–563. [Google Scholar] [CrossRef]
- Harris, S.P.; Bowles, W.R.; Fok, A.; McClanahan, S.B. An anatomic investigation of the mandibular first molar using micro-computed tomography. J. Endod. 2013, 39, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.R.; Almeida, B.M.; Neves, M.A.; Moreno, J.O.; Rocas, I.N.; Siqueira, J.F., Jr. Disinfecting oval-shaped root canals: Effectiveness of different supplementary approaches. J. Endod. 2011, 37, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.K.; R’Oris, A.; Barkis, D.; Wesselink, P.R. Prevalence and extent of long oval canals in the apical third. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2000, 89, 739–743. [Google Scholar] [CrossRef]
- Paqué, F.; Balmer, M.; Attin, T.; Peters, O.A. Preparation of oval-shaped root canals in mandibular molars using nickel-titanium rotary instruments: A micro-computed tomography study. J. Endod. 2010, 36, 703–707. [Google Scholar] [CrossRef]
- Hongo, T.; Watanabe, S.; Yao, K.; Satake, K.; Okiji, T. Evaluation of cleaning efficacy-related properties of root canal irrigant activation using a computer-controlled hot tip powered with a diode laser. Asian Pac. J. Dent. 2019, 19, 9–15. [Google Scholar] [CrossRef]
- van der Sluis, L.W.; Versluis, M.; Wu, M.K.; Wesselink, P.R. Passive ultrasonic irrigation of the root canal: A review of the literature. Int. Endod. J. 2007, 40, 415–426. [Google Scholar] [CrossRef]
- Vendramini, Y.; Salles, A.; Portella, F.F.; Brew, M.C.; Steier, L.; de Figueiredo, J.A.P.; Bavaresco, C.S. Antimicrobial effect of photodynamic therapy on intracanal biofilm: A systematic review of in vitro studies. Photodiagnosis Photodyn. Ther. 2020, 32, 102025. [Google Scholar] [CrossRef]
- Shrestha, A.; Kishen, A. Antibiofilm efficacy of photosensitizer-functionalized bioactive nanoparticles on multispecies biofilm. J. Endod. 2014, 40, 1604–1610. [Google Scholar] [CrossRef]
- Zhang, Y.; Das, G.K.; Vijayaragavan, V.; Xu, Q.C.; Padmanabhan, P.; Bhakoo, K.K.; Tamil Selvan, S.; Tan, T.T.Y. “Smart” theranostic lanthanide nanoprobes with simultaneous up-conversion fluorescence and tunable T1–T2 magnetic resonance imaging contrast and near-infrared activated photodynamic therapy. Nanoscale 2014, 6, 12609–12617. [Google Scholar] [CrossRef]
- Qiu, H.; Tan, M.; Ohulchanskyy, T.Y.; Lovell, J.F.; Chen, G. Recent Progress in Upconversion Photodynamic Therapy. Nanomaterials 2018, 8, 344. [Google Scholar] [CrossRef] [PubMed]
- Asnaashari, M.; Sadeghian, A.; Hazrati, P. The Effect of High-Power Lasers on Root Canal Disinfection: A Systematic Review. J. Lasers Med. Sci. 2022, 13, e66. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Wei, Y.; Wu, B.; Chen, Q.; Xing, D. Pyropheophorbide a and C(RGDyK) Comodified Chitosan-Wrapped Upconversion Nanoparticle for Targeted Near-Infrared Photodynamic Therapy. Mol. Pharm. 2012, 9, 1580–1589. [Google Scholar] [CrossRef]
- Wang, X.; Liu, K.; Yang, G.; Cheng, L.; He, L.; Liu, Y.; Li, Y.; Guo, L.; Liu, Z. Near-Infrared Light Triggered Photodynamic Therapy in Combination With Gene Therapy Using Upconversion Nanoparticles for Effective Cancer Cell Killing. Nanoscale 2014, 6, 9198. [Google Scholar] [CrossRef] [PubMed]
- Kishen, A.; Asundi, A. Photomechanical investigations on post endodontically rehabilitated teeth. J. Biomed. Opt. 2002, 7, 262–270. [Google Scholar] [CrossRef]
- Boutsioukis, C.; Gogos, C.; Verhaagen, B.; Versluis, M.; Kastrinakis, E.; Van der Sluis, L.W. The effect of apical preparation size on irrigant flow in root canals evaluated using an unsteady Computational Fluid Dynamics model. Int. Endod. J. 2010, 43, 874–881. [Google Scholar] [CrossRef]
- Rodrigues, R.C.V.; Zandi, H.; Kristoffersen, A.K.; Enersen, M.; Mdala, I.; Ørstavik, D.; Rôças, I.N.; Siqueira, J.F., Jr. Influence of the apical preparation size and the irrigant type on bacterial reduction in root canal-treated teeth with apical periodontitis. J. Endod. 2017, 43, 1058–1063. [Google Scholar] [CrossRef]
- Baasch, A.; Ramírez-Muñoz, A.; Navarrete, N.; Navarro-Candel, M.; Koury-González, J.M.; Esteves-Nieves, O.; Vieira, G.C.S.; Pérez, A.R. Influence of irrigation needle design on cleaning efficiency in 3D mandibular molar models. Odontology 2025. [Google Scholar] [CrossRef]
- Robinson, J.P.; Macedo, R.G.; Verhaagen, B.; Versluis, M.; Cooper, P.R.; van der Sluis, L.W.M.; Walmsley, A.D. Cleaning lateral morphological features of the root canal: The role of streaming and cavitation. Int. Endod. J. 2018, 51 (Suppl. 1), e55–e64. [Google Scholar] [CrossRef]
- Lee, O.Y.S.; Khan, K.; Li, K.Y.; Shetty, H.; Abiad, R.S.; Cheung, G.S.P.; Neelakantan, P. Influence of apical preparation size and irrigation technique on root canal debridement: A histological analysis of round and oval root canals. Int. Endod. J. 2019, 52, 1366–1376. [Google Scholar] [CrossRef] [PubMed]
- Boutsioukis, C.; Gogos, C.; Verhaagen, B.; Versluis, M.; Kastrinakis, E.; Van der Sluis, L.W. The effect of root canal taper on the irrigant flow: Evaluation using an unsteady Computational Fluid Dynamics model. Int. Endod. J. 2010, 43, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Boutsioukis, C.; Lambrianidis, T.; Verhaagen, B.; Versluis, M.; Kastrinakis, E.; Wesselink, P.R.; van der Sluis, L.W. The effect of needle-insertion depth on the irrigant flow in the root canal: Evaluation using an unsteady computational fluid dynamics model. J. Endod. 2010, 36, 1664–1668. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Araujo, M.C.; Garcia, P.F.; Fraga, R.C.; Dantas, C.J. Histological evaluation of the effectiveness of five instrumentation techniques for cleaning the apical third of root canals. J. Endod. 1997, 23, 499–502. [Google Scholar] [CrossRef]
- Boutsioukis, C.; Gutierrez Nova, P. Syringe Irrigation in Minimally Shaped Root Canals Using 3 Endodontic Needles: A Computational Fluid Dynamics Study. J. Endod. 2021, 47, 1487–1495. [Google Scholar] [CrossRef]
- Limoeiro, A.G.d.S.; Souza, C.; Bueno, C.; Kato, A.; Fontana, C.E.; Pelegrine, R. Efficacy of Passive Ultrasonic Irrigation, Continuous Ultrasonic Irrigation Versus Irrigation With Reciprocating Activation Device in Penetration Into Main and Simulated Lateral Canals. J. Conserv. Dent. 2019, 22, 155. [Google Scholar] [CrossRef]
- Tse, M.C.C.; Cheung, G.S.P. Spatial Cleaning Action of Ultrasonic Irrigation on Enterococcus Faecalis Biofilm. Dent. J. 2020, 8, 42. [Google Scholar] [CrossRef]
- Plotino, G.; Grande, N.M.; Isufi, A.; Ioppolo, P.; Pedullà, E.; Bedini, R.; Gambarini, G.; Testarelli, L. Fracture Strength of Endodontically Treated Teeth with Different Access Cavity Designs. J. Endod. 2017, 43, 995–1000. [Google Scholar] [CrossRef]
- Vieira, G.C.S.; Pérez, A.R.; Alves, F.R.F.; Provenzano, J.C.; Mdala, I.; Siqueira, J.F., Jr.; Rôças, I.N. Impact of Contracted Endodontic Cavities on Root Canal Disinfection and Shaping. J. Endod. 2020, 46, 655–661. [Google Scholar] [CrossRef]
- Teed, C.; Hussein, H.; Kishen, A. Synchronized Microbubble Photodynamic Activation to Disinfect Minimally Prepared Root Canals. J. Endod. 2023, 49, 198–204. [Google Scholar] [CrossRef]
- Card, S.J.; Sigurdsson, A.; Ørstavik, D.; Trope, M. The effectiveness of increased apical enlargement in reducing intracanal bacteria. J. Endod. 2002, 28, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.; Kumar, A.; Andrabi, S.; Mishra, S.K.; Tewari, R.K. Effect of Apical Third Enlargement to Different Preparation Sizes and Tapers on Postoperative Pain and Outcome of Primary Endodontic Treatment: A Prospective Randomized Clinical Trial. J. Endod. 2021, 47, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Alves, F.R.; Almeida, B.M.; de Oliveira, J.C.; Rocas, I.N. Ability of chemomechanical preparation with either rotary instruments or self-adjusting file to disinfect oval-shaped root canals. J. Endod. 2010, 36, 1860–1865. [Google Scholar] [CrossRef]
- De-Deus, G.; Belladonna, F.G.; de Siqueira Zuolo, A.; Perez, R.; Carvalho, M.S.; Souza, E.M.; Lopes, R.T.; Silva, E. Micro-CT comparison of XP-endo Finisher and passive ultrasonic irrigation as final irrigation protocols on the removal of accumulated hard-tissue debris from oval shaped-canals. Clin. Oral Investig. 2019, 23, 3087–3093. [Google Scholar] [CrossRef]
- Arnold, M.; Ricucci, D.; Siqueira, J.F., Jr. Infection in a complex network of apical ramifications as the cause of persistent apical periodontitis: A case report. J. Endod. 2013, 39, 1179–1184. [Google Scholar] [CrossRef]
- Ricucci, D.; Siqueira, J.F., Jr.; Bate, A.L.; Pitt Ford, T.R. Histologic investigation of root canal-treated teeth with apical periodontitis: A retrospective study from twenty-four patients. J. Endod. 2009, 35, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Antunes, H.S.; Pérez, A.R.; Alves, F.R.F.; Mdala, I.; Silva, E.; Belladonna, F.G.; Rôças, I.N. The Apical Root Canal System of Teeth with Posttreatment Apical Periodontitis: Correlating Microbiologic, Tomographic, and Histopathologic Findings. J. Endod. 2020, 46, 1195–1203. [Google Scholar] [CrossRef]
- Kessler, A.; Hickel, R.; Reymus, M. 3D Printing in Dentistry-State of the Art. Oper. Dent. 2020, 45, 30–40. [Google Scholar] [CrossRef]
- Cui, Z.; Wei, Z.; Du, M.; Yan, P.; Jiang, H. Shaping ability of protaper next compared with waveone in late-model three-dimensional printed teeth. BMC Oral Health 2018, 18, 115. [Google Scholar] [CrossRef]
- Reymus, M.; Fotiadou, C.; Kessler, A.; Heck, K.; Hickel, R.; Diegritz, C. 3D printed replicas for endodontic education. Int. Endod. J. 2019, 52, 123–130. [Google Scholar] [CrossRef]
- Kooanantkul, C.; Shelton, R.M.; Camilleri, J. Comparison of obturation quality in natural and replica teeth root-filled using different sealers and techniques. Clin. Oral Investig. 2023, 27, 2407–2417. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Muñoz, A.; Escribano-Capdevila, M.; Navarrete, N.; Vieira, G.C.S.; Salamanca-Ramos, M.; Ortolani-Seltenerich, P.S.; Aranguren, J.; Pérez, A.R. Comparative Micro-CT Analysis of Minimally Invasive Endodontic Systems Using 3D-Printed Replicas and Natural Teeth. Materials 2024, 17, 5279. [Google Scholar] [CrossRef] [PubMed]
- Nassri, M.R.; Carlik, J.; da Silva, C.R.; Okagawa, R.E.; Lin, S. Critical analysis of artificial teeth for endodontic teaching. J. Appl. Oral Sci. 2008, 16, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Macedo, R.G.; Robinson, J.P.; Verhaagen, B.; Walmsley, A.D.; Versluis, M.; Cooper, P.R.; van der Sluis, L.W. A novel methodology providing insights into removal of biofilm-mimicking hydrogel from lateral morphological features of the root canal during irrigation procedures. Int. Endod. J. 2014, 47, 1040–1051. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rôças, I.N. Clinical implications and microbiology of bacterial persistence after treatment procedures. J. Endod. 2008, 34, 1291–1301.e1293. [Google Scholar] [CrossRef]
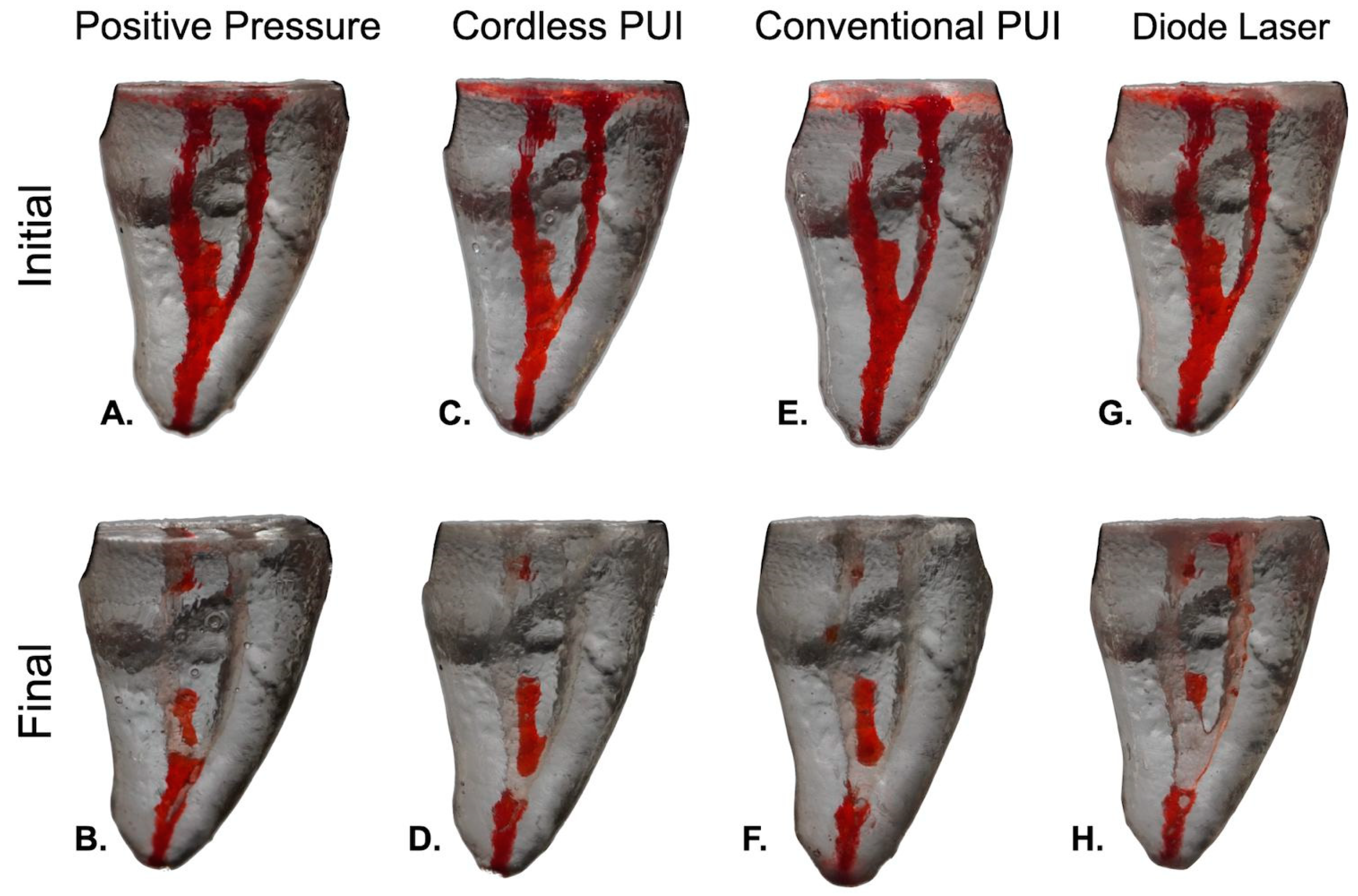
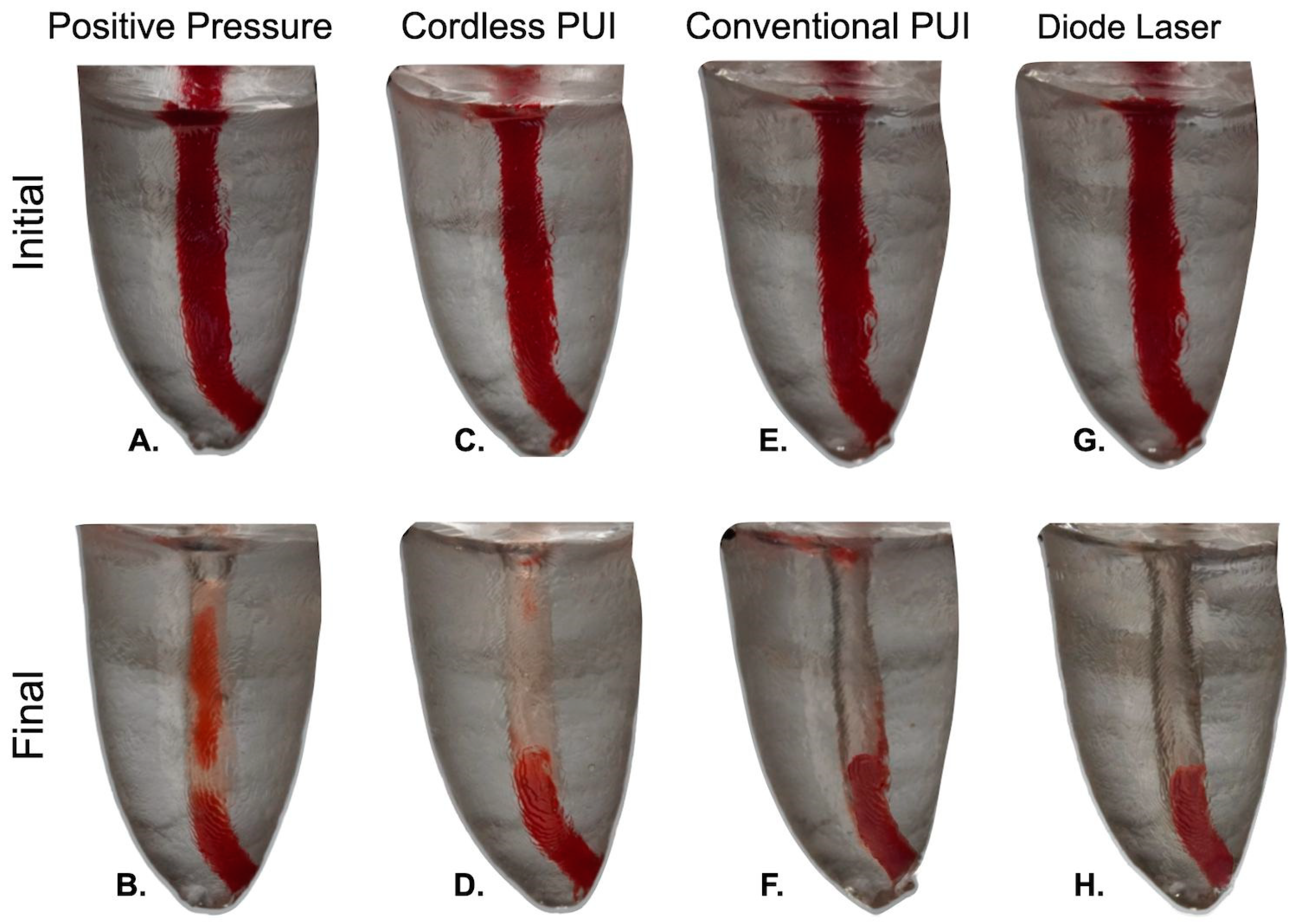
| Features | Conventional Syringe | Cordless PUI | Conventional PUI | Laser 1W |
|---|---|---|---|---|
| Tissue remnants | ||||
| Full canal | ||||
| Surface (mm3) | ||||
| Initial surface | 17,453.2 (16,862.4; 6997–26,698) | 17,168.4 (15,986.3; 5909–28,076) | 17,568.1 (16,536.3; 6408–27,458) | 17,877.4 (16,398.2; 6205–27,809) |
| Final | 6800.2 (6375.2; 2012–16,034) | 5837.4 (5640.3; 0–13,482) | 6944.2 (6530.3; 0–14,488) | 6625.4 (6909.1; 0–16,164) |
| Δ% | 36.1 (36.3; 11–62) | 29.1 (23.2; 0–65) | 37.3 (37.2; 0–55) | 35.4 (37.4; 0–80) |
| Apical | ||||
| Surface (mm3) | ||||
| Initial | 3749.3 (3692.4; 933–9456) | 3567.3 (3479.3; 628–8413) | 3460.2 (3707.4; 107–4763) | 3549.1 (3208.2; 853–8514) |
| Final | 1574.3 (870.2; 69–8192) | 1246.1 (889.4; 0–3923) | 484.6 (398.2; 0–6052) | 1035.3 (965.1; 0–4128) |
| Δ% | 42.2 (40.3; 7–87) a | 30.4 (25.2; 0–84) b | 14.1 (1.3; 0–87) c | 29.3 (22.2; 0–85) b |
| Middle | ||||
| Surface (mm3) | ||||
| Initial | 5708.8 (5679.1; 2301–9934) | 5494.2 (5463.4; 2460–9527) | 5413.4 (5254.3; 2614–9644) | 5277.2 (5086.1; 2795–9547) |
| Final | 1951.3 (1566.2; 0–5603) | 745.2 (500.4; 0–4726) | 777.2 (694.3; 0–4875) | 659.2 (654.3; 0–4769) |
| Δ% | 34.3 (31.1; 0–85) a | 13.4 (10.2; 0–53) b | 14.3 (12.2; 0–67) b | 12.1 (10.1; 0–57) b |
| Coronal | ||||
| Surface(mm3) | ||||
| Inicial | 8387.7 (8229.2; 3936–11,567) | 8470.4 (8342.1; 3857–11,760) | 8153.1 (8174.1; 3466–11,834) | 8595.4 (8063.2; 3305–11,618) |
| Final | 2995.8 (2557.1; 0–8219) | 2559.4 (2483.1; 0–7609) | 2453.6 (2396.3; 0–8539) | 2590.3 (2457.2; 0–7997) |
| Δ% | 35.1 (34.1; 0–84) | 30.4 (29.1; 0–75) | 30.2 (33.2; 0–81) | 30.1 (34.2; 0–83) a |
| Features (Mean-Median-Range) | Conventional Syringe | Cordless PUI | Conventional PUI | Laser 1W |
|---|---|---|---|---|
| Tissue remnants | ||||
| All canal | ||||
| Surface (mm2) | ||||
| Initial surface | 14,166.2 (13,983.3; 6695–22,107) | 14,037.2 (13,019.2; 6416–22,449) | 14,587.3 (13,761.2; 6353–22,642) | 14,163.3 (13,045.1; 6522–22,445) |
| Final | 6670.1 (4787.1; 606–17,830) | 5486.3 (4719.2; 0–13,341) | 5570.3 (4676.1; 0–18,473) | 5285.2 (4841.2; 0–15,702) |
| Δ% | 47.2 (41.2; 9–88) | 39.4 (32.3; 0–85) | 38.4 (26.3; 0–77) | 37.4 (23.1; 0–91) |
| Apical | ||||
| Surface (mm2) | ||||
| Initial | 2788.3 (2483.2; 695–5483) | 2356.4 (2129.3; 678–5266) | 2425.2 (2262.2; 674–5160) | 2420.3 (2396.1; 658–5381) |
| Final | 1080.4 (945.3; 216–3131) | 951.3 (835.4; 0–3375) | 970.1 (801.3; 0–3732) | 859.2 (730.2; 0–2549) |
| Δ% | 42.3 (41.2; 13–71) | 41.2 (31.3; 0–86) | 40.3 (37.2; 0–85) | 35.2 (31.2; 0–83) |
| Middle | ||||
| Surface(mm2) | ||||
| Initial | 5620.3 (4720.1; 1700–8417) | 5397.2 (4587.5 (1807–8646) | 5558.4 (4565.2; 1622–8599) | 5424.1 (4583.4; 1738–8506) |
| Final | 1345.4 (1293.4; 0–5248) | 1771.2 (1760.1; 0–5302) | 1389.3 (103.2; 0–1168) | 1387.4 (1223.1; 0–5919) |
| Δ% | 24.1 (21.2; 0–85) | 32.3 (21.2; 0–86) | 24.4 (22.1; 0–89) | 23.3 (22.2; 0–88) |
| Coronal | ||||
| Surface(mm2) | ||||
| Inicial | 7636.3 (7098.1; 4795–9894) | 7480.2 (7297.3; 4550–9515) | 7780.3 (7584.1; 4798–9400) | 7713.3 (7576.3; 4355–9502) |
| Final | 3715.2 (3084.3; 0–9255) | 3170.2 (2885.4; 0–8616) | 3568.4 (3308.2; 0–8692) | 3631.2 (3389.1; 0–8415) |
| Δ% | 49.1 (45.3; 0–94) | 41.3 (38.2; 0–97) | 45.3 (41.2; 0–96) | 47.3 (43.2; 0–96) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiscareño, A.; Ortolani-Seltenerich, P.S.; Ramírez-Muñoz, A.; Pérez-Ron, O.; Mendez S, P.M.; Leal-Moya, C.; Malvicini, G.; Vieira, G.C.S.; Pérez, A.R. Minimally Invasive Root Canal Cleaning: Evaluating Supplementary Irrigation Techniques. Dent. J. 2025, 13, 192. https://doi.org/10.3390/dj13050192
Tiscareño A, Ortolani-Seltenerich PS, Ramírez-Muñoz A, Pérez-Ron O, Mendez S PM, Leal-Moya C, Malvicini G, Vieira GCS, Pérez AR. Minimally Invasive Root Canal Cleaning: Evaluating Supplementary Irrigation Techniques. Dentistry Journal. 2025; 13(5):192. https://doi.org/10.3390/dj13050192
Chicago/Turabian StyleTiscareño, Alissa, P. S. Ortolani-Seltenerich, Ana Ramírez-Muñoz, Omar Pérez-Ron, Pedro M. Mendez S, Carmen Leal-Moya, Giulia Malvicini, Gaya C. S. Vieira, and Alejandro R. Pérez. 2025. "Minimally Invasive Root Canal Cleaning: Evaluating Supplementary Irrigation Techniques" Dentistry Journal 13, no. 5: 192. https://doi.org/10.3390/dj13050192
APA StyleTiscareño, A., Ortolani-Seltenerich, P. S., Ramírez-Muñoz, A., Pérez-Ron, O., Mendez S, P. M., Leal-Moya, C., Malvicini, G., Vieira, G. C. S., & Pérez, A. R. (2025). Minimally Invasive Root Canal Cleaning: Evaluating Supplementary Irrigation Techniques. Dentistry Journal, 13(5), 192. https://doi.org/10.3390/dj13050192







