How Does Allergen Sensitization Affect Orthodontic Tooth Movement-Associated Phenomena? A Systematic Review of Animal Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Development
2.2. Eligibility Criteria
- Population (P): Every kind of experimental animal having undergone any kind of allergen sensitization.
- Intervention (I): Any kind of orthodontic intervention.
- Comparator (C): Animals without allergen sensitization but receiving orthodontic intervention.
- Outcomes (O): Primarily, root resorption (extent/severity; assessed using linear measurements or grading scales or percentages); secondarily, the amount of tooth movement (assessed with filler gauges, CT, on histological sections), histologic changes, and biomolecular levels.
- Study design (S): Prospective controlled studies (case-control, cohort etc.).
2.3. Information Sources and Search Strategy
2.4. Study Selection
2.5. Data Collection and Data Items
2.6. Risk of Bias in Individual Studies
2.7. Synthesis of Results, Risk of Bias Across Studies, and Additional Analyses
3. Results
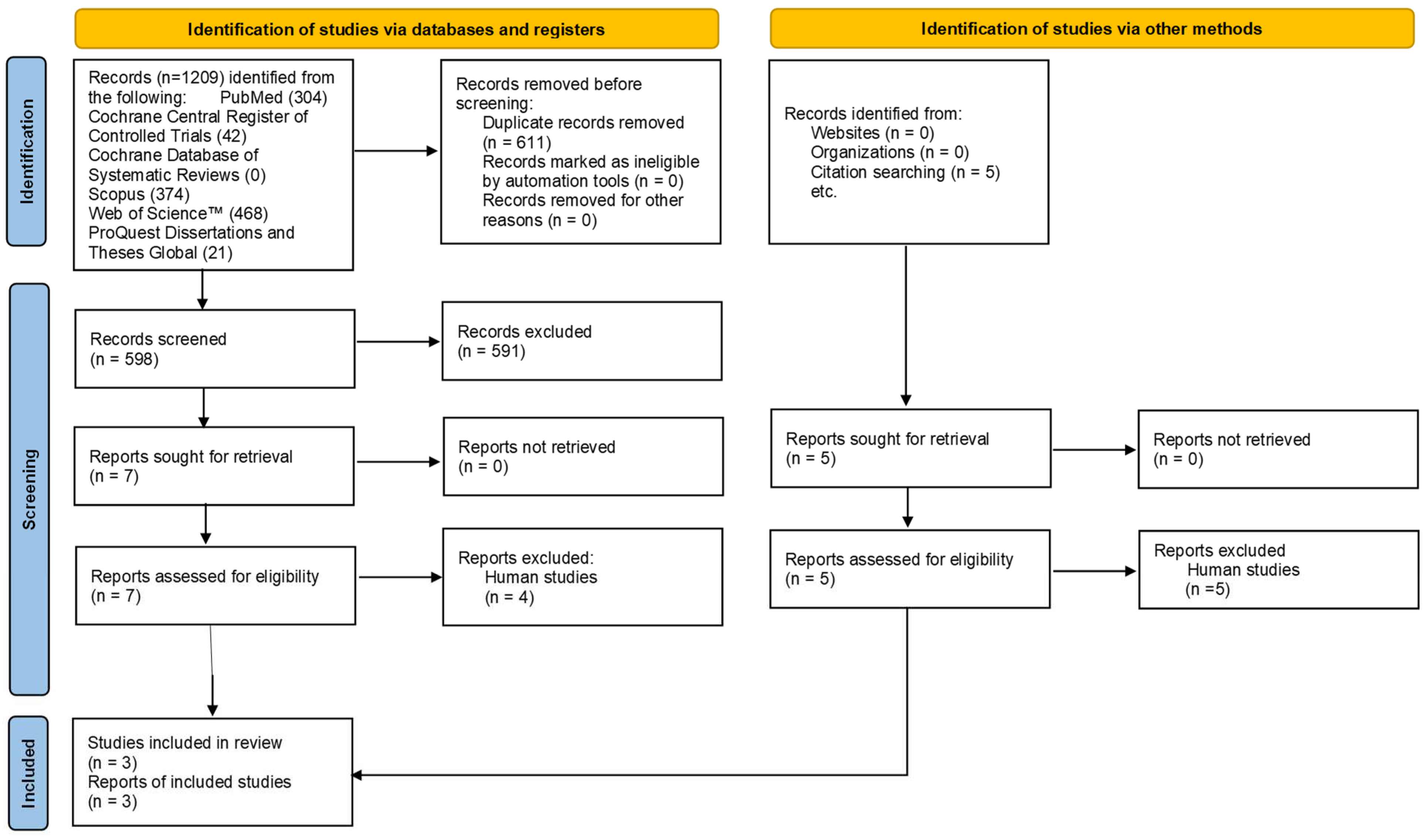
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias Within Studies
3.4. Results of Individual Studies
4. Discussion
4.1. Strengths and Limitations
4.2. Recommendations for Future Research
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, Y.; Jacox, L.A.; Little, S.H.; Ko, C.C. Orthodontic tooth movement: The biology and clinical implications. Kaohsiung J. Med. Sci. 2018, 34, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Linares, A.; Hartsfield, J.K., Jr. Cellular and Molecular Pathways Leading to External Root Resorption. J. Dent. Res. 2017, 96, 145–152. [Google Scholar] [CrossRef]
- Wang, Z.; McCauley, L.K. Osteoclasts and odontoclasts: Signaling pathways to development and disease. Oral Dis. 2011, 17, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Hienz, S.A.; Paliwal, S.; Ivanovski, S. Mechanisms of bone resorption in periodontitis. J. Immunol. Res. 2015, 2015, 615486. [Google Scholar] [CrossRef]
- Proffit, W.R.; Fields, H.W., Jr.; Sarver, D.M. Contemporary Orthodontics, 6th ed.; Mosby Elsevier: St. Louis, MO, USA, 2018. [Google Scholar]
- McNab, S.; Battistutta, D.; Taverne, A.; Symons, A.L. External apical root resorption of posterior teeth in asthmatics after orthodontic treatment. Am. J. Orthod. Dentofacial Orthop. 1999, 116, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Roscoe, M.G.; Meira, J.B.; Cattaneo, P.M. Association of orthodontic force system and root resorption: A systematic review. Am. J. Orthod. Dentofac. Orthop. 2015, 147, 610–626. [Google Scholar] [CrossRef]
- Weltman, B.; Vig, K.W.; Fields, H.W.; Shanker, S.; Kaizar, E.E. Root resorption associated with orthodontic tooth movement: A systematic review. Am. J. Orthod. Dentofac. Orthop. 2010, 137, 462–476. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Mishima, H. The Role of RANKL and Involvement of Cementum in Orthodontic Root Resorption. Appl. Sci. 2021, 11, 7244. [Google Scholar] [CrossRef]
- Kaklamanos, E.G.; Makrygiannakis, M.A.; Athanasiou, A.E. Does Medication Administration Affect the Rate of Orthodontic Tooth Movement and Root Resorption Development in Humans? A Systematic Review. Eur. J. Orthod. 2020, 42, 407–414. [Google Scholar] [CrossRef]
- Makrygiannakis, M.A.; Kaklamanos, E.G.; Athanasiou, A.E. Effects of Systemic Medication on Root Resorption Associated with Orthodontic Tooth Movement: A Systematic Review of Animal Studies. Eur. J. Orthod. 2019, 41, 346–359. [Google Scholar] [CrossRef]
- Davidovitch, Z.; Lee, Y.J.; Counts, A.L.; Park, Y.G.; Bursac, Z. The Immune System Possibly Modulates Orthodontic Root Resorption. In Biological Mechanisms of Tooth Movement and Craniofacial Adaptation; Davidovitch, Z., Mah, J., Eds.; Harvard Society for the Advancement of Orthodontics: Cambridge, MA, USA, 2000; pp. 207–217. [Google Scholar]
- Krishnan, V.; Zahrowski, J.J.; Davidovitch, Z. The Effect of Drugs, Hormones, and Diet on Orthodontic Tooth Movement. In Biological Mechanisms of Tooth Movement, 3rd ed.; Krishnan, V., Kuijpers-Jagtman, A.M., Davidovitch, Z., Eds.; Willey Blackwel: Hoboken, NJ, USA, 2021; pp. 199–218. [Google Scholar]
- GBD 2015 Chronic Respiratory Disease Collaborators. Global, Regional, and National Deaths, Prevalence, Disability-Adjusted Life Years, and Years Lived with Disability for Chronic Obstructive Pulmonary Disease and Asthma, 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med. 2017, 5, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Enilari, O.; Sinha, S. The Global Impact of Asthma in Adult Populations. Ann. Glob. Health 2019, 85, 2. [Google Scholar] [CrossRef] [PubMed]
- Madore, A.M.; Laprise, C. Immunological and genetic aspects of asthma and allergy. J. Asthma Allergy 2010, 3, 107–121. [Google Scholar] [PubMed]
- Molet, S.; Hamid, Q.; Davoine, F.; Nutku, E.; Taha, R.; Pagé, N.; Olivenstein, R.; Elias, J.; Chakir, J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J. Allergy Clin. Immunol. 2001, 108, 430–438. [Google Scholar] [CrossRef]
- Dos Santos, C.; Bellini-Pereira, S.A.; Medina, M.; Normando, D. Allergies/asthma and root resorption: A systematic review. Prog. Orthod. 2021, 22, 8. [Google Scholar] [CrossRef]
- Al-Saqi, R.; Athanasiou, A.E.; Makrygiannakis, M.A.; Kaklamanos, E.G. Are Asthma and Allergy Associated with Increased Root Resorption Following Orthodontic Treatment? A Meta-Analysis. PLoS ONE 2023, 18, e0285309. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Prisma-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, 7647. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; PRISMA-S Group. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, version 6.5 (updated August 2024); Cohrane: London, UK, 2024; Available online: www.training.cochrane.org/handbook (accessed on 3 February 2025).
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- De Melo, G.A.R.S.A.; Martelli, K.; de Freitas, K.M.S.; Pinelli Valarelli, F.; Cançado, R.H. Influence of Asthma on the Degree of Apical Root Resorption in Orthodontically Treated Patients. Iran. J. Orthod. 2018, 13, e9518. [Google Scholar]
- Malan, B. Factors Associated with Orthodontically Induced Apical Root Resorption of Maxillary Incisors. Master’s Thesis, Loma Linda University, Loma Linda, CA, USA, 2017. [Google Scholar]
- Pastro, J.D.V.; Nogueira, A.C.A.; de Freitas, K.M.S.; Valarelli, F.P.; Cançado, R.H.; de Oliveira, R.C.G.; de Oliveira, R.C.G. Factors Associated to Apical Root Resorption after Orthodontic Treatment. Open Dent. J. 2018, 12, 331–339. [Google Scholar] [CrossRef]
- Aghili, H.; Ardekani, M.D.; Meybodi, S.A.R.F.; Toodehzaeim, M.H.; Modaresi, J.; Mansouri, R.; Momeni, E. The effect of ovalbumin on orthodontic induced root resorption. Dent. Res. J. 2013, 10, 630–635. [Google Scholar]
- Machado, C.C.; Nojima, M.; Rodrigues e Silva, P.M.; Mandarim-de-Lacerda, C.A. Histomorphometric study of the periodontal ligament in the initial period of orthodontic movement in Wistar rats with induced allergic asthma. Am. J. Orthod. Dentofac. Orthop. 2012, 142, 333–338. [Google Scholar] [CrossRef]
- Murata, N.; Ioi, H.; Ouchi, M.; Takao, T.; Oida, H.; Aijima, R.; Yamaza, T.; Kido, M. Effect of Allergen Sensitization on External Root Resorption. J. Dent. Res. 2013, 92, 641–647. [Google Scholar] [CrossRef]
- Broide, D.H. Molecular and cellular mechanisms of allergic disease. J. Allergy Clin. Immunol. 2001, 108, S65–S71. [Google Scholar] [CrossRef]
- Verna, C.; Hartig, L.E.; Kalia, S.; Melsen, B. Influence of steroid drugs on orthodontically induced root resorption. Orthod. Craniofac. Res. 2006, 9, 57–62. [Google Scholar] [CrossRef]
- Nishioka, M.; Ioi, H.; Nakata, S.; Nakasima, A.; Counts, A. Root resorption and immune system factors in the Japanese. Angle Orthod. 2006, 76, 103–108. [Google Scholar]
- Nanekrungsan, K.; Patanaporn, V.; Janhom, A.; Korwanich, N. External apical root resorption in maxillary incisors in orthodontic patients: Associated factors and radiographic evaluation. Imaging Sci. Dent. 2012, 42, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Owman-Moll, P.; Kurol, J. Root resorption after orthodontic treatment in high- and low-risk patients: Analysis of allergy as a possible predisposing factor. Eur. J. Orthod. 2000, 22, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Sondeijker, C.F.W.; Lamberts, A.; Beckmann, A.; Kuitert, S.H.; van Westing, R.B.; Persoon, K.; Kuijpers-Jagtman, A.M. Development of a clinical practice guideline for orthodontically induced external apical root resorption. Eur. J. Orthod. 2019, 42, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Malmgren, O.; Goldson, L.; Hill, C.; Orwin, A.; Petrini, L.; Lundberg, M. Root resorption after orthodontic treatment of traumatized teeth. Am. J. Orthod. 1982, 82, 487–491. [Google Scholar] [CrossRef]
- Chan, E.K.; Darendeliler, M.A. Exploring the third dimension in root resorption. Orthod. Craniofac. Res. 2004, 7, 64–70. [Google Scholar] [CrossRef]
- Levander, E.; Malmgren, O. Evaluation of the risk of root resorption during orthodontic treatment: A study of upper incisors. Eur. J. Orthod. 1998, 10, 30–38. [Google Scholar] [CrossRef]
- Topkara, A.; Karaman, A.I.; Kau, C.H. Apical root resorption caused by orthodontic forces: A brief review and a long-term observation. Eur. J. Dent. 2012, 6, 445–453. [Google Scholar] [CrossRef]
- Linge, L.; Ohm Linge, B.; Skien, D. Patient characteristics and treatment variables associated with apical root resorption during orthodontic treatment. Am. J. Orthod. Dentofac. Orthop. 1991, 99, 35–43. [Google Scholar] [CrossRef]
- Sameshima, G.T.; Sinclair, P.M. Predicting and preventing root resorption: Part I. Diagnostic factors. Am. J. Orthod. Dentofac. Orthop. 2001, 119, 505–510. [Google Scholar] [CrossRef]
- Kjaer, I. Morphological characteristics of dentitions developing excessive root resorption during orthodontic treatment. Eur. J. Orthod. 1995, 17, 25–34. [Google Scholar] [CrossRef]
- Lee, R.Y.; Artun, J.; Alonzo, T.A. Are dental anomalies risk factors for apical root resorption in orthodontic patients? Am. J. Orthod. Dentofac. Orthop 1999, 116, 187–195. [Google Scholar] [CrossRef]
- Komori, T. Animal models for osteoporosis. Eur. J. Pharmacol. 2015, 759, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, D.; Xanthos, T.; Dontas, I.; Lelovas, P.; Perrea, D. The use of mice and rats as animal models for cardiopulmonary resuscitation research. Lab. Anim. 2008, 42, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Kucharewicz, I.; Bodzenta- Łukaszyk, A.; Buczko, W. Experimental asthma in rats. Pharmacol. Rep. 2008, 60, 783–788. [Google Scholar]
- Kucharewicz, I.; Kasacka, I.; Pawlak, D.; Tankiewicz-Kwedlo, A.; Mroczko, B.; Buczko, W.; Bodzenta-Lukaszyk, A. The concentration of kynurenine in rat model of asthma. Folia Histochem. Cytobiol. 2008, 46, 199–203. [Google Scholar] [CrossRef] [PubMed]
| Database [23 December 2024] | Search Strategy | Hits |
|---|---|---|
| PubMed | (orthodon* OR “orthodontic force” OR “mechanical force”) AND (“tooth movement” OR “orthodontic movement” OR “orthodontic anchorage” OR “root resorption”) AND (allergy OR allergic* OR sensitiv* OR hypersensitiv* OR ovalbumin OR OVA OR “Dust Mite” OR HDM OR ascaris OR aspergill* OR “cotton dust” OR latex OR DRA OR cockroach OR asthma* OR airway) | 304 |
| Cochrane Central Register of Controlled Trials | (orthodon* OR “orthodontic force” OR “mechanical force”) AND (“tooth movement” OR “orthodontic movement” OR “orthodontic anchorage” OR “root resorption”) AND (allergy OR allergic* OR sensitiv* OR hypersensitiv* OR ovalbumin OR OVA OR “Dust Mite” OR HDM OR ascaris OR aspergill* OR “cotton dust” OR latex OR DRA OR cockroach OR asthma* OR airway) in Title Abstract Keyword (word variations were searched) | 42 |
| Cochrane Database of Systematic Reviews | (orthodon* OR “orthodontic force” OR “mechanical force”) AND (“tooth movement” OR “orthodontic movement” OR “orthodontic anchorage” OR “root resorption”) AND (allergy OR allergic* OR sensitiv* OR hypersensitiv* OR ovalbumin OR OVA OR “Dust Mite” OR HDM OR ascaris OR aspergill* OR “cotton dust” OR latex OR DRA OR cockroach OR asthma* OR airway) in Title Abstract Keyword (word variations were searched) | 0 |
| Scopus | TITLE-ABS-KEY ((orthodon* OR “orthodontic force” OR “mechanical force”) AND (“tooth movement” OR “orthodontic movement” OR “orthodontic anchorage” OR “root resorption”) AND (allergy OR allergic* OR sensitiv* OR hypersensitiv* OR ovalbumin OR ova OR “Dust Mite” OR hdm OR ascaris OR aspergill* OR “cotton dust” OR latex OR dra OR cockroach OR asthma* OR airway)) | 374 |
| Web of Science™ | (orthodon* OR “orthodontic force” OR “mechanical force”) AND (“tooth movement” OR “orthodontic movement” OR “orthodontic anchorage” OR “root resorption”) AND (allergy OR allergic* OR sensitiv* OR hypersensitiv* OR ovalbumin OR OVA OR “Dust Mite” OR HDM OR ascaris OR aspergill* OR “cotton dust” OR latex OR DRA OR cockroach OR asthma* OR airway) (Topic) and Preprint Citation Index (Exclude—Database) | 468 |
| ProQuest Dissertations and Theses Global | title((orthodon* OR “orthodontic force” OR “mechanical force”) AND (“tooth movement” OR “orthodontic movement” OR “orthodontic anchorage” OR “root resorption”) AND (allergy OR allergic* OR sensitiv* OR hypersensitiv* OR ovalbumin OR OVA OR “Dust Mite” OR HDM OR ascaris OR aspergill* OR “cotton dust” OR latex OR DRA OR cockroach OR asthma* OR airway)) OR abstract((orthodon* OR “orthodontic force” OR “mechanical force”) AND (“tooth movement” OR “orthodontic movement” OR “orthodontic anchorage” OR “root resorption”) AND (allergy OR allergic* OR sensitiv* OR hypersensitiv* OR ovalbumin OR OVA OR “Dust Mite” OR HDM OR ascaris OR aspergill* OR “cotton dust” OR latex OR DRA OR cockroach OR asthma* OR airway)) [Full text] | 21 |
| Study | Animal Characteristics and Allergen Sensitization Method | Group Characteristics | Tooth Movement Model | Measurement Methodology |
|---|---|---|---|---|
| Machado et al. 2012 [31] | Wistar rats male, 180–200 g OVA + Al(OH)3: SC; on d 1 and 14 [reinforcement] OVA: NI; for 3 d [7 d after reinforcement] | EG1: 8; OVA EG2: 8; OVA + OTM CG1: 8 CG2: 8; OTM Sample size calculation: NM | NiTi CCS from L Mx I to FM [40 g] Force application:
| Histological analysis: OTM: PDL area [FM mesial root] |
| Murata et al. 2013 [32] | Brown Norway rats male, 6 w old, 110–140 g OVA + Al(OH)3: SC; on d 1 OVA: IP; 7 d later | EG1: 7; OVA EG2: 14; OVA + OTM EG3: 14; OVA + OTM + aspirin CG1: 7 CG2: 14; OTM Sample size calculation: NM | NiTi CCS from R Mx I to FM [≅10 g] Force application:
| Histological analysis: RR [area]; number of odontoclasts and osteoclasts [FM distopalatal root] Micro-CT: OTM [μm]: distal of FM to mesial of SM ELISA: TNF-α, IL-1β, IL-6, RANKL, leukotrienes, synthases 5-LOX and LTA4 hydrolase, receptors BLT1 and BLT2 |
| Aghili et al. 2013 [30] | Wistar rats male, 3 m old, 330–350 g OVA + alume: IP; daily for 3 ds OVA: IP; weekly for 3 w | EG1: 15; OVA EG2: 15; OVA + OTM CG1: 15 CG2: 15; OTM Sample size calculation: NM | NiTi CCS from R Mx I to FM [50 g] Force application:*
| Histological analysis: RR [%]; sum of length of resorptive lacunae to mean length of root periphery [FM mesial root] |
| Signaling Questions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Machado et al. 2012 [31] | high | low | high | high | high | high | high | low | unclear | unclear |
| Murata et al. 2013 [32] | high | low | high | high | high | high | high | low | unclear | unclear |
| Aghili et al. 2013 [30] | unclear | low | unclear | high | high | high | high | unclear | unclear | unclear |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bineshaq, F.S.; Athanasiou, A.E.; Makrygiannakis, M.A.; Kalfas, S.; Kaklamanos, E.G. How Does Allergen Sensitization Affect Orthodontic Tooth Movement-Associated Phenomena? A Systematic Review of Animal Studies. Dent. J. 2025, 13, 166. https://doi.org/10.3390/dj13040166
Bineshaq FS, Athanasiou AE, Makrygiannakis MA, Kalfas S, Kaklamanos EG. How Does Allergen Sensitization Affect Orthodontic Tooth Movement-Associated Phenomena? A Systematic Review of Animal Studies. Dentistry Journal. 2025; 13(4):166. https://doi.org/10.3390/dj13040166
Chicago/Turabian StyleBineshaq, Fatima Saeed, Athanasios E. Athanasiou, Miltiadis A. Makrygiannakis, Sotirios Kalfas, and Eleftherios G. Kaklamanos. 2025. "How Does Allergen Sensitization Affect Orthodontic Tooth Movement-Associated Phenomena? A Systematic Review of Animal Studies" Dentistry Journal 13, no. 4: 166. https://doi.org/10.3390/dj13040166
APA StyleBineshaq, F. S., Athanasiou, A. E., Makrygiannakis, M. A., Kalfas, S., & Kaklamanos, E. G. (2025). How Does Allergen Sensitization Affect Orthodontic Tooth Movement-Associated Phenomena? A Systematic Review of Animal Studies. Dentistry Journal, 13(4), 166. https://doi.org/10.3390/dj13040166







