Influence of Time Interval, Temperature, and Storage Condition on Fluoride Release and Recharge from Silorane-Based Restorative Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Specimen Preparation
2.2. Release of Fluoride
2.3. Recharge of Fluoride
2.4. Statistical Analysis
3. Results
3.1. For Fluoride Release
3.2. For Fluoride Recharge
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SBC | Silorane-based composites |
| MBC | Methacrylate-based composites |
| GIC | Glass ionomer cement |
| TISAB | Total ionic strength adjustment buffer |
| NaF | Sodium fluoride |
| FR | Fluoride release |
| PLR | Powder-to-liquid ratio |
| LED | Light-emitting diode |
| AS | Artificial saliva |
| DW | Distilled water |
| APF | Acidulated phosphate fluoride |
References
- Eick, J.D.; Kostoryz, E.L.; Rozzi, S.M.; Jacobs, D.W.; Oxman, J.D.; Chappelow, C.C.; Glaros, A.G.; Yourtee, D.M. In vitro biocompatibility of oxirane/polyol dental composites with promising physical properties. Dent. Mater. 2002, 18, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.M.; Ghinea, R.; Ugarte-Alván, L.I.; Pulgar, R.; Paravina, R.D. Color and translucency in silorane-based resin composite compared to universal and nanofilled composites. J. Dent. 2010, 38, e110–e116. [Google Scholar] [CrossRef]
- Madhyastha, P.S.; Bhat, K.M.; Padma, D.; Bangera, M.K.; Naik, D.G.; Srikant, N. Cytotoxicity of Silorane and Methacrylate based Dental Composites on Human Pulp Cells. J. Orofac. Sci. 2021, 13, 13. [Google Scholar] [CrossRef]
- Hollanders, A.C.C. Closing the Gap: Modifying Factors in Secondary Caries. Ph.D. Dissertation, Radboud University, Nijmegen, The Netherlands, 27 June 2022. [Google Scholar]
- Clarkson, J.J.; McLoughlin, J. Role of fluoride in oral health promotion. Int. Dent. J. 2000, 50, 119–128. [Google Scholar] [CrossRef]
- Bratthall, D.; Hänsel-Petersson, G.; Sundberg, H. Reasons for the caries decline: What do the experts believe? Eur. J. Oral. Sci. 1996, 104, 416–422. [Google Scholar] [CrossRef]
- Burke, F.M.; Ray, N.J.; McConnell, R.J. Fluoride-containing restorative materials. Int. Dent. J. 2006, 56, 33–43. [Google Scholar] [CrossRef]
- Wiegand, A.; Buchalla, W.; Attin, T. Review on fluoride-releasing restorative materials-fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent. Mater. 2007, 23, 343–362. [Google Scholar] [CrossRef]
- Yan, Z.; Sidhu, S.K.; Mahmoud, G.A.; Carrick, T.E.; McCabe, J.F. Effects of temperature on the fluoride release and recharging ability of glass ionomers. Oper. Dent. 2007, 32, 138–143. [Google Scholar] [CrossRef]
- Yoda, A.; Nikaido, T.; Ikeda, M.; Sonoda, H.; Foxton, R.M.; Tagami, J. Effect of curing method and storage condition on fluoride ion release from a fluoride-releasing resin cement. Dent. Mater. J. 2006, 25, 261–266. [Google Scholar] [CrossRef]
- Preston, A.J.; Mair, L.H.; Agalamanyi, E.A.; Higham, S.M. Fluoride release from aesthetic dental materials. J. Oral. Rehabil. 1999, 26, 123–129. [Google Scholar] [CrossRef]
- Al-Naimi, O.T.; Itota, T.; Hobson, R.S.; McCabe, J.F. Fluoride release for restorative materials and its effect on biofilm formation in natural saliva. J. Mater. Sci. Mater. Med. 2008, 19, 1243–1248. [Google Scholar] [PubMed]
- Xu, X.; Burgess, J.O. Compressive strength, fluoride release and recharge of fluoride-releasing materials. Biomaterials 2003, 24, 2451–2461. [Google Scholar] [PubMed]
- Kelić, K.; Par, M.; Peroš, K.; Šutej, I.; Tarle, Z. Fluoride-Releasing Restorative Materials: The Effect of a Resinous Coat on Ion Release. Acta Stomatol. Croat. 2020, 54, 371–381. [Google Scholar]
- Panpisut, P.; Monmaturapoj, N.; Srion, A.; Angkananuwat, C.; Krajangta, N.; Panthumvanit, P. The effect of powder to liquid ratio on physical properties and fluoride release of glass ionomer cements containing pre-reacted spherical glass fillers. Dent. Mater. J. 2020, 39, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Turjanski, S.; Par, M.; Bergman, L.; Soče, M.; Grego, T.; Klarić Sever, E. Influence of Ionizing Radiation on Fluoride-Releasing Dental Restorative Materials. Polymers 2023, 15, 632. [Google Scholar] [CrossRef]
- Singh, H.; Rashmi, S.; Pai, S.; Kini, S. Comparative Evaluation of Fluoride Release from Two Different Glass Ionomer Cement and a Novel Alkasite Restorative Material-An in Vitro Study. Pesqui. Bras. Em Odontopediatria E Clínica Integr. 2020, 20, 1590. [Google Scholar]
- Nedeljkovic, I.; De Munck, J.; Vanloy, A.; Declerck, D.; Lambrechts, P.; Peumans, M.; Teughels, W.; Van Meerbeek, B.; Van Landuyt, K.L. Secondary caries: Prevalence, characteristics, and approach. Clin. Oral Investig. 2020, 24, 683–691. [Google Scholar]
- Eskandarizadeh, A.; Mohammadzadeh, I.; Shahravan, A.; Bavafa, M.; Kakooei, S.; Torabi, M. Prevention of secondary caries by a new antibacterial compound. Dent. Res. J. 2020, 17, 40–47. [Google Scholar]
- Al Rabiah, A.; Zahrah, A.; Malath, T.; Ebtihal, A.D.; Daniyah, A.S.; Abdullah, A.Q. Dental Composite Restorations Repair: A Systematic Review and Meta-Analysis. J. Pharm. Res. Int. 2021, 33, 707–738. [Google Scholar]
- Kumari, P.D.; Khijmatgar, S.; Chowdhury, A.; Lynch, E.; Chowdhury, C.R. Factors influencing fluoride release in atraumatic restorative treatment (ART) materials: A review. J. Oral Biol. Craniofacial Res. 2019, 9, 315–320. [Google Scholar] [CrossRef]
- Hadi, M.R. Effect of Increased Fluoride Contents on Fluoride Release from Glass Ionomer Cements. Syst. Rev. Pharm. 2020, 11, 440–443. [Google Scholar]
- Braden, M.; Clarke, R.L. Water absorption characteristics of dental microfine composite filling materials: I. Proprietary materials. Biomaterials 1984, 5, 369–372. [Google Scholar] [PubMed]
- Damen, J.J.; Buijs, M.J.; Ten Cate, J.M. Uptake and release of fluoride by saliva-coated glass ionomer cement. Caries Res. 1996, 30, 454–457. [Google Scholar] [PubMed]
- Carvalho, A.S.; Cury, J.A. Fluoride release from some dental materials in different solutions. Oper. Dent. 1999, 24, 14–19. [Google Scholar]
- Garcez, R.M.; Buzalaf, M.A.; de Araújo, P.A. Fluoride release of six restorative materials in water and pH-cycling solutions. J. Appl. Oral. Sci. 2007, 15, 406–411. [Google Scholar]
- Moreau, J.L.; Xu, H.H. Fluoride releasing restorative materials: Effects of pH on mechanical properties and ion release. Dent. Mater. 2010, 26, e227–e235. [Google Scholar]
- Jabin, Z.; Nasim, I.; Vishnu Priya, V. Quantitative Analysis and Effect of SDF, APF, NaF on Demineralized Human Primary Enamel Using SEM, XRD, and FTIR. Int. J. Clin. Pediatr. Dent. 2021, 14, 537–541. [Google Scholar]
- Yan, I.G.; Zheng, F.M.; Gao, S.S.; Duangthip, D.; Lo, E.C.M.; Chu, C.H. Fluoride Delivered via a Topical Application of 38% SDF and 5% NaF. Int. Dent. J. 2022, 72, 773–778. [Google Scholar]
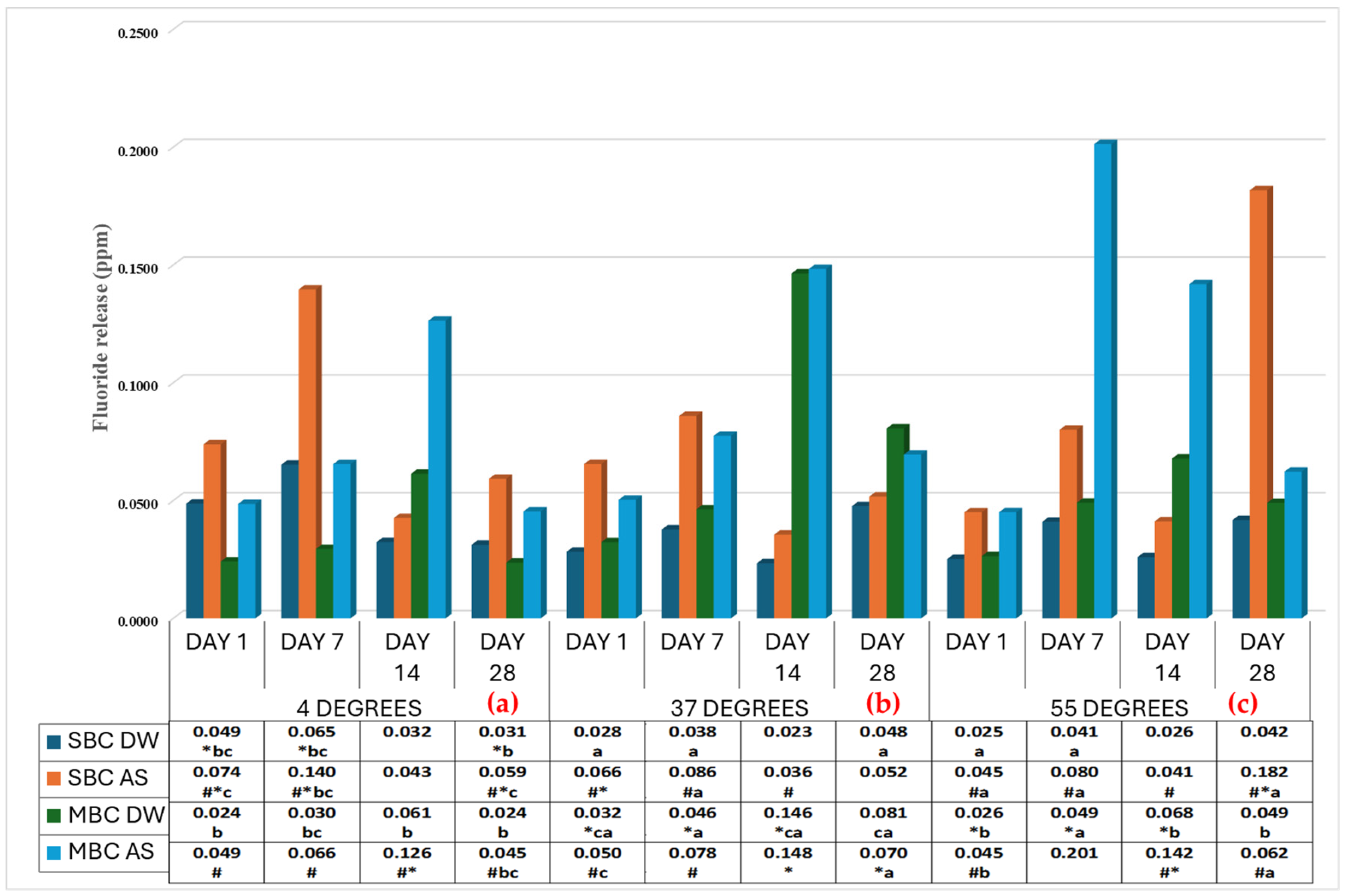
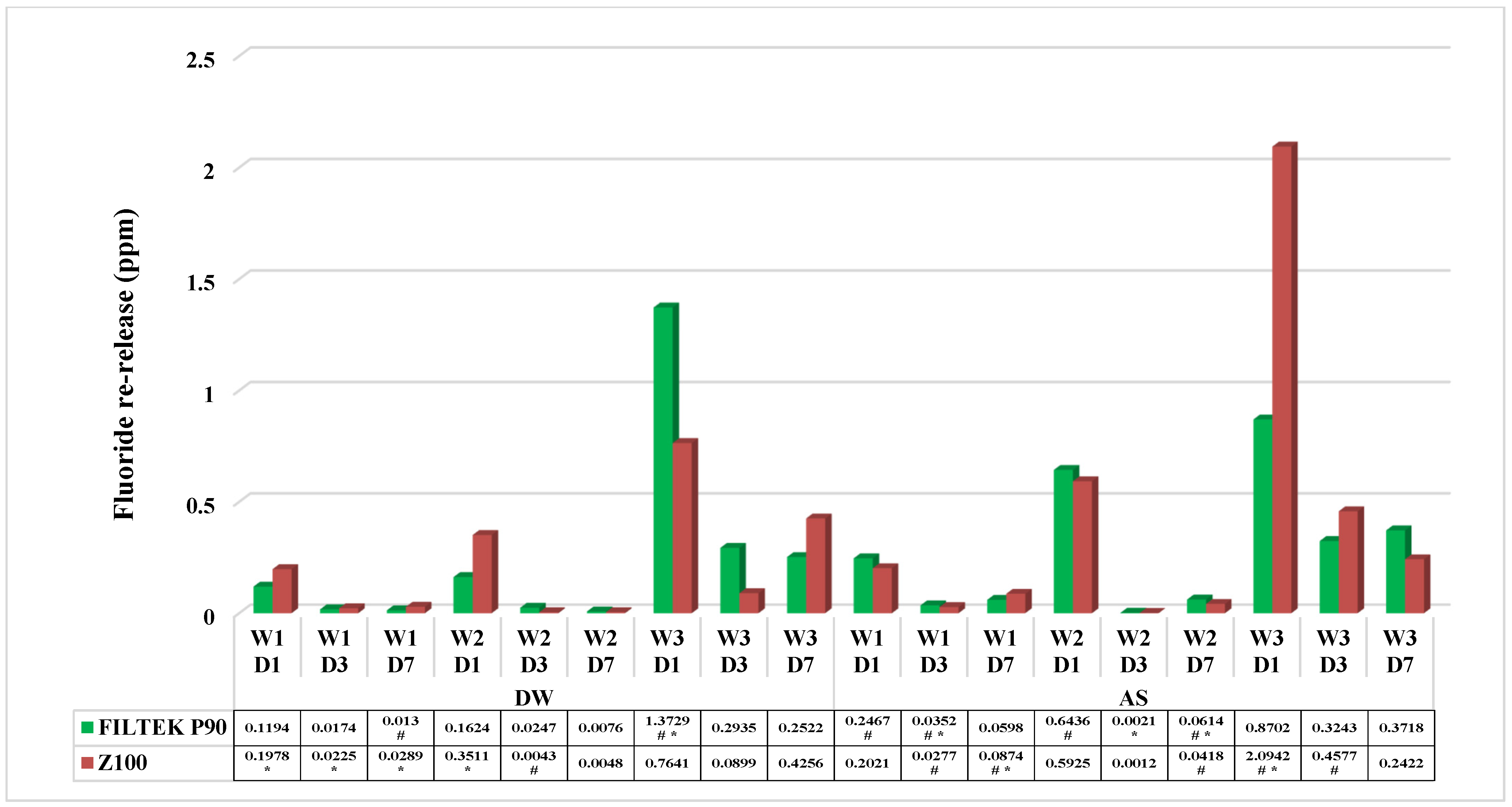
| Name | Type | Manufacturer | Shade | Organic Matrix | Inorganic Fillers | Filler Content [vol.%] |
|---|---|---|---|---|---|---|
| Filtek P90 [SBC] | Silorane-based micro hybrid composite | 3M/ESPE, St. Paul, MN, USA | A2 | 3,4-Epoxycyclohexylethyl cyclopolymethylsiloxane, bis-3,4-poxycyclohexylethylphenylmethylsilane | Silanized quartz, yttrium fluoride | 55 |
| Z100 [MBC] | Methacrylate-based hybrid composite | 3M/ESPE, St. Paul, MN, USA | A2 | Bis-GMA and TEGDMA | Zirconium, silica | 66 |
| AS (Mean ± sd) | DW (Mean ± sd) | t | p Value | |||
|---|---|---|---|---|---|---|
| 4 Degrees | SBC | DAY 1 | 0.074 ± 0.028 | 0.049 ± 0.017 | 2.425 | 0.028 |
| DAY 7 | 0.14 ± 0.066 | 0.065 ± 0.033 | 3.182 | 0.007 | ||
| DAY 14 | 0.043 ± 0.011 | 0.032 ± 0.016 | 1.691 | 0.11 | ||
| DAY 28 | 0.059 ± 0.015 | 0.031 ± 0.006 | 5.368 | <0.001 | ||
| MBC | DAY 1 | 0.049 ± 0.005 | 0.024 ± 0.002 | 14.96 | <0.001 | |
| DAY 7 | 0.065 ± 0.004 | 0.029 ± 0.002 | 26.935 | <0.001 | ||
| DAY 14 | 0.126 ± 0.05 | 0.061 ± 0.052 | 2.865 | 0.01 | ||
| DAY 28 | 0.045 ± 0.002 | 0.024 ± 0.001 | 27.801 | <0.001 | ||
| 37 Degrees | SBC | DAY 1 | 0.066 ± 0.02 | 0.028 ± 0.002 | 5.837 | <0.001 |
| DAY 7 | 0.086 ± 0.003 | 0.038 ± 0.002 | 41.226 | <0.001 | ||
| DAY 14 | 0.036 ± 0.005 | 0.023 ± 0.001 | 6.903 | <0.001 | ||
| DAY 28 | 0.052 ± 0.001 | 0.048 ± 0.014 | 0.907 | 0.388 | ||
| MBC | DAY 1 | 0.05 ± 0.003 | 0.032 ± 0.004 | 11.538 | <0.001 | |
| DAY 7 | 0.077 ± 0.013 | 0.046 ± 0.007 | 6.914 | <0.001 | ||
| DAY 14 | 0.148 ± 0.032 | 0.146 ± 0.035 | 0.127 | 0.9 | ||
| DAY 28 | 0.07 ± 0.014 | 0.081 ± 0.049 | −0.688 | 0.506 | ||
| 55 Degrees | SBC | DAY 1 | 0.045 ± 0.002 | 0.025 ± 0.001 | 35.242 | <0.001 |
| DAY 7 | 0.08 ± 0.004 | 0.041 ± 0.004 | 23.386 | <0.001 | ||
| DAY 14 | 0.041 ± 0.004 | 0.026 ± 0.001 | 11.269 | <0.001 | ||
| DAY 28 | 0.182 ± 0.022 | 0.042 ± 0.013 | 17.58 | <0.001 | ||
| MBC | DAY 1 | 0.045 ± 0.004 | 0.026 ± 0 | 15.683 | <0.001 | |
| DAY 7 | 0.201 ± 0.274 | 0.049 ± 0.003 | 1.756 | 0.113 | ||
| DAY 14 | 0.142 ± 0.01 | 0.068 ± 0.039 | 5.775 | <0.001 | ||
| DAY 28 | 0.062 ± 0.007 | 0.049 ± 0.005 | 5.121 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madhyastha, P.S.; Naik, D.G.; Natarajan, S.; Vinodhini, R.S. Influence of Time Interval, Temperature, and Storage Condition on Fluoride Release and Recharge from Silorane-Based Restorative Materials. Dent. J. 2025, 13, 197. https://doi.org/10.3390/dj13050197
Madhyastha PS, Naik DG, Natarajan S, Vinodhini RS. Influence of Time Interval, Temperature, and Storage Condition on Fluoride Release and Recharge from Silorane-Based Restorative Materials. Dentistry Journal. 2025; 13(5):197. https://doi.org/10.3390/dj13050197
Chicago/Turabian StyleMadhyastha, Prashanthi S., Dilip G. Naik, Srikant Natarajan, and Rachel Sarah Vinodhini. 2025. "Influence of Time Interval, Temperature, and Storage Condition on Fluoride Release and Recharge from Silorane-Based Restorative Materials" Dentistry Journal 13, no. 5: 197. https://doi.org/10.3390/dj13050197
APA StyleMadhyastha, P. S., Naik, D. G., Natarajan, S., & Vinodhini, R. S. (2025). Influence of Time Interval, Temperature, and Storage Condition on Fluoride Release and Recharge from Silorane-Based Restorative Materials. Dentistry Journal, 13(5), 197. https://doi.org/10.3390/dj13050197






