Electrolytic Cleaning of Dental Implants: A Scoping Review of Clinical Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Method
2.2. Selection of Papers
2.3. Tool for Risk of Bias Assessment
3. Results
3.1. Article Selection
3.2. Study Design, Number of Patients and Implants, and Evaluation Period
3.3. Patients’ Characteristics and Treatment Protocol
3.3.1. Periodontal and Peri-Implant Disease
3.3.2. Initial Clinical Findings
3.3.3. Treatment Protocol
Suprastructure Removal
Timing of Surgery and Electrolytic Cleaning
Evaluation Methods
3.4. Surgical Protocols
3.4.1. Flap Design
3.4.2. Bone Augmentation
3.4.3. Barrier Membrane
3.4.4. Membrane Fixation Method
3.4.5. Suture
3.5. Mechanical Decontamination, Antiseptic and/or Antibiotic Treatment
3.5.1. Powder Spray System
3.5.2. Antiseptics
3.5.3. Antibiotics
3.6. Study Results
3.6.1. Disease Resolution
3.6.2. Re-Osseointegration
3.6.3. Peri-Implantitis Recurrence
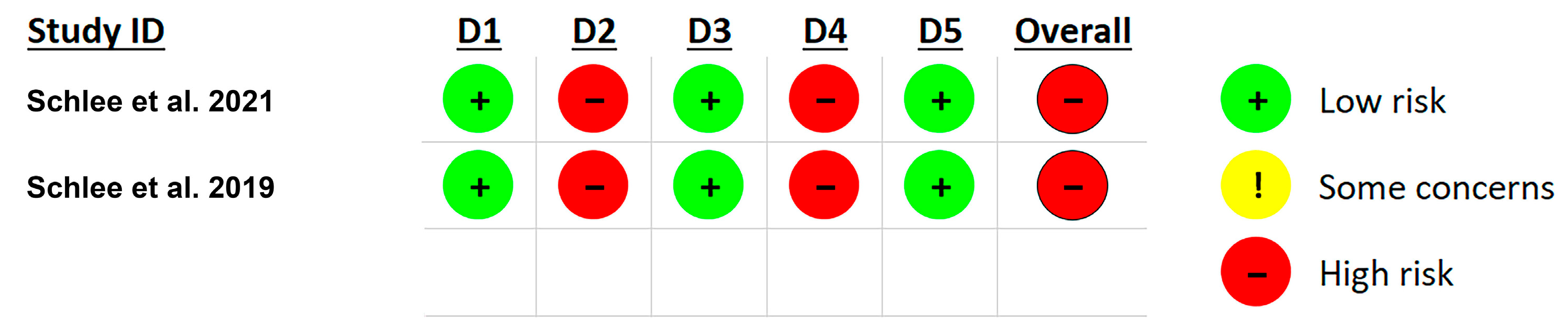
3.7. Risk of Bias Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BL | Bone level |
| BoP | Bleeding on probing |
| CBCT | Cone beam computed tomography |
| GBR | Guided bone regeneration |
| GTR | Guided tissue regeneration |
| HA | Hydroxyapatite |
| PI | Plaque index |
| PD | Probing depth |
| P-B | Distance from the implant platform to the most apical position of bone |
| PGCL | Polyglycolic acid copolymer |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PSS | Powder spray system |
| PTFE | Polytetrafluoroethylene |
| RC | Regular connection |
| RCT | Randomized controlled trial |
| SLA | Sandblasted, large-grid, acid-etched |
| Ti | Titanium |
| T00 | Pre-treatment baseline |
| T0 | Initial time point |
| T1 | First time point |
| T2 | Second time point |
| T3 | Third time point |
References
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Huttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Albrektsson, T.; Branemark, P.I.; Hansson, H.A.; Lindstrom, J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral. Implant. Res. 2009, 20 (Suppl. 4), 172–184. [Google Scholar] [CrossRef] [PubMed]
- Rompen, E.; Domken, O.; Degidi, M.; Pontes, A.E.; Piattelli, A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: A literature review. Clin. Oral. Implant. Res. 2006, 17 (Suppl. 2), 55–67. [Google Scholar] [CrossRef]
- Yamano, S.; Al-Sowygh, Z.H.; Gallucci, G.O.; Wada, K.; Weber, H.P.; Sukotjo, C. Early peri-implant tissue reactions on different titanium surface topographies. Clin. Oral. Implant. Res. 2011, 22, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Donos, N.; Hamlet, S.; Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Bosshardt, D.D.; Ivanovski, S. Gene expression profile of osseointegration of a hydrophilic compared with a hydrophobic microrough implant surface. Clin. Oral. Implant. Res. 2011, 22, 365–372. [Google Scholar] [CrossRef]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin. Oral. Implant. Res. 2011, 22, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Wall, I.; Donos, N.; Carlqvist, K.; Jones, F.; Brett, P. Modified titanium surfaces promote accelerated osteogenic differentiation of mesenchymal stromal cells in vitro. Bone 2009, 45, 17–26. [Google Scholar] [CrossRef]
- Schwarz, F.; Herten, M.; Wieland, M.; Dard, M.; Becker, J. Chemically modified, ultra-hydrophilic titanium implant surfaces. Mund. Kiefer Gesichtschir. 2007, 11, 11–17. [Google Scholar] [CrossRef]
- Rupp, F.; Gittens, R.A.; Scheideler, L.; Marmur, A.; Boyan, B.D.; Schwartz, Z.; Geis-Gerstorfer, J. A review on the wettability of dental implant surfaces I: Theoretical and experimental aspects. Acta Biomater. 2014, 10, 2894–2906. [Google Scholar] [CrossRef]
- Hori, N.; Iwasa, F.; Tsukimura, N.; Sugita, Y.; Ueno, T.; Kojima, N.; Ogawa, T. Effects of UV photofunctionalization on the nanotopography enhanced initial bioactivity of titanium. Acta Biomater. 2011, 7, 3679–3691. [Google Scholar] [CrossRef] [PubMed]
- Puisys, A.; Schlee, M.; Linkevicius, T.; Petrakakis, P.; Tjaden, A. Photo-activated implants: A triple-blinded, split-mouth, randomized controlled clinical trial on the resistance to removal torque at various healing intervals. Clin. Oral. Investig. 2020, 24, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Reasons for failures of oral implants. J. Oral. Rehabil. 2014, 41, 443–476. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Henningsen, A.; Jung, O.; Heiland, M.; Hammacher, C.; Stein, J.M. Definition, etiology, prevention and treatment of peri-implantitis—A review. Head. Face Med. 2014, 10, 34. [Google Scholar] [CrossRef]
- Derks, J.; Schaller, D.; Hakansson, J.; Wennstrom, J.L.; Tomasi, C.; Berglundh, T. Effectiveness of Implant Therapy Analyzed in a Swedish Population: Prevalence of Peri-implantitis. J. Dent. Res. 2016, 95, 43–49. [Google Scholar] [CrossRef]
- Ramanauskaite, A.; Fretwurst, T.; Schwarz, F. Efficacy of alternative or adjunctive measures to conventional non-surgical and surgical treatment of peri-implant mucositis and peri-implantitis: A systematic review and meta-analysis. Int. J. Implant. Dent. 2021, 7, 112. [Google Scholar] [CrossRef]
- Diaz, P.; Gonzalo, E.; Villagra, L.J.G.; Miegimolle, B.; Suarez, M.J. What is the prevalence of peri-implantitis? A systematic review and meta-analysis. BMC Oral. Health 2022, 22, 449. [Google Scholar] [CrossRef]
- Zipprich, H.; Weigl, P.; Di Gianfilippo, R.; Steigmann, L.; Henrich, D.; Wang, H.L.; Schlee, M.; Ratka, C. Comparison of decontamination efficacy of two electrolyte cleaning methods to diode laser, plasma, and air-abrasive devices. Clin. Oral. Investig. 2022, 26, 4549–4558. [Google Scholar] [CrossRef]
- Renvert, S.; Hirooka, H.; Polyzois, I.; Kelekis-Cholakis, A.; Wang, H.L.; Working, G. Diagnosis and non-surgical treatment of peri-implant diseases and maintenance care of patients with dental implants-Consensus report of working group 3. Int. Dent. J. 2019, 69, 12–17. [Google Scholar] [CrossRef]
- Schwarz, F.; Schmucker, A.; Becker, J. Efficacy of alternative or adjunctive measures to conventional treatment of peri-implant mucositis and peri-implantitis: A systematic review and meta-analysis. Int. J. Implant. Dent. 2015, 1, 22. [Google Scholar] [CrossRef]
- Schwarz, F.; Jepsen, S.; Obreja, K.; Galarraga-Vinueza, M.E.; Ramanauskaite, A. Surgical therapy of peri-implantitis. Periodontology 2000 2022, 88, 145–181. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Wennstrom, J.L.; Lindhe, J. Long-term outcome of surgical treatment of peri-implantitis. A 2-11-year retrospective study. Clin. Oral. Implant. Res. 2018, 29, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Roccuzzo, M.; Fierravanti, L.; Pittoni, D.; Dalmasso, P.; Roccuzzo, A. Implant survival after surgical treatment of peri-implantitis lesions by means of deproteinized bovine bone mineral with 10% collagen: 10-year results from a prospective study. Clin. Oral. Implant. Res. 2020, 31, 768–776. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A.; Salvi, G.E.; Mombelli, A.; Loup, P.J.; Heitz, F.; Kruger, E.; Lang, N.P. Supportive peri-implant therapy following anti-infective surgical peri-implantitis treatment: 5-year survival and success. Clin. Oral. Implant. Res. 2018, 29, 1–6. [Google Scholar] [CrossRef]
- Carcuac, O.; Derks, J.; Abrahamsson, I.; Wennstrom, J.L.; Petzold, M.; Berglundh, T. Surgical treatment of peri-implantitis: 3-year results from a randomized controlled clinical trial. J. Clin. Periodontol. 2017, 44, 1294–1303. [Google Scholar] [CrossRef]
- Schwarz, F.; John, G.; Schmucker, A.; Sahm, N.; Becker, J. Combined surgical therapy of advanced peri-implantitis evaluating two methods of surface decontamination: A 7-year follow-up observation. J. Clin. Periodontol. 2017, 44, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskaite, A.; Becker, K.; Juodzbalys, G.; Schwarz, F. Clinical outcomes following surgical treatment of peri-implantitis at grafted and non-grafted implant sites: A retrospective analysis. Int. J. Implant. Dent. 2018, 4, 27. [Google Scholar] [CrossRef]
- Schneider, S.; Rudolph, M.; Bause, V.; Terfort, A. Electrochemical removal of biofilms from titanium dental implant surfaces. Bioelectrochemistry 2018, 121, 84–94. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Gianfreda, F.; Punzo, A.; Pistilli, V.; Bollero, P.; Cervino, G.; D’Amico, C.; Cairo, F.; Cicciu, M. Electrolytic Cleaning and Regenerative Therapy of Peri-implantitis in the Esthetic Area: A Case Report. Eur. J. Dent. 2022, 16, 950–956. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Brodbeck, U.R.; Rathe, F.; Stumpf, T.; Imber, J.C.; Weigl, P.; Schlee, M. Evidence of re-osseointegration after electrolytic cleaning and regenerative therapy of peri-implantitis in humans: A case report with four implants. Clin. Oral. Investig. 2022, 26, 3735–3746. [Google Scholar] [CrossRef]
- Schlee, M.; Rathe, F.; Brodbeck, U.; Ratka, C.; Weigl, P.; Zipprich, H. Treatment of Peri-implantitis-Electrolytic Cleaning Versus Mechanical and Electrolytic Cleaning-A Randomized Controlled Clinical Trial-Six-Month Results. J. Clin. Med. 2019, 8, 1909. [Google Scholar] [CrossRef] [PubMed]
- Schlee, M.; Wang, H.L.; Stumpf, T.; Brodbeck, U.; Bosshardt, D.; Rathe, F. Treatment of Periimplantitis with Electrolytic Cleaning versus Mechanical and Electrolytic Cleaning: 18-Month Results from a Randomized Controlled Clinical Trial. J. Clin. Med. 2021, 10, 3475. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S313–S318. [Google Scholar] [CrossRef]
- Monje, A.; Pons, R.; Amerio, E.; Wang, H.L.; Nart, J. Resolution of peri-implantitis by means of implantoplasty as adjunct to surgical therapy: A retrospective study. J. Periodontol. 2022, 93, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Serino, G.; Sato, H.; Holmes, P.; Turri, A. Intra-surgical vs. radiographic bone level assessments in measuring peri-implant bone loss. Clin. Oral. Implant. Res. 2017, 28, 1396–1400. [Google Scholar] [CrossRef]
- Kerkfeld, V.; Meyer, U. Higher Resolution in Cone Beam Computed Tomography Is Accompanied by Improved Bone Detection in Peri-implant Bone Despite Metal Artifact Presence. Int. J. Oral. Maxillofac. Implant. 2018, 33, 1331–1338. [Google Scholar] [CrossRef]
- Ritter, L.; Elger, M.C.; Rothamel, D.; Fienitz, T.; Zinser, M.; Schwarz, F.; Zoller, J.E. Accuracy of peri-implant bone evaluation using cone beam CT, digital intra-oral radiographs and histology. Dentomaxillofac Radiol. 2014, 43, 20130088. [Google Scholar] [CrossRef]
- Schwarz, F.; Herten, M.; Sager, M.; Bieling, K.; Sculean, A.; Becker, J. Comparison of naturally occurring and ligature-induced peri-implantitis bone defects in humans and dogs. Clin. Oral. Implant. Res. 2007, 18, 161–170. [Google Scholar] [CrossRef]
- Monje, A.; Pons, R.; Insua, A.; Nart, J.; Wang, H.L.; Schwarz, F. Morphology and severity of peri-implantitis bone defects. Clin. Implant. Dent. Relat. Res. 2019, 21, 635–643. [Google Scholar] [CrossRef]
- Peev, S.; Yotsova, R.; Parushev, I. Histomorphometric Analysis of Osseointegrated Intraosseous Dental Implants Using Undecalcified Specimens: A Scoping Review. Biomimetics 2024, 9, 672. [Google Scholar] [CrossRef] [PubMed]
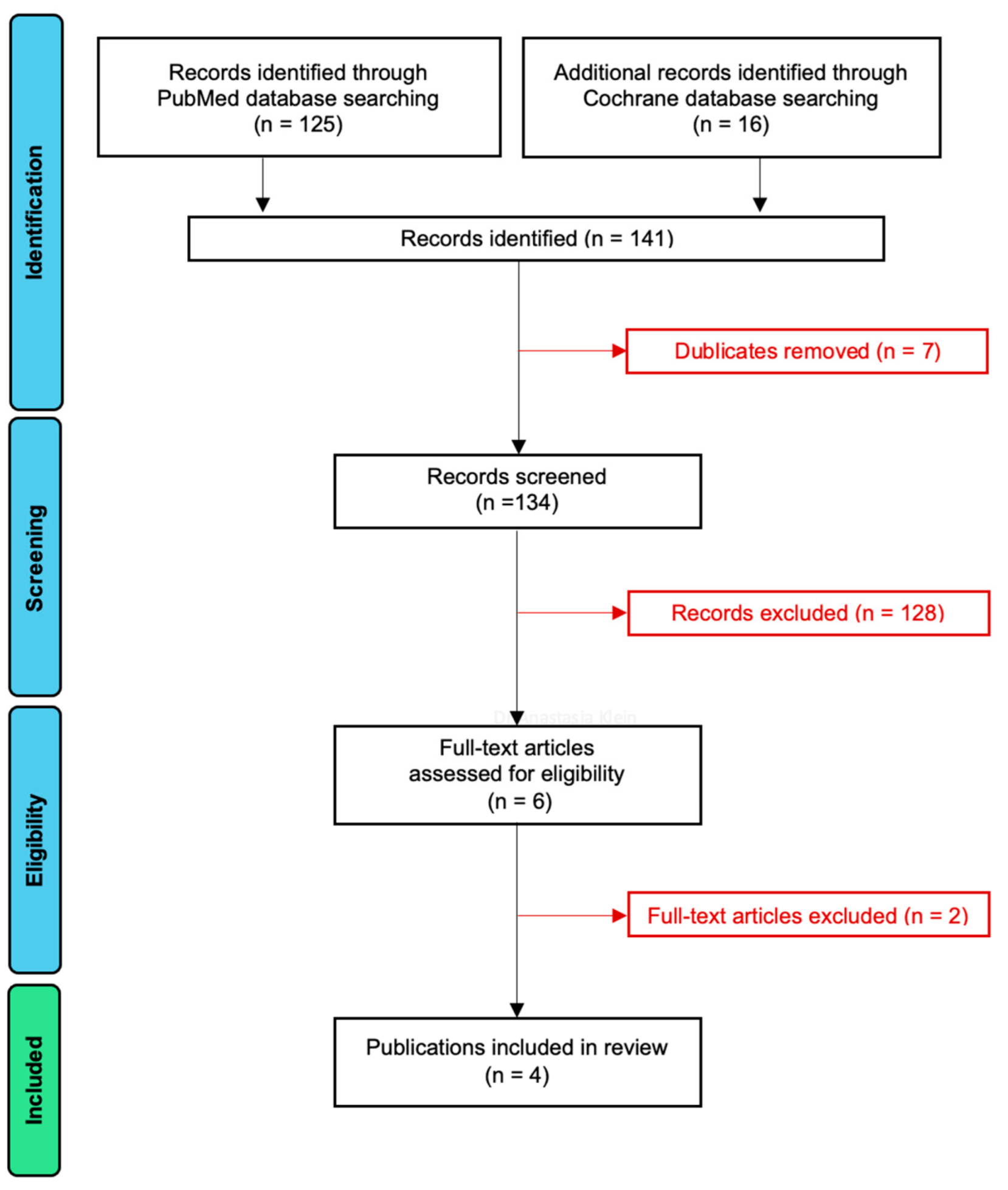
| Study | Study Design | No. Patients | No. Implants | Implant Type | Evaluation Period |
|---|---|---|---|---|---|
| Gianfreda et al., 2022 [30] | Case Report | 1 | 1 | 1 Bredent, Senden, Germany | 2 years |
| Bosshardt et al., 2022 [31] | Case Report | 3 | 4 | 1 Steri Oss HA-coated 3,8/12 mm 1 Straumann BL, RC, SLActive 4,1/12 mm 1 Straumann BL, RC, SLActive, Ti + G3 4,8/12 mm 1 Straumann BL, RC, SLActive, Ti 4,1/14 mm | 6–13 months |
| w | Randomized Controlled Clinical Trial | 24 | 24 | 5 Astra TX 2 Astra EV 2 Straumann tissue level 1 Straumann bone level 2 Conelog 2 Camlog 2 Ankylos 1 Sky 2 Branemark 1 Xive 1 Steri Oss 2 Zimmer 1 Nobel Active | 18 Months |
| Schlee et al., 2019. [32] | Randomized Controlled Clinical Trial, Proof of Principle | 24 | 24 | 5 Astra TX 2 Astra EV 2 Straumann tissue level 1 Straumann bone level 2 Conelog 2 Camlog 2 Ankylos 1 Sky 2 Branemark 1 Xive 1 Steri Oss 2 Zimmer 1 Nobel Active | 6 Months |
| Study | Diagnosis | Initial Clinical Findings | Treatment Protocol | Evaluation Methods |
|---|---|---|---|---|
| Gianfreda et al., 2022 [30] | Peri-implantitis | Good periodontal health; 6-month maintenance; Periodontal chart 62 y.o.; Male; BoP 0%; PI 5%; Implant PD 9 mm; Implant CBCT 5.5 mm Bone deficit; | Periapical X-ray, PD, CBCT T00: Professional oral hygiene + Periostat® gel T0 (1 week): Implant crown removal; Cover screw insertion + iodoform paste; Maryland bridge T1 (4 weeks): Defect degranulation + rifampicin; Electrolytic cleaning + GBR + platelet aggregates | Periapical X-ray Probing depth (PD) CBCT Bleeding on probing (BoP) |
| Bosshardt et al., 2022 [31] | Peri-implantitis | 55–62 y.o.; Females Implant PD | T0: Suprastructure removal; PD, BoP, radiographs; Electrolytic cleaning + GBR; Cover screw placement T1 (6 months): Suprastructure placement; PD; BoP; radiographs; T2 (13 months): PD; BoP; radiographs; explantation | Probing depth (PD): 6 points (m, mb, b, db, d, dl) Bleeding on probing (BoP): 6 points (m, mb, b, db, d, dl) P-B distance: 6 points (m, mb, b, db, d, dl) Radiographic bone level Histology Histomorphometry |
| Schlee et al., 2021 [33] | Peri-implantitis | No periodontitis Mean age 50% male/50% female BoP < 20% PI < 20% Implant BoP 100% Implant Pus 100% Implant mean PD < 10 cigarettes/day | Test group (12 patients; 12 implants): electrolytic cleaning Control group (12 patients; 12 implants): powder spray + electrolytic cleaning T2 (after 12 months): PD; BoP; pus; recessions; photos; radiographs T3 (after 18 months): PD; BoP; pus; recessions; photos; radiographs | Probing depth (PD): 6 points (m, mb, b, db, d, dl) Bleeding on probing (BoP): 6 points (m, mb, b, db, d, dl) P-B distance: 6 points (m, mb, b, db, d, dl) Radiographic bone level Statistical analysis |
| Schlee et al., 2019 [32] | Peri-implantitis | No periodontitis Mean age 50% male/50% female BoP < 20% PI < 20% Implant BoP 100% Implant Pus 100% Implant mean PD < 10 cigarettes/day | Test group (12 patients; 12 implants): electrolytic cleaning Control group (12 patients; 12 implants): powder spray + electrolytic cleaning T00: Suprastructure removal; PSS cleaning + chlorhexidine; Cover screw placement T0 (14 days after): Photos; PPD; BoP; pus; radiographs; Electrolytic cleaning & GBR T1 (6 months after): Photos; radiographs; P-D distance; BoP; pus, recessions; Exposed parts cleaned with cleaning paste; Suprastructure placement | Standardized photos (occlusal, buccal, lingual view) Probing depth (PD): 6 points (m, mb, b, db, d, dl) Bleeding on probing (BoP): 6 points (m, mb, b, db, d, dl) P-B distance: 6 points (m, mb, b, db, d, dl) Bone gain VAS assessment |
| Study | Flap Design | Bone Augmentation | Membranes | Pins/Miniscrews | Suture |
|---|---|---|---|---|---|
| Gianfreda et al., 2022 [30] |
| Autogenous bone and BioOss® | Cytoplast Ti-250® | 2 mini-screws | PTFE 5.0 PGCL 6.0 |
| Bosshardt et al., 2022 [31] | No information | 50:50 autogenous bone and 3 BioOss® 1 Maxgraft® | 3 BioGide® 1 Jason® | 0/2/3 umbrella screws | No information |
| Schlee et al., 2021 [33] |
| 50:50 autogenous bone and BioOss® | BioGide® | Umbrella screws | Propylene monofilamens 6.0 |
| Schlee et al., 2019 [32] |
| 50:50 autogenous bone and BioOss® | BioGide® | Umbrella screws | Propylene monofilamens 6.0 |
| Study | Powder Spray System | Antiseptics | Antibiotics |
|---|---|---|---|
| Gianfreda et al., 2022 [30] | AirFlow Master Piezon® (1 week before implant crown removal) | Iodoform paste (before cover screw placement) Curasept® 0.12% (twice daily for 2 weeks after surgery) | 2 mini-screws |
| Bosshardt et al., 2022 [31] | No | No | No |
| Schlee et al., 2021 [33] | PerioFlow®, erythritol, EMS® (after suprastructure removal, 2 weeks before surgery) AirFlow Plus®, EMS® for control group | Chlorhexamed Forte® 0.2% (2 weeks before surgery) | No |
| Schlee et al., 2019 [32] | PerioFlow®, erythritol, EMS® (after suprastructure removal, 2 weeks before surgery) AirFlow Plus®, EMS® for control group | Chlorhexamed Forte® 0.2% (2 weeks before surgery and in case of exposure after surgery) | No |
| Study | Results | Disease Resolution | Re-Osseointegration | Peri-Implantitis Recurrence |
|---|---|---|---|---|
| Gianfreda et al., 2022 [30] | No clinical problems Stable periodontal indices Bone gain | Yes | Yes | No |
| Bosshardt et al., 2022 [31] | Radiographic and histological bone gain; Reduced PD and BoP | No | Yes | Yes |
| Schlee et al., 2021 [33] | 18 implants assessed Statistically significant radiologic bone gain compared to T0; T2 and T3: No statistically significant change in BoP and Pus; Significant PD reduction; Cleaning with PSS: no additional benefit | No | Yes | Yes |
| Schlee et al., 2019 [32] | Bone gain: no statistically significant difference between groups Significant clinical bone fill | No | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, A.; Rasperini, G.; Gruber, R.; Andrukhov, O.; Rausch-Fan, X. Electrolytic Cleaning of Dental Implants: A Scoping Review of Clinical Studies. Dent. J. 2025, 13, 172. https://doi.org/10.3390/dj13040172
Klein A, Rasperini G, Gruber R, Andrukhov O, Rausch-Fan X. Electrolytic Cleaning of Dental Implants: A Scoping Review of Clinical Studies. Dentistry Journal. 2025; 13(4):172. https://doi.org/10.3390/dj13040172
Chicago/Turabian StyleKlein, Anastasia, Giulio Rasperini, Reinhard Gruber, Oleh Andrukhov, and Xiaohui Rausch-Fan. 2025. "Electrolytic Cleaning of Dental Implants: A Scoping Review of Clinical Studies" Dentistry Journal 13, no. 4: 172. https://doi.org/10.3390/dj13040172
APA StyleKlein, A., Rasperini, G., Gruber, R., Andrukhov, O., & Rausch-Fan, X. (2025). Electrolytic Cleaning of Dental Implants: A Scoping Review of Clinical Studies. Dentistry Journal, 13(4), 172. https://doi.org/10.3390/dj13040172






