Guided Bone Regeneration Using Carbonated Apatite Granules and L-Lactic Acid/ε-Caprolactone Membranes: A Case Series and Histological Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Series
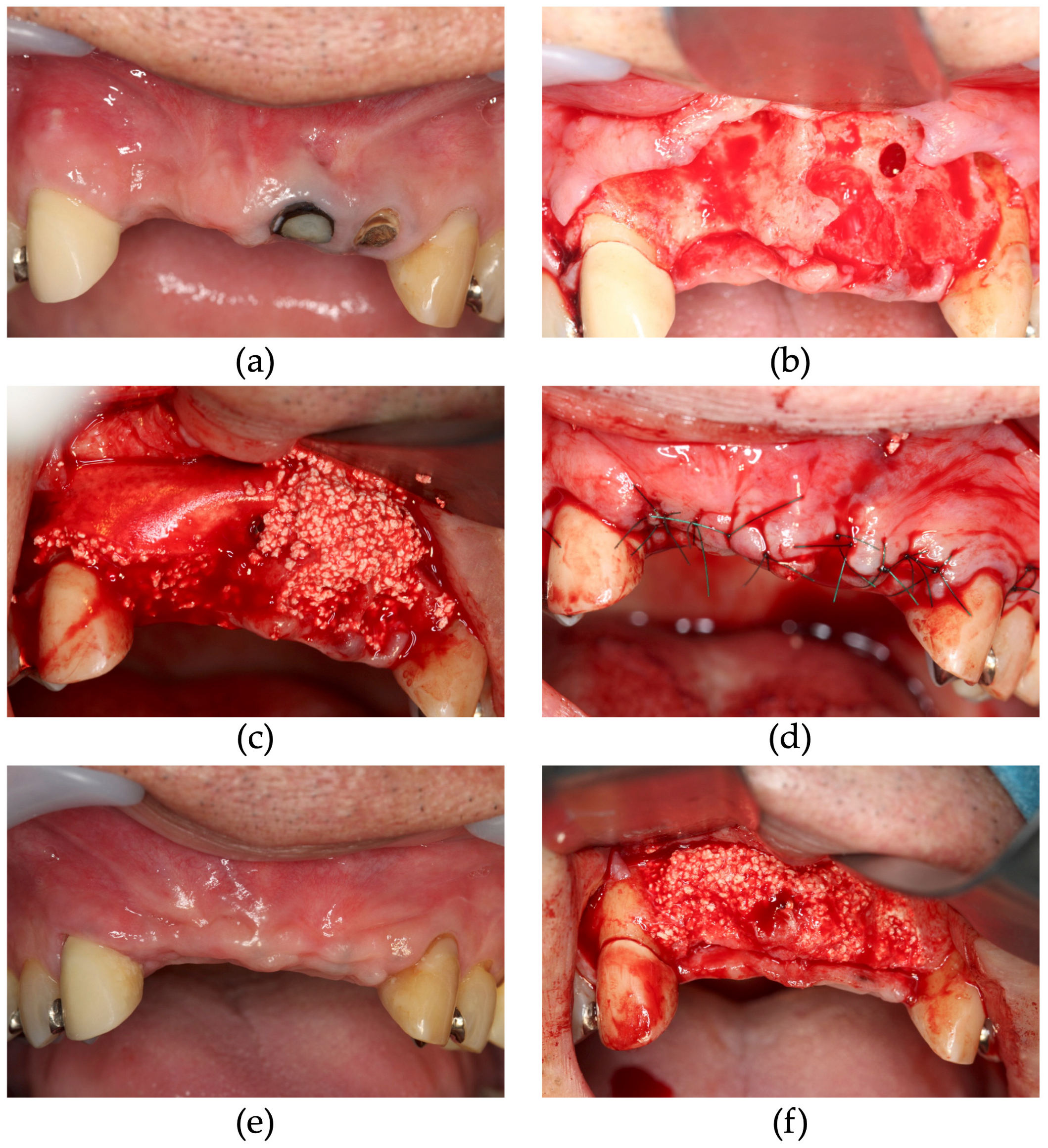
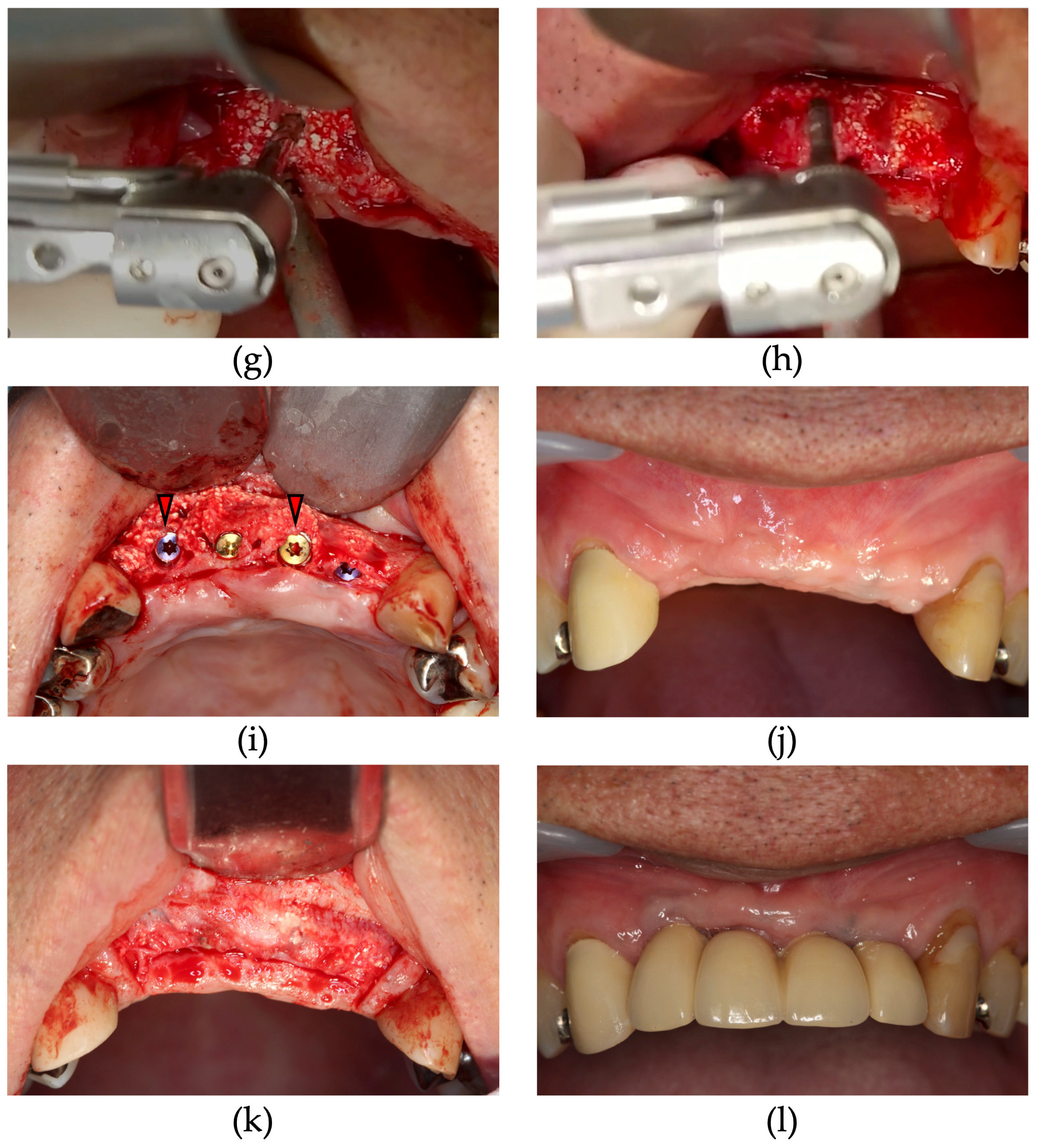
2.2. Surgical Procedure
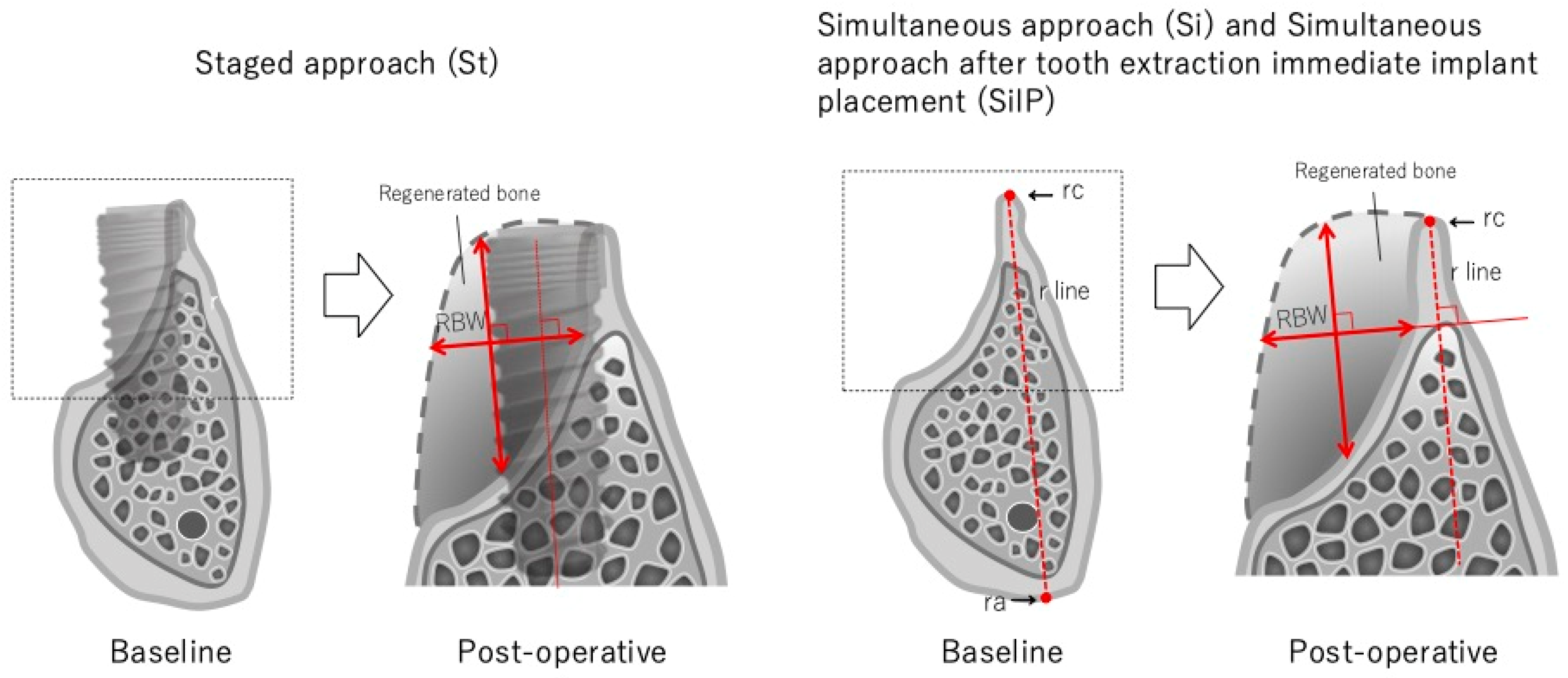
2.3. Measurements Using CBCT
2.4. Clinical Measurement
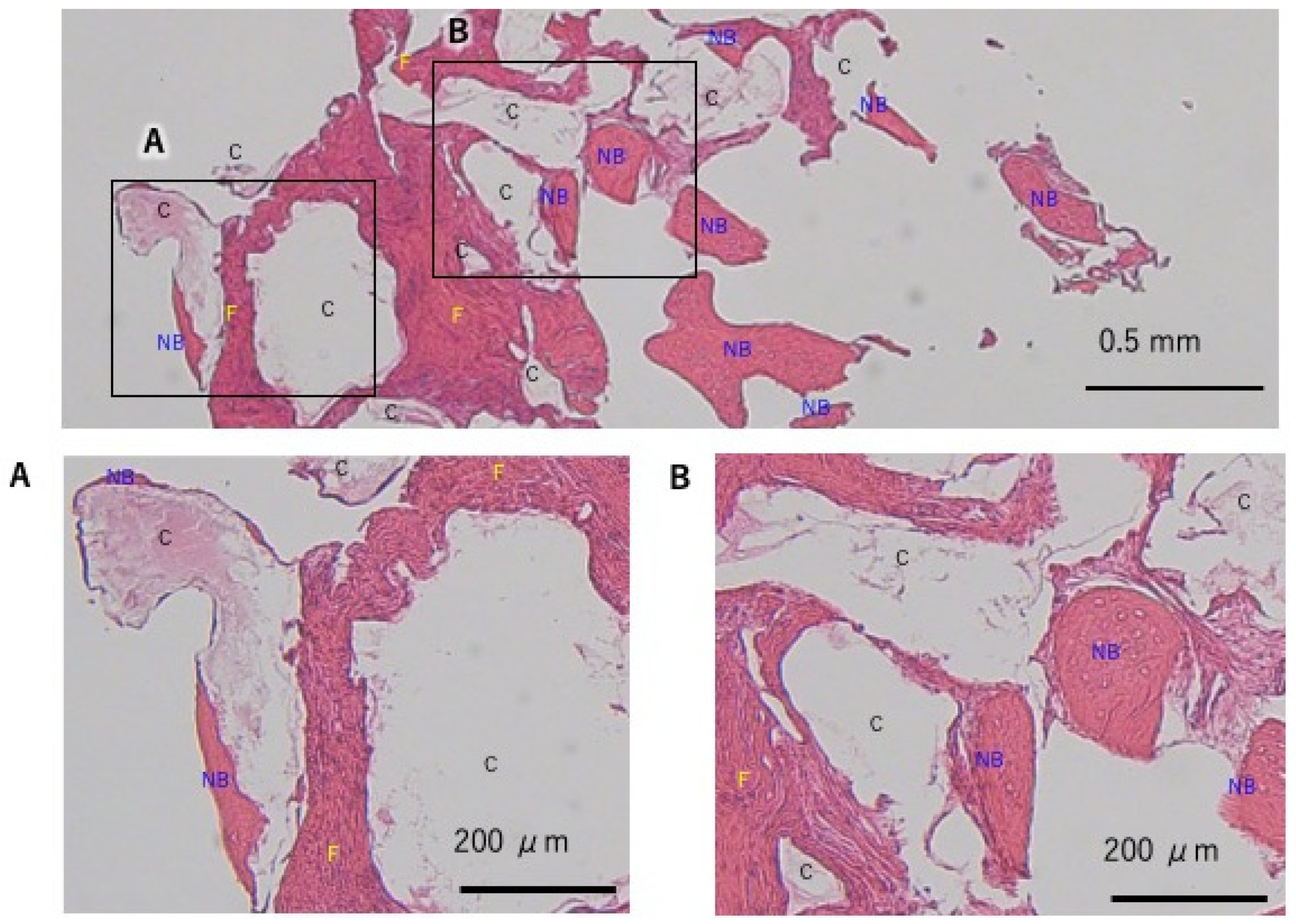
2.5. Histological Evaluation
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mizraji, G.; Davidzohn, A.; Gursoy, M.; Gursoy, U.; Shapira, L.; Wilensky, A. Membrane barriers for guided bone regeneration: An overview of available biomaterials. Periodontology 2000 2013, 93, 56–76. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, L.; Ngai, T. Multiphasic membranes/scaffolds for periodontal guided tissue regeneration. Macromol. Mater. Eng. 2023, 308, 2300081. [Google Scholar] [CrossRef]
- Hämmerle, C.H.; Jung, R.E. Bone augmentation by means of barrier membranes. Periodontology 2000 2003, 33, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, J.M.; Martín, I.S.; Santos, A.; Pujol, A.; Sanz-Moliner, J.D.; Nart, J. High-density polytetrafluoroethylene membranes in guided bone and tissue regeneration procedures: A literature review. Int. J. Oral Maxillofac. Surg. 2014, 43, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Ronda, M.; Rebaudi, A.; Torelli, L.; Stacchi, C. Expanded vs. dense polytetrafluoroethylene membranes in vertical ridge augmentation around dental implants: A prospective randomized controlled clinical trial. Clin. Oral Implants Res. 2014, 25, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.A.; Monje, A.; Wang, H.L. Vertical ridge augmentation and soft tissue reconstruction of the anterior atrophic maxillae: A case series. Int. J. Periodontics Restor. Dent. 2015, 35, 613–623. [Google Scholar] [CrossRef]
- Soldatos, N.K.; Stylianou, P.; Koidou, V.P.; Angelov, B.; Yukna, R.; Romanos, G.E. Limitations and options using resorbable versus nonresorbable membranes for successful guided bone regeneration. Quintessence Int. 2017, 48, 131–147. [Google Scholar]
- Urban, I.A.; Monje, A. Guided bone regeneration in alveolar bone reconstruction. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Chacón, G.; Saleh, M.H.A.; Decker, A.; Kan, J.Y.K.; Wang, H.L. Vertical ridge augmentation using collagen membrane and tenting screws in the esthetic zone: A case series. Int. J. Periodontics Restor. Dent. 2024, 44, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Abe, G.L.; Sasaki, J.I.; Katata, C.; Kohno, T.; Tsuboi, R.; Kitagawa, H.; Imazato, S. Fabrication of novel poly(lactic acid/caprolactone) bilayer membrane for GBR application. Dent. Mater. 2020, 36, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Kudoh, K.; Fukuda, N.; Kasugai, S.; Tachikawa, N.; Koyano, K.; Matsushita, Y.; Ogino, Y.; Ishikawa, K.; Miyamoto, Y. Maxillary sinus floor augmentation using low-crystalline carbonate apatite granules with simultaneous implant installation: First-in-human clinical trial. J. Oral Maxillofac. Surg. 2019, 77, 985.e1–985.e11. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Katagiri, H.; Kono, M.; Schaller, B.; Iizuka, T.; Safi, A.F. The impact of the size of bone substitute granules on macrophage and osteoblast behaviors in vitro. Clin. Oral Investig. 2021, 25, 4949–4958. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Miyamoto, Y.; Tsuchiya, A.; Hayashi, K.; Tsuru, K.; Ohe, G. Physical and histological comparison of hydroxyapatite, carbonate apatite, and β-tricalcium phosphate bone substitutes. Materials 2018, 11, 1993. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, M.; Tsuru, K.; Fukuda, N.; Sakemi, Y.; Nakashima, Y.; Ishikawa, K. Evaluation of carbonate apatite blocks fabricated from dicalcium phosphate dihydrate blocks for reconstruction of rabbit femoral and tibial defects. J. Mater. Sci. Mater. Med. 2017, 28, 85. [Google Scholar] [CrossRef] [PubMed]
- Ogino, Y.; Ayukawa, Y.; Tachikawa, N.; Shimogishi, M.; Miyamoto, Y.; Kudoh, K.; Fukuda, N.; Ishikawa, K.; Koyano, K. Staged sinus floor elevation using novel low-crystalline carbonate apatite granules: Prospective results after 3-year functional loading. Materials 2021, 14, 5760. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Koyanagi, T.; Takagi, T.; Kitanaka, Y.; Aoki, A.; Iwata, T. Ridge preservation and augmentation using a carbonated apatite bone graft substitute: A case series. Dent. J. 2024, 12, 55. [Google Scholar] [CrossRef]
- Shido, R.; Ohba, S.; Tominaga, R.; Sumita, Y.; Asahina, I. A Prospective Study of the Assessment of the Efficacy of a Biodegradable Poly (l-lactic acid/ε-caprolactone) Membrane for Guided Bone Regeneration. J. Clin. Med. 2023, 12, 5994. [Google Scholar] [CrossRef] [PubMed]
- Beitlitum, I.; Artzi, Z.; Nemcovsky, C.E. Clinical evaluation of particulate allogeneic with and without autogenous bone grafts and resorbable collagen membranes for bone augmentation of atrophic alveolar ridges. Clin. Oral Implants Res. 2010, 21, 1242–1250. [Google Scholar] [CrossRef]
- Von Arx, T.; Buser, D. Horizontal ridge augmentation using autogenous block grafts and the guided bone regeneration technique with collagen membranes: A clinical study with 42 patients. Clin. Oral Implant Res. 2006, 17, 359–366. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Sawada, K.; Yamada, A.; Mizutani, K.; Meinzer, W.; Iwata, T.; Izumi, Y.; Aoki, A. Er:YAG laser-assisted bone regenerative therapy for implant placement: A case series. Int. J. Periodontics Restor. Dent. 2021, 41, e137–e175. [Google Scholar] [CrossRef] [PubMed]
- Ersanli, S.; Olgac, V.; Leblebicioglu, B. Histologic analysis of alveolar bone following guided bone regeneration. J. Periodontol. 2004, 75, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Bassi, M.A.; Andrisani, C.; Lico, S.; Ormanier, Z.; Ottria, L.; Gargari, M. Guided bone regeneration via a preformed titanium foil: Clinical, histological and histomorphometric outcome of a case series. Oral Implantol. 2016, 9, 164–174. [Google Scholar]
- Čandrlić, M.; Perić Kačarević, Ž.; Ivanišević, Z.; Tomas, M.; Včev, A.; Faj, D.; Matijević, M. Histological and radiological features of a four-phase injectable synthetic bone graft in guided bone regeneration: A case report. Int. J. Environ. Res. Public Health 2020, 18, 206. [Google Scholar] [CrossRef]




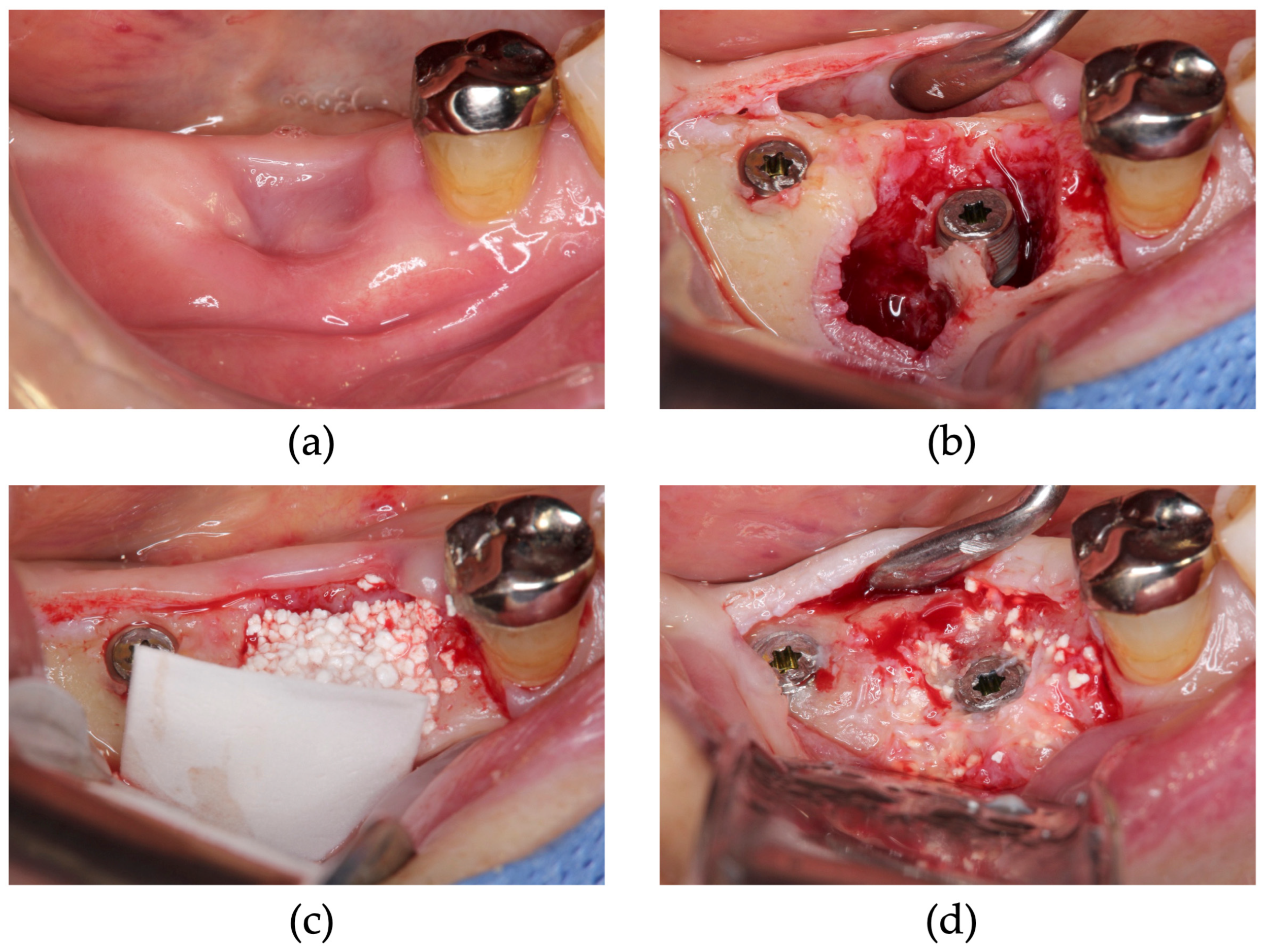
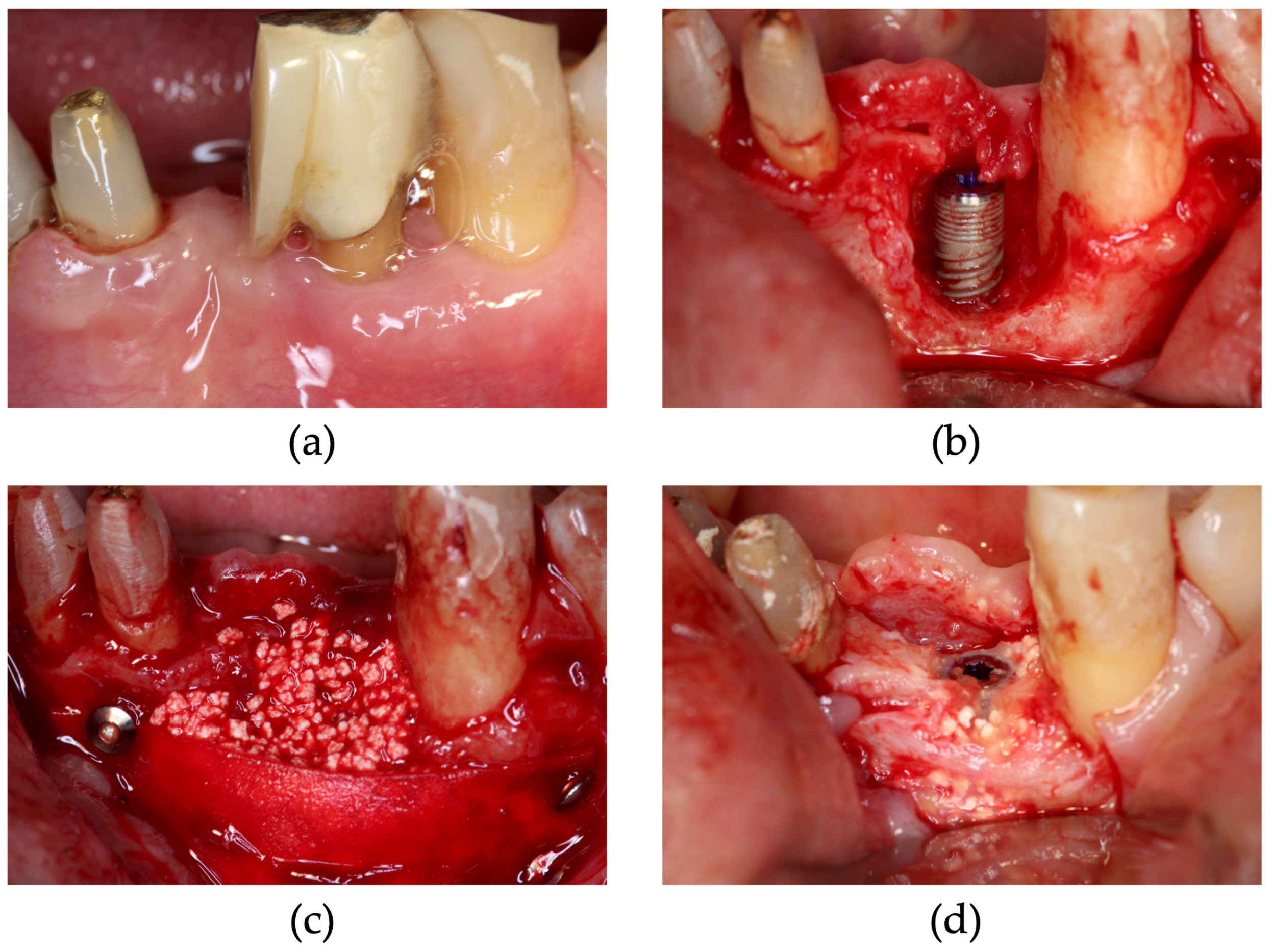
| Patient Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| Age (Y O) | 63 | 63 | 21 | 21 | 65 | 65 | 54 | 41 | 56 | 54 | 54 | 73 | 61 | 68 | 68 |
| Sex (Male = m, Female = f) | m | m | f | F | f | f | M | m | M | f | f | f | m | m | m |
| Number of treatment sites (FDI) | 12 | 13 | 12 | 13 | 11 | 12 | 35 | 46 | 37 | 21 | 22 | 46 | 33 | 35 | 36 |
| Procedure | St | St | St | St | St | St | SiIP | SiIP | SiIP | Si | Si | Si | SiIP | Si | Si |
| implant diameter (mm) | 3.3 | 4 | 3.3 | 3.3 | 3.3 | 4 | 3.3 | 4 | 4 | 3.3 | 3.3 | 4 | 3.3 | 4 | 4 |
| Pre-Operative Bone Width | Post-Operative Bone Width | Regenerated Bone Width Without Implant Diameter | Regenerated Bone Width Within Implant Diameter | |
|---|---|---|---|---|
| (Mean ± SD mm) | (Mean ± SD mm) | (Mean ± SD mm) | (Mean ± SD mm) | |
| All procedure (N = 15) | 2.81 ± 1.67 | 9.14 ± 2.25 * | 4.14 ± 1.99 | 6.36 ± 1.83 |
| Si (N = 5) | 3.06 ± 0.66 | 9.33 ± 2.48 | 2.60 ± 2.42 | 6.34 ± 2.64 |
| SiIP (N = 4) | 1.81 ± 1.09 | 9.37 ± 2.08 * | 3.90 ± 0.78 | 7.55 ± 1.17 |
| St (N = 6) | 3.27 ± 2.37 | 8.84 ± 2.54 | 5.57 ± 1.08 |
| Bone Quantity Achievement (Median) | Regenerated Bone Quality (Median) | Pre-Operative Oral Vestibular Condition (Median) | Post-Operative Oral Vestibular Condition (Median) | |
|---|---|---|---|---|
| All Procedure (N = 15) | 5 | 5 | 4 | 3 |
| Procedure | ||||
| Si (N = 5) | 5 | 5 | 4 | 3 |
| SilM (N = 4) | 5 | 5 | 4 | 2.5 |
| St (N = 6) | 5 | 5 | 3 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taniguchi, Y.; Koyanagi, T.; Kitanaka, Y.; Yamada, A.; Aoki, A.; Iwata, T. Guided Bone Regeneration Using Carbonated Apatite Granules and L-Lactic Acid/ε-Caprolactone Membranes: A Case Series and Histological Evaluation. Dent. J. 2025, 13, 85. https://doi.org/10.3390/dj13020085
Taniguchi Y, Koyanagi T, Kitanaka Y, Yamada A, Aoki A, Iwata T. Guided Bone Regeneration Using Carbonated Apatite Granules and L-Lactic Acid/ε-Caprolactone Membranes: A Case Series and Histological Evaluation. Dentistry Journal. 2025; 13(2):85. https://doi.org/10.3390/dj13020085
Chicago/Turabian StyleTaniguchi, Yoichi, Tatsuro Koyanagi, Yutaro Kitanaka, Azusa Yamada, Akira Aoki, and Takanori Iwata. 2025. "Guided Bone Regeneration Using Carbonated Apatite Granules and L-Lactic Acid/ε-Caprolactone Membranes: A Case Series and Histological Evaluation" Dentistry Journal 13, no. 2: 85. https://doi.org/10.3390/dj13020085
APA StyleTaniguchi, Y., Koyanagi, T., Kitanaka, Y., Yamada, A., Aoki, A., & Iwata, T. (2025). Guided Bone Regeneration Using Carbonated Apatite Granules and L-Lactic Acid/ε-Caprolactone Membranes: A Case Series and Histological Evaluation. Dentistry Journal, 13(2), 85. https://doi.org/10.3390/dj13020085








