Calcium and Microhardness Quantification in Healthy and Fluorotic Dentin Conditioned with a Self-Etching System: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Samples
2.2. Atomic Absorption Spectrometry Test
2.3. Vickers Microhardness Test
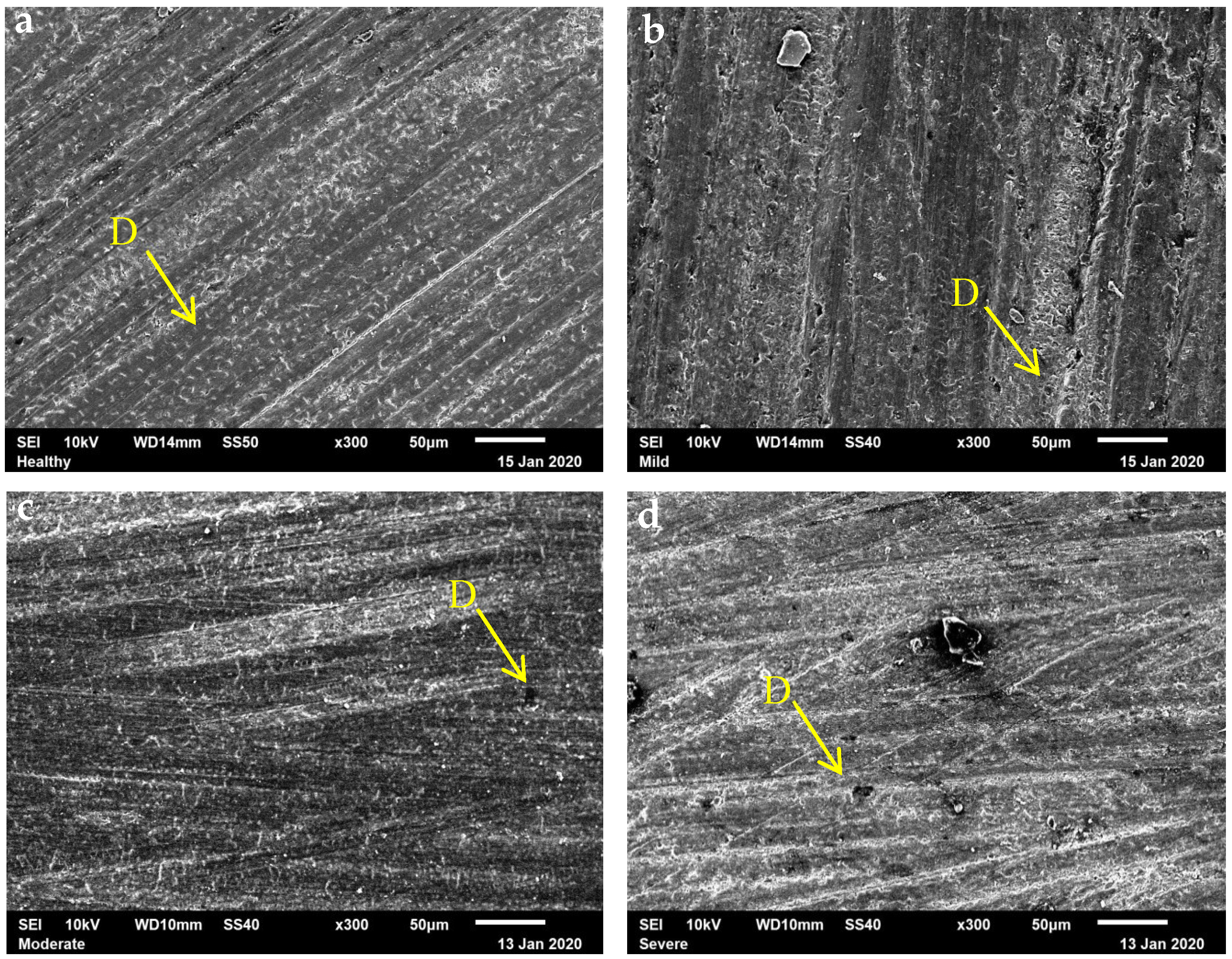
2.4. Scanning Electron Microscopy Evaluation
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kitahara, S.; Shimizu, S.; Takagaki, T.; Inokoshi, M.; Abdou, A.; Burrow, M.F.; Nikaido, T. Dentin Bonding Durability of Four Different Recently Introduced Self-Etch Adhesives. Materials 2024, 17, 4296. [Google Scholar] [CrossRef] [PubMed]
- Khabadze, Z.; Dashtieva, M.Y.; Meremkulov, R.A.; Kubrin, A.; Sheibanian, M.; Fedotova, N.; Pilshchikova, O.; Meremkulov, A.; Mordanov, O.; Malikovna, A.S. MDP-10 as the Most Important Functional Monomer of the Last Generation Self-Etching Adhesive Systems. J. Int. Dent. Med. Res. 2024, 17, 1. [Google Scholar]
- Zhai, Y.; Shi, Z.; Premaraj, T.; Premaraj, S.; Karpova, T.; Dong, P.; Gu, L. Effects of Acid Etching on the Microstructure and Stiffness of Human Teeth. J. Eng. Sci. Med. Diagn. Ther. 2025, 8, 3. [Google Scholar] [CrossRef]
- Oliveira, S.S.A.; Marshall, S.J.; Hilton, J.F.; Marshall, G.W. Etching Kinetics of a Self-Etching Primer. Biomaterials 2002, 23, 4105–4112. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H. Aggressiveness of Contemporary Self-Etching Systems: I. Depth of Penetration beyond Dentin Smear Layers. Dent. Mater. 2001, 17, 296–308. [Google Scholar] [CrossRef]
- Marshall Jr, G.W.; Inai, N.; Magidi, I.C.; Balooch, M.; Kinney, J.H.; Tagami, J.; Marshall, S.J. Dentin Demineralization: Effects of Dentin Depth, pH and Different Acids. Dent. Mater. 1997, 13, 338–343. [Google Scholar] [CrossRef]
- Garchitorena Ferreira, M.I. Materiales Bioactivos en la Remineralización Dentinaria. Odontoestomatologia 2016, 18, 11–19. [Google Scholar]
- Yoshida, Y.; Van Meerbeek, B.; Nakayama, Y.; Yoshioka, M.; Snauwaert, J.; Abe, Y.; Lambrechts, P.; Vanherle, G.; Okazaki, M. Adhesion to and Decalcification of Hydroxyapatite by Carboxylic Acids. J. Dent. Res. 2001, 80, 1565–1569. [Google Scholar] [CrossRef]
- Kinney, J.H.; Balooch, M.; Haupt, D.L.; Marshall, S.J.; Marshall, G.W. Mineral Distribution and Dimensional Changes in Human Dentin during Demineralization. J. Dent. Res. 1995, 74, 1179–1184. [Google Scholar] [CrossRef]
- Vieira, A.P.G.F.; Hanocock, R.; Eggertsson, H.; Everett, E.T.; Grynpas, M.D. Tooth Quality in Dental Fluorosis: Genetic and Environmental Factors. Calcif. Tissue Int. 2005, 76, 17–25. [Google Scholar] [CrossRef]
- Fejerskov, O.; Larsen, M.J.; Richards, A.; Baelum, V. Dental Tissue Effects of Fluoride. Adv. Dent. Res. 1994, 8, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Banu Ermis, R.; Gokay, N. Effect of Fluorosis on Dentine Shear Bond Strength of a Self-Etching Bonding System. J. Oral Rehabil. 2003, 30, 1090–1094. [Google Scholar] [CrossRef]
- Giriyappa, R.H.; Chandra, B.S. Comparative Evaluation of Self-Etching Primers with Fourth and Fifth Generation Dentin-Bonding Systems on Carious and Normal Dentin Substrates: An In Vitro Shear Bond Strength Analysis. J. Conserv. Dent. Endod. 2008, 11, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Giannini, M.; Makishi, P.; Ayres, A.P.; Vermelho, P.M.; Fronza, B.M.; Nikaido, T.; Tagami, J. Self-Etch Adhesive Systems: A Literature Review. Braz. Dent. J. 2015, 26, 3–10. [Google Scholar] [CrossRef]
- Migliau, G. Classification Review of Dental Adhesive Systems: From the IV Generation to the Universal Type. Ann. Stomatol. 2017, 8, 1. [Google Scholar] [CrossRef]
- Kumar, J.V.; Swango, P.A.; Opima, P.N.; Green, E.L. Dean’s Fluorosis Index: An Assessment of Examiner Reliability. J. Public Health Dent. 2000, 60, 57–59. [Google Scholar] [CrossRef] [PubMed]
- DenBesten, P.; Li, W. Chronic Fluoride Toxicity: Dental Fluorosis. Monogr. Oral Sci. 2011, 22, 81–96. [Google Scholar] [CrossRef]
- Zavala-Alonso, V.; Aguilera-Flores, R.; Patino-Marin, N.; Martinez-Castanon, G.A.; Anusavice, K.J.; Loyola-Rodriguez, J.P. Nanostructure Evaluation of Healthy and Fluorotic Dentin by Atomic Force Microscopy Before and After Phosphoric Acid Etching. Dent. Mater. J. 2011, 30, 546–553. [Google Scholar] [CrossRef]
- Monjarás-Ávila, A.J.; Zavala-Alonso, N.V.; Martínez-Castañón, G.A.; Patiño-Marín, N.; Flores, D.S.H.; Ruíz, F. Sodium Hypochlorite as Fluorotic Dentin Pretreatment of Two-Step Self-Etch Adhesive with Silver Nanoparticle: Atomic Force Microscope and Adhesive Microtensile Bond Strength Evaluation. J. Nanomater. 2017, 2017, 1381929. [Google Scholar] [CrossRef]
- Sayin, T.C.; Serper, A.; Cehreli, Z.C.; Kalayci, S. Calcium Loss from Root Canal Dentin Following EDTA, EGTA, EDTAC, and Tetracycline-HCl Treatment with or without Subsequent NaOCl Irrigation. J. Endod. 2007, 33, 581–584. [Google Scholar] [CrossRef]
- Bohaty, B.S.; Sene, F. Clinical Presentation: Reconstruction Using Composite Materials. In Material-Tissue Interfacial Phenomena: Contributions from Dental and Craniofacial Reconstructions; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 1–20. [Google Scholar] [CrossRef]
- Patel, P.; Kapadia, U.; Vyas, J.; Mhay, S.; Nalliah, R.P. Determining the Failure Rate of Direct Restorations—Chart Review versus Electronic Health Record Reports. Dent. J. 2024, 12, 250. [Google Scholar] [CrossRef]
- Revelo-Mejía, I.A.; Hardisson, A.; Rubio, C.; Gutiérrez, Á.J.; Paz, S. Dental Fluorosis: The Risk of Misdiagnosis—A Review. Biol. Trace Elem. Res. 2021, 199, 1762–1770. [Google Scholar] [CrossRef] [PubMed]
- Perdigão, J.; Geraldeli, S.; Hodges, J.S. Total-Etch versus Self-Etch Adhesives: Effect on Postoperative Sensitivity. J. Am. Dent. Assoc. 2003, 134, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Wang, R.; Zhu, M. Comparative Evaluation of Bonding Performance Between Universal and Self-Etch Adhesives: In Vitro Study. Heliyon 2024, 10, e12650. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.L.; Snauwaert, J.; De Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Systematic Review of the Chemical Composition of Contemporary Dental Adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef]
- Hashimoto, M.; Ohno, H.; Kaga, M.; Endo, K.; Sano, H.; Oguchi, H. In Vivo Degradation of Resin-Dentin Bonds in Humans over 1 to 3 Years. J. Dent. Res. 2000, 79, 1385–1391. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Yoshihara, K.; Van Landuyt, K.; Yoshida, Y.; Peumans, M. From Buonocore’s pioneering acid-etch technique to self-adhering restoratives: A status perspective of rapidly advancing dental adhesive technology. J. Adhes. Dent. 2020, 22, 7–34. [Google Scholar]
- Sadek, F.T.; Pashley, D.H.; Ferrari, M.; Tay, F.R. Tubular Occlusion of Dentin by Restorative Materials. Am. J. Dent. 2007, 20, 155–162. [Google Scholar]
- Okuyama, K.; Murata, Y.; Pereira, P.N.; Miguez, P.A.; Komatsu, H.; Sano, H. Fluoride Release and Uptake by Various Dental Materials after Fluoride Application. Am. J. Dent. 2006, 19, 123–127. [Google Scholar]
- Mazzoni, A.; Pashley, D.H.; Nishitani, Y.; Breschi, L.; Mannello, F.; Tjäderhane, L.; Toledano, M.; Pashley, E.L.; Tay, F.R. Reactivity of Dentin-Pretreatment Agents and a Phosphorylated Adhesive with Mineralized and Demineralized Dentin. Dent. Mater. 2009, 25, 884–892. [Google Scholar] [CrossRef]
- Vieira, A.P.; Hancock, R.; Dumitriu, M.; Schwartz, M.; Limeback, H.; Grynpas, M. How does fluoride affect dentin microhardness and mineralization? J. Dent. Res. 2005, 84, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Kirsten, G.A.; Takahashi, M.K.; Rached, R.N.; Giannini, M.; Souza, E.M. Microhardness of Dentin Underneath Fluoride-Releasing Adhesive Systems Subjected to Cariogenic Challenge and Fluoride Therapy. J. Dent. 2010, 38, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Yamashita, S.; Mendonca, M.; Brueckner, S.; Achong-Bowe, R.; Thompson, J.; Kuriki, N.; Mizuhira, M.; Benjamin, Y.; Duncan, H.F.; et al. Ultrastructural Evaluation of Adverse Effects on Dentine Formation from Systemic Fluoride Application in an Experimental Mouse Model. Int. Endod. J. 2025, 58, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, Z.; Zhang, B.; Peng, W.; Guo, L. Effects of dimethyl sulfoxide pretreatment on the bonding properties of fluorotic dentin of different severity: An in vitro study. J. Prosthet. Dent. 2024, 131, 508–517. [Google Scholar] [CrossRef]

| Technique | System | Content | Indications |
|---|---|---|---|
| Self-etch primer application | OptiBond Versa (Kerr, CA, USA) | Primer Monomers: glycerol phosphate dimethacrylate (GPDM). Hydrophilic comonomers including mono and difunctional methacrylate monomers. Solvents: water, acetone, and ethyl alcohol. Photoinitiator: camphorquinone (CQ) based. Adhesive Monomers: hydrophobic, structural, and cross-linking monomers. Solvents: ethyl alcohol. Photoinitiator: CQ based. Fillers: 0.4-micron barium glass nano-silica. Fluoride: sodium hexafluorosilicate. | Apply with a micro brush for 20 s and then dry with oil-free air for 5 s. |
| Fluorosis Degree | Amount Ion Calcium Mean ± SD | Sig. | Hardness Before Mean ± SD | Hardness After Mean ± SD | Sig. | |
|---|---|---|---|---|---|---|
| Healthy | 0.343 ± 0.361 | Mild | 0.000 | 53.95 ± 2.37 | 49.34 ± 5.57 | 0.030 |
| Moderate | 0.009 | |||||
| Severe | 0.001 | |||||
| Mild | 1.045 ± 0.215 | Healthy | 0.000 | 56.54 ± 5.20 | 51.67 ± 7.92 | 0.107 |
| Moderate | 0.524 | |||||
| Severe | 0.961 | |||||
| Moderate | 0.840 ± 0.552 | Healthy | 0.009 | 58.76 ± 3.68 | 53.70 ± 4.39 | 0.263 |
| Mild | 0.524 | |||||
| Severe | 0.814 | |||||
| Severe | 0.972 ± 0.237 | Healthy | 0.001 | 60.17 ± 4.97 | 60.07 ± 1.93 | 0.979 |
| Mild | 0.961 | |||||
| Moderate | 0.814 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzaga, J.A.R.; Ávila, A.J.M.; Hardan, L.; Alonso, N.V.Z.; Suárez, C.E.C.; Nassar, N.; Holiel, A.A.; Kharouf, N.; Haikel, Y.; Bourgi, R. Calcium and Microhardness Quantification in Healthy and Fluorotic Dentin Conditioned with a Self-Etching System: An In Vitro Study. Dent. J. 2025, 13, 168. https://doi.org/10.3390/dj13040168
Gonzaga JAR, Ávila AJM, Hardan L, Alonso NVZ, Suárez CEC, Nassar N, Holiel AA, Kharouf N, Haikel Y, Bourgi R. Calcium and Microhardness Quantification in Healthy and Fluorotic Dentin Conditioned with a Self-Etching System: An In Vitro Study. Dentistry Journal. 2025; 13(4):168. https://doi.org/10.3390/dj13040168
Chicago/Turabian StyleGonzaga, José Alejandro Rivera, Ana Josefina Monjarás Ávila, Louis Hardan, Norma Verónica Zavala Alonso, Carlos Enrique Cuevas Suárez, Nicolas Nassar, Ahmed A. Holiel, Naji Kharouf, Youssef Haikel, and Rim Bourgi. 2025. "Calcium and Microhardness Quantification in Healthy and Fluorotic Dentin Conditioned with a Self-Etching System: An In Vitro Study" Dentistry Journal 13, no. 4: 168. https://doi.org/10.3390/dj13040168
APA StyleGonzaga, J. A. R., Ávila, A. J. M., Hardan, L., Alonso, N. V. Z., Suárez, C. E. C., Nassar, N., Holiel, A. A., Kharouf, N., Haikel, Y., & Bourgi, R. (2025). Calcium and Microhardness Quantification in Healthy and Fluorotic Dentin Conditioned with a Self-Etching System: An In Vitro Study. Dentistry Journal, 13(4), 168. https://doi.org/10.3390/dj13040168













