Changes in Upper Airway Airflow After Rapid Maxillary Expansion Beyond the Peak Period of Adenoidal Growth—A CBCT Study Using Computer Fluid Dynamics and Considering Adenoidal Dimensions as a Factor
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. CBCT Assessment
2.2.1. Preliminary Definition of Mid-Palatal Suture (MPS) Maturation
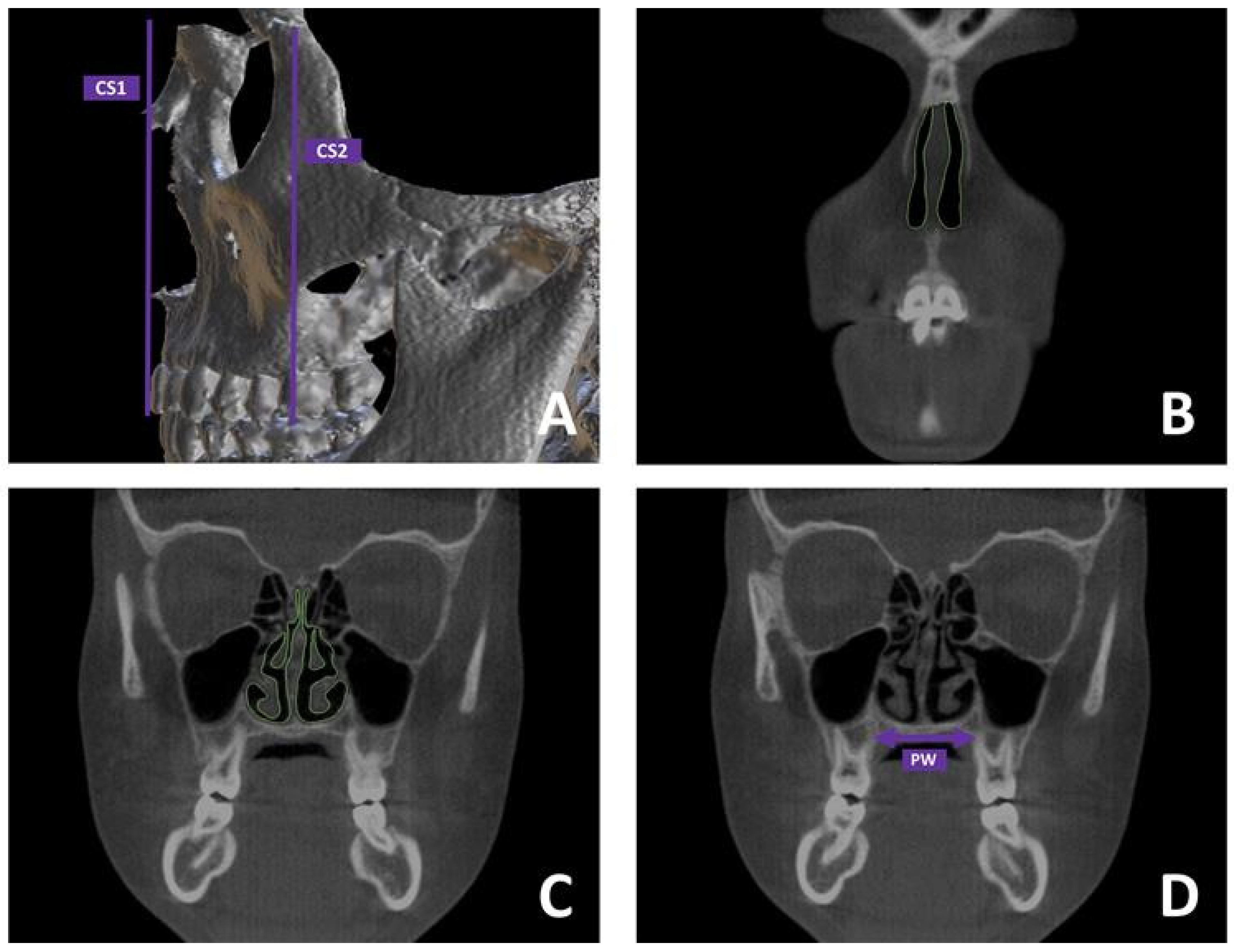
2.2.2. Segmentation Protocol of Upper Airways (UAs)
2.2.3. Skeletal Measurements
2.2.4. CFD Analysis
2.3. Statistics
3. Results
3.1. Reliability Assessment
3.2. Baseline Data
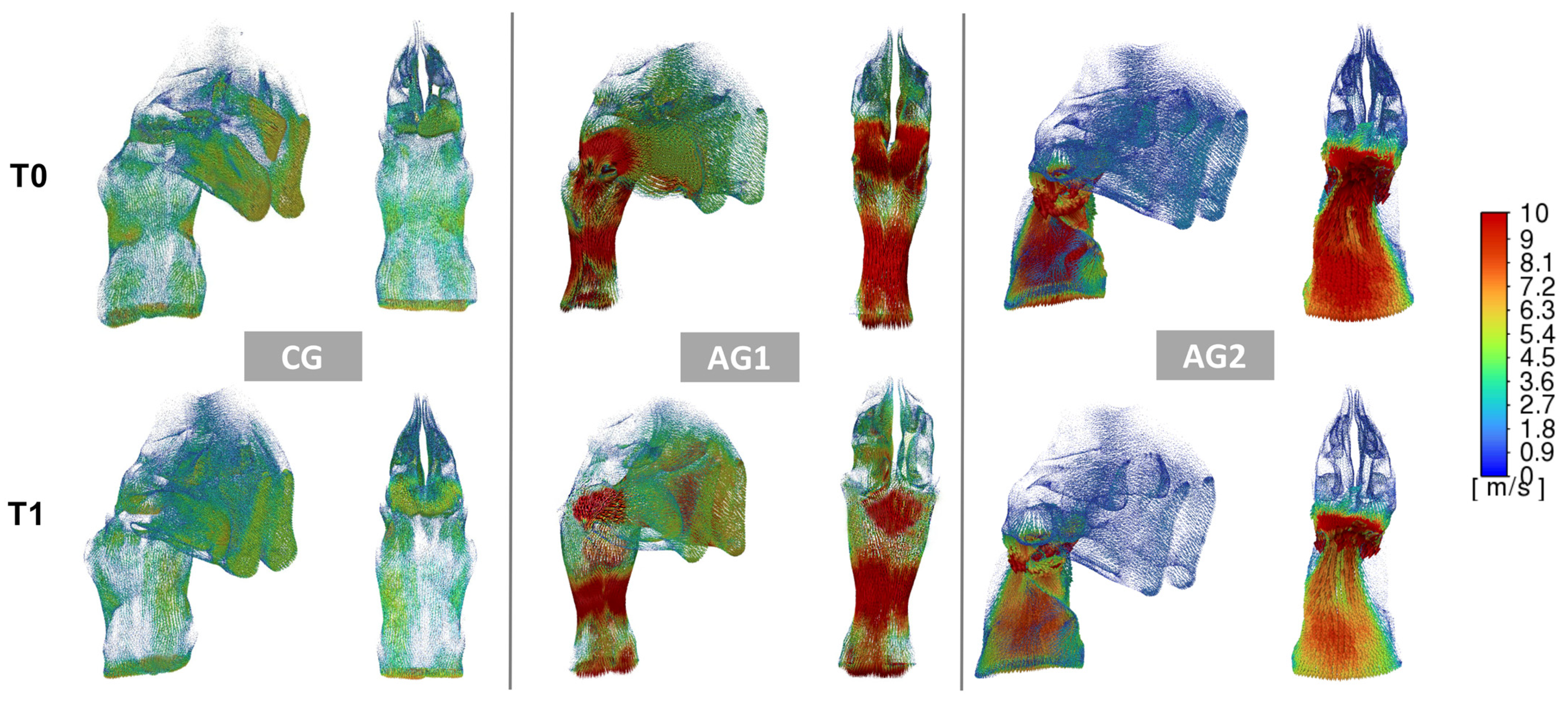
3.3. Inter-Timing Changes (T0–T1)
3.4. Comparison of the Changes Among CG, AG1 and AG2 Groups
3.5. Correlation Between Ventilation Parameters and Skeletal Parameters
4. Discussion
4.1. Concerning
4.2. Limitations
- -
- The primary limitation of this study is the absence of an untreated control group, which would have provided insights into the natural developmental changes of the upper airways in growing individuals. However, the use of CBCT scans in untreated controls raises ethical concerns due to unnecessary exposure to ionizing radiation in pediatric subjects [40,41]. While the control group in this study cannot fully replace an untreated control group, it did allow for the differentiation between two distinct pharyngeal tissue conditions: normal (adenoidal obstruction < 25%) and hypertrophic (AG1 and AG2).
- -
- The results of this study cannot be generalized for several reasons: (1) the study focused on adenoidal hypertrophy as the primary factor, excluding other potential causes of obstruction (e.g., palatine tonsils, nasal valve collapse, soft tissue changes, etc.); (2) the sample was a retrospective orthodontic cohort that did not include otolaryngological examination. Future studies are encouraged to evaluate the effects of RME on upper airway ventilation with and without otolaryngological intervention, and (3) the anterior portion of the nasal cavity was excluded from the segmentation and CFD analysis due to low accuracy in 3D modeling of this region. Although including this area could have affected CFD patterns, such differences are likely of minimal comparative significance, as the main differences between the groups were linked to the patency of the posterior airways.
5. Conclusions
- -
- Twelve months after treatment (T1), there was a slight improvement in the ventilation conditions (pressure, velocity, and obstructions) in both CG, AG1, and AG2. However, such increment was significantly greater in AG1 compared to the other two groups.
- -
- The alterations in the adenotonsillar region likely represent the most substantial factors influencing UA airflow changes.
- -
- A general weak tendency toward inverse correlation was found between the increment of CS2 and the improvement of ventilation parameters only in AG1.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UA | upper airways |
| RME | rapid maxillary expansion |
| CBCT | Cone-Beam Computed Tomography |
| CFD | computational fluid dynamics |
| MPS | maturation stage of the mid-palatal suture |
| NSD | nasal septum deviation |
| FH | Frankfort plane |
| ICC | Intraclass Correlation Coefficient |
| ENT | Ear, Nose, and Throat |
References
- Bucci, R.; D’Anto, V.; Rongo, R.; Valletta, R.; Martina, R.; Michelotti, A. Dental and skeletal effects of palatal expansion techniques: A systematic review of the current evidence from systematic reviews and meta-analyses. J. Oral Rehabil. 2016, 43, 543–564. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xu, T.; Zou, W. Effects of rapid maxillary expansion on the midpalatal suture: A systematic review. Eur. J. Orthod. 2015, 37, 651–655. [Google Scholar] [CrossRef]
- Zhou, Y.; Long, H.; Ye, N.; Xue, J.; Yang, X.; Liao, L.; Lai, W. The effectiveness of non-surgical maxillary expansion: A meta-analysis. Eur. J. Orthod. 2014, 36, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Bucci, R.; Montanaro, D.; Rongo, R.; Valletta, R.; Michelotti, A.; D’Anto, V. Effects of maxillary expansion on the upper airways: Evidence from systematic reviews and meta-analyses. J. Oral Rehabil. 2019, 46, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Lo Giudice, A.; Fastuca, R.; Portelli, M.; Militi, A.; Bellocchio, M.; Spinuzza, P.; Briguglio, F.; Caprioglio, A.; Nucera, R. Effects of rapid vs. slow maxillary expansion on nasal cavity dimensions in growing subjects: A methodological and reproducibility study. Eur. J. Paediatr. Dent. 2017, 18, 299–304. [Google Scholar]
- Motro, M.; Schauseil, M.; Ludwig, B.; Zorkun, B.; Mainusch, S.; Ateş, M.; Küçükkeleş, N.; Korbmacher-Steiner, H. Rapid-maxillary-expansion induced rhinological effects: A retrospective multicenter study. Eur. Arch. Otorhinolaryngol. 2016, 273, 679–687. [Google Scholar] [CrossRef]
- Garrocho-Rangel, A.; Rosales-Berber, M.; Ballesteros-Torres, A.; Hernández-Rubio, Z.; Flores-Velázquez, J.; Yáñez-González, E.; Ruiz-Rodríguez, S.; Pozos-Guillén, A. Rapid maxillary expansion and its consequences on the nasal and oropharyngeal anatomy and breathing function of children and adolescents: An umbrella review. Int. J. Pediatr. Otorhinolaryngol. 2023, 171, 111633. [Google Scholar] [CrossRef]
- Baratieri, C.; Alves, M., Jr.; de Souza, M.M.; de Souza Araújo, M.T.; Maia, L.C. Does rapid maxillary expansion have long-term effects on airway dimensions and breathing? Am. J. Orthod. Dentofac. Orthop. 2011, 140, 146–156. [Google Scholar] [CrossRef]
- Kikuchi, M. Orthodontic treatment in children to prevent sleep-disordered breathing in adulthood. Sleep Breath 2005, 9, 146–158. [Google Scholar] [CrossRef]
- Calvo-Henriquez, C.; Capasso, R.; Chiesa-Estomba, C.; Liu, S.Y.; Martins-Neves, S.; Castedo, E.; O’Connor-Reina, C.; Ruano-Ravina, A.; Kahn, S. The role of pediatric maxillary expansion on nasal breathing. A systematic review and metanalysis. Int. J. Pediatr. Otorhinolaryngol. 2020, 135, 110139. [Google Scholar] [CrossRef]
- Doruk, C.; Sökücü, O.; Sezer, H.; Canbay, E.I. Evaluation of nasal airway resistance during rapid maxillary expansion using acoustic rhinometry. Eur. J. Orthod. 2004, 26, 397–401. [Google Scholar] [CrossRef] [PubMed]
- De Felippe, N.L.O.; Da Silveira, A.C.; Viana, G.; Kusnoto, B.; Smith, B.; Evans, C.A. Relationship between rapid maxillary expansion and nasal cavity size and airway resistance: Short- and long-term effects. Am. J. Orthod. Dentofac. Orthop. 2008, 134, 370–382. [Google Scholar] [CrossRef]
- Leboulanger, N. Nasal obstruction in children. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2016, 133, 183–186. [Google Scholar] [CrossRef]
- Sakoda-Iwata, R.; Iwasaki, T.; Tsujii, T.; Hisagai, S.; Oku, Y.; Ban, Y.; Sato, H.; Ishii, H.; Kanomi, R.; Yamasaki, Y. Does rapid maxillary expansion improve nasal airway obstruction? A computer fluid dynamics study in patients with nasal mucosa hypertrophy and obstructive adenoids. Am. J. Orthod. Dentofac. Orthop. 2023, 164, e1–e13. [Google Scholar] [CrossRef]
- Papaioannou, G.; Kambas, I.; Tsaoussoglou, M.; Panaghiotopoulou-Gartagani, P.; Chrousos, G.; Kaditis, A.G. Age-dependent changes in the size of adenotonsillar tissue in childhood: Implications for sleep-disordered breathing. J. Pediatr. 2013, 162, 269–274.e264. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Di Carlo, G.; Cornelis, M.A.; Cattaneo, P.M. Three-dimensional analyses of short- and long-term effects of rapid maxillary expansion on nasal cavity and upper airway: A systematic review and meta-analysis. Orthod. Craniofac. Res. 2020, 23, 250–276. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Saitoh, I.; Takemoto, Y.; Inada, E.; Kanomi, R.; Hayasaki, H.; Yamasaki, Y. Improvement of nasal airway ventilation after rapid maxillary expansion evaluated with computational fluid dynamics. Am. J. Orthod. Dentofac. Orthop. 2012, 141, 269–278. [Google Scholar] [CrossRef]
- Iwasaki, T.; Papageorgiou, S.N.; Yamasaki, Y.; Ali Darendeliler, M.; Papadopoulou, A.K. Nasal ventilation and rapid maxillary expansion (rme): A randomized trial. Eur. J. Orthod. 2021, 43, 283–292. [Google Scholar] [CrossRef]
- Gurani, S.F.; Cattaneo, P.M.; Rafaelsen, S.R.; Pedersen, M.R.; Thorn, J.J.; Pinholt, E.M. The effect of altered head and tongue posture on upper airway volume based on a validated upper airway analysis-an mri pilot study. Orthod. Craniofac. Res. 2020, 23, 102–109. [Google Scholar] [CrossRef]
- Angelieri, F.; Cevidanes, L.H.; Franchi, L.; Gonçalves, J.R.; Benavides, E.; McNamara, J.A., Jr. Midpalatal suture maturation: Classification method for individual assessment before rapid maxillary expansion. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 759–769. [Google Scholar] [CrossRef]
- Iwasaki, T.; Yanagisawa-Minami, A.; Suga, H.; Shirazawa, Y.; Tsujii, T.; Yamamoto, Y.; Ban, Y.; Sato-Hashiguchi, M.; Sato, H.; Kanomi, R.; et al. Rapid maxillary expansion effects of nasal airway in children with cleft lip and palate using computational fluid dynamics. Orthod. Craniofac. Res. 2019, 22, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Ronsivalle, V.; Leonardi, R.; Lagravere, M.; Flores-Mir, C.; Grippaudo, C.; Alessandri Bonetti, G.; Lo Giudice, A. Medium-term effects of rapid maxillary expansion on nasal cavity and pharyngeal airway volumes considering age as a factor: A retrospective study. J. Dent. 2024, 144, 104934. [Google Scholar] [CrossRef] [PubMed]
- Lo Giudice, A.; Ronsivalle, V.; Gastaldi, G.; Leonardi, R. Assessment of the accuracy of imaging software for 3d rendering of the upper airway, usable in orthodontic and craniofacial clinical settings. Prog. Orthod. 2022, 23, 22. [Google Scholar] [CrossRef] [PubMed]
- De Backer, J.W.; Vanderveken, O.M.; Vos, W.G.; Devolder, A.; Verhulst, S.L.; Verbraecken, J.A.; Parizel, P.M.; Braem, M.J.; Van de Heyning, P.H.; De Backer, W.A. Functional imaging using computational fluid dynamics to predict treatment success of mandibular advancement devices in sleep-disordered breathing. J. Biomech. 2007, 40, 3708–3714. [Google Scholar] [CrossRef]
- Gamiño, B.; Aguillón, J. Numerical simulation of syngas combustion with a multi-spark ignition system in a diesel engine adapted to work at the otto cycl. Fuel 2010, 89, 581–591. [Google Scholar] [CrossRef]
- Kobayashi, R.; Miyazaki, S.; Karaki, M.; Kobayashi, E.; Karaki, R.; Akiyama, K.; Matsubara, A.; Mori, N. Measurement of nasal resistance by rhinomanometry in 892 japanese elementary school children. Auris Nasus Larynx 2011, 38, 73–76. [Google Scholar] [CrossRef]
- Behrents, R.G.; Shelgikar, A.V.; Conley, R.S.; Flores-Mir, C.; Hans, M.; Levine, M.; McNamara, J.A.; Palomo, J.M.; Pliska, B.; Stockstill, J.W.; et al. Obstructive sleep apnea and orthodontics: An american association of orthodontists white paper. Am. J. Orthod. Dentofac. Orthop. 2019, 156, 13–28.e11. [Google Scholar] [CrossRef]
- Handelman, C.S.; Osborne, G. Growth of the nasopharynx and adenoid development from one to eighteeen years. Angle Orthod. 1976, 46, 243–259. [Google Scholar]
- Yoon, A.; Abdelwahab, M.; Bockow, R.; Vakili, A.; Lovell, K.; Chang, I.; Ganguly, R.; Liu, S.Y.; Kushida, C.; Hong, C. Impact of rapid palatal expansion on the size of adenoids and tonsils in children. Sleep Med. 2022, 92, 96–102. [Google Scholar] [CrossRef]
- Pereira, L.; Monyror, J.; Almeida, F.T.; Almeida, F.R.; Guerra, E.; Flores-Mir, C.; Pachêco-Pereira, C. Prevalence of adenoid hypertrophy: A systematic review and meta-analysis. Sleep Med. Rev. 2018, 38, 101–112. [Google Scholar] [CrossRef]
- Langer, M.R.; Itikawa, C.E.; Valera, F.C.; Matsumoto, M.A.; Anselmo-Lima, W.T. Does rapid maxillary expansion increase nasopharyngeal space and improve nasal airway resistance? Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 122–125. [Google Scholar] [CrossRef]
- Marcus, C.L.; Moore, R.H.; Rosen, C.L.; Giordani, B.; Garetz, S.L.; Taylor, H.G.; Mitchell, R.B.; Amin, R.; Katz, E.S.; Arens, R.; et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N. Engl. J. Med. 2013, 368, 2366–2376. [Google Scholar] [CrossRef]
- Hoxha, S.; Kaya-Sezginer, E.; Bakar-Ates, F.; Köktürk, O.; Toygar-Memikoğlu, U. Effect of semi-rapid maxillary expansion in children with obstructive sleep apnea syndrome: 5-month follow-up study. Sleep Breath 2018, 22, 1053–1061. [Google Scholar] [CrossRef]
- Villarroel, T.; Yagnam, S.; Vicuña, D.; Concha, G.; Oyonarte, R. Midpalatal suture maturation in 15- to 35-year-olds: Morphological assessment in the coronal plane using cbct-an exploratory study. Odontology 2023, 112, 647–656. [Google Scholar] [CrossRef]
- Alsufyani, N.A.; Al-Saleh, M.A.; Major, P.W. Cbct assessment of upper airway changes and treatment outcomes of obstructive sleep apnoea: A systematic review. Sleep Breath 2013, 17, 911–923. [Google Scholar] [CrossRef]
- Buck, L.M.; Dalci, O.; Darendeliler, M.A.; Papageorgiou, S.N.; Papadopoulou, A.K. Volumetric upper airway changes after rapid maxillary expansion: A systematic review and meta-analysis. Eur. J. Orthod. 2017, 39, 463–473. [Google Scholar] [CrossRef]
- Principato, J.J.; Wolf, P. Pediatric nasal resistance. Laryngoscope 1985, 95, 1067–1069. [Google Scholar] [CrossRef]
- Xie, B.; Zhang, L.; Lu, Y. The role of rapid maxillary expansion in pediatric obstructive sleep apnea: Efficacy, mechanism and multidisciplinary collaboration. Sleep Med. Rev. 2023, 67, 101733. [Google Scholar] [CrossRef]
- Crouse, U.; Laine-Alava, M.T.; Warren, D.W. Nasal impairment in prepubertal children. Am. J. Orthod. Dentofac. Orthop. 2000, 118, 69–74. [Google Scholar] [CrossRef]
- Oenning, A.C.; Jacobs, R.; Salmon, B. Aladaip, beyond alara and towards personalized optimization for paediatric cone-beam ct. Int. J. Paediatr. Dent. 2021, 31, 676–678. [Google Scholar] [CrossRef]
- Jaju, P.P.; Jaju, S.P. Cone-beam computed tomography: Time to move from alara to alada. Imaging Sci. Dent. 2015, 45, 263–265. [Google Scholar] [CrossRef] [PubMed]


| Sample | Total (n = 67) | Control Group (CG; n = 24) | Adenoids Group 1 (AG1; n = 22) | Adenoids Group 2 (AG2; n = 21) | Significance * | |
|---|---|---|---|---|---|---|
| Characteristics | ||||||
| Mean/n | Mean/n | Mean/n | ||||
| Mean age | 11.8 (± 1.3) | 10.9 (± 1.5) | 11.2 (± 1.6) | NS | ||
| Gender | NS | |||||
| Male | 10 | 9 | 9 | |||
| Female | 14 | 13 | 12 | |||
| MPS Stage | NS | |||||
| Stage A | 11 | 14 | 10 | |||
| Stage B | 13 | 8 | 11 | |||
| NSD | ||||||
| Yes | 6 | 7 | 8 | NS | ||
| No | 18 | 15 | 13 | |||
| CG (n = 24) (a) | AG 1 (n =22) (b) | AG 2 (n = 21) (c) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | p Value * | Median | IQR | p Value * | Median | IQR | p Value * | p Value ** | |||
| Airway Pressure (Pa) | ||||||||||||
| T0 | 82.625 | (54.25–102.43) | <0.05 | 198.24 | (157.57–240.98) | <0.05 | 254.6 | (236.73–297.10) | 0.066 | <0.05 (a-b, a-c) | ||
| T1 | 70.62 | (42.53–88.56) | 159.22 | (127.90–181.25) | 231.44 | (187.11–264.46) | <0.05 (a-b, a-c, b-c) | |||||
| T1-T2 | 12.01 | (−3.74–22.21) | - | 39.02 | (4.69–60.16) | - | 23.16 | (8.59–66.09) | - | <0.05 (b-a, b-c) | ||
| Change (%) | 14.53 | 19.68 | 9.10 | |||||||||
| Airway Velocity (m/s) | ||||||||||||
| T0 | 6.65 | (5.54–8.31) | 0.092 | 15.54 | (9.07–21.22) | <0.05 | 27.42 | (16.51–31.06) | 0.074 | <0.05 (a-b, a-c, b-c) | ||
| T1 | 5.93 | (4.11–7.43) | 12.68 | (6.39–15.92) | 25.47 | (22.55–35.75) | <0.05 (a-b, a-c, b-c) | |||||
| T1-T2 | 0.72 | (0.51–3.15) | - | 2.86 | (1.06–7.47) | - | 1.95 | (−6.06–6.96) | - | <0.05 (b-a, b-c) | ||
| Change (%) | 10.83 | 18.40 | 7.11 | |||||||||
| PW (mm) | ||||||||||||
| T0 | 24.16 | (22.64–29.13) | <0.001 | 22.64 | (20.89–25.19) | <0.001 | 23.68 | (21.37–25.98) | <0.05 | 0.231 | ||
| T1 | 26.87 | (23.95–31.59) | 24.99 | (22.81–27.48) | 26.04 | (24.53–28.14) | 0.379 | |||||
| T1-T2 | 2.71 | (0.77–3.34) | - | 2.35 | (0.97–2.91) | - | 2.36 | (0.61–4.46) | - | 0.406 | ||
| Change (%) | 11.22 | 10.38 | 9.97 | |||||||||
| CS1 (mm2) | ||||||||||||
| T0 | 166.09 | (140.07–191.28) | <0.001 | 156.40 | (126.28–175.10) | <0.001 | 198.11 | (169.18–213.41) | <0.001 | 0.092 | ||
| T1 | 199.32 | (176.64–218.49) | 192.76 | (178.26–204.32) | 228.56 | (189.96–248.93) | 0.121 | |||||
| T1-T2 | 33.23 | (11.15–54.03) | - | 36.36 | (15.01–50.50) | - | 30.45 | (18.02–45.11) | - | 0.532 | ||
| Change (%) | 20.01 | 23.25 | 15.37 | |||||||||
| CS2 (mm2) | ||||||||||||
| T0 | 235.4 | (222.65–271.08) | <0.001 | 198.67 | (190.01–235.91) | <0.05 | 217.25 | (195.25–228.99) | <0.05 | 0.256 | ||
| T1 | 270.89 | (236.83–312.50) | 228.56 | (209.10–251.27) | 236.17 | (202.53–261.31) | 0.441 | |||||
| T1-T2 | 35.49 | (9.10–46.66) | - | 29.89 | (6.40–44.13) | - | 18.92 | (0.75–32.32) | - | 0.358 | ||
| Change (%) | 15.08 | 15.05 | 8.71 | |||||||||
| Obstruction (n) | T0 | T1 | p value *** | T0 | T1 | p value **** | T0 | T1 | p value *** | p value **** | ||
| YES | 7 | 1 | <0.001 | 16 | 8 | 0.151 | 16 | 14 | 0.606 | <0.05 | ||
| NO | 17 | 23 | 6 | 16 | 5 | 7 | ||||||
| Groups | Timing | Airway Ventilation | PW | CS1 | CS2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T1-T0 | T0 | T1 | T1-T0 | T0 | T1 | T1-T0 | |||||
| CG | T0 | Pressure | −0.103 | - | −0.074 | - | −0.484 * | - | |||||
| Velocity | −0.062 | −0.265 | −0.230 | ||||||||||
| T1 | Pressure | - | −0.194 | - | - | −0.264 | - | - | −0.571 * | - | |||
| Velocity | −0.065 | −0.347 | −0.331 | ||||||||||
| T1-T0 | Pressure | - | −0.327 | - | −0.067 | - | −0.021 | ||||||
| Velocity | −0.25 | −0.15 | −0.072 | ||||||||||
| AG1 | T0 | Pressure | −0.169 | - | −0.227 | - | −0.408 | - | |||||
| Velocity | 0.033 | 0.109 | −0.495 * | ||||||||||
| T1 | Pressure | - | 0.104 | - | - | −0.234 | - | - | −0.403 * | - | |||
| Velocity | −0.204 | −0.277 | −0.580 * | ||||||||||
| T1-T0 | Pressure | - | −0.283 | - | −0.146 | - | −0.415 * | ||||||
| Velocity | 0.114 | −0.118 | −0.498 * | ||||||||||
| AG2 | T0 | Pressure | −0.025 | −0.125 | −0.361 | ||||||||
| Velocity | −0.274 | 0.026 | −0.302 | ||||||||||
| T1 | Pressure | −0.028 | −0.235 | −0.391 | |||||||||
| Velocity | −0.121 | −0.341 | −0.317 | ||||||||||
| T1-T0 | Pressure | −0.027 | −0.131 | −0.204 | |||||||||
| Velocity | −0.26 | 0.164 | −0.378 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palazzo, G.; Leonardi, R.; Isola, G.; Lagravere, M.; Lo Giudice, A. Changes in Upper Airway Airflow After Rapid Maxillary Expansion Beyond the Peak Period of Adenoidal Growth—A CBCT Study Using Computer Fluid Dynamics and Considering Adenoidal Dimensions as a Factor. Dent. J. 2025, 13, 209. https://doi.org/10.3390/dj13050209
Palazzo G, Leonardi R, Isola G, Lagravere M, Lo Giudice A. Changes in Upper Airway Airflow After Rapid Maxillary Expansion Beyond the Peak Period of Adenoidal Growth—A CBCT Study Using Computer Fluid Dynamics and Considering Adenoidal Dimensions as a Factor. Dentistry Journal. 2025; 13(5):209. https://doi.org/10.3390/dj13050209
Chicago/Turabian StylePalazzo, Giuseppe, Rosalia Leonardi, Gaetano Isola, Manuel Lagravere, and Antonino Lo Giudice. 2025. "Changes in Upper Airway Airflow After Rapid Maxillary Expansion Beyond the Peak Period of Adenoidal Growth—A CBCT Study Using Computer Fluid Dynamics and Considering Adenoidal Dimensions as a Factor" Dentistry Journal 13, no. 5: 209. https://doi.org/10.3390/dj13050209
APA StylePalazzo, G., Leonardi, R., Isola, G., Lagravere, M., & Lo Giudice, A. (2025). Changes in Upper Airway Airflow After Rapid Maxillary Expansion Beyond the Peak Period of Adenoidal Growth—A CBCT Study Using Computer Fluid Dynamics and Considering Adenoidal Dimensions as a Factor. Dentistry Journal, 13(5), 209. https://doi.org/10.3390/dj13050209










