Effects of Bromelain and Papain in Tooth Whitening and Caries Removal: A Literature Review
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
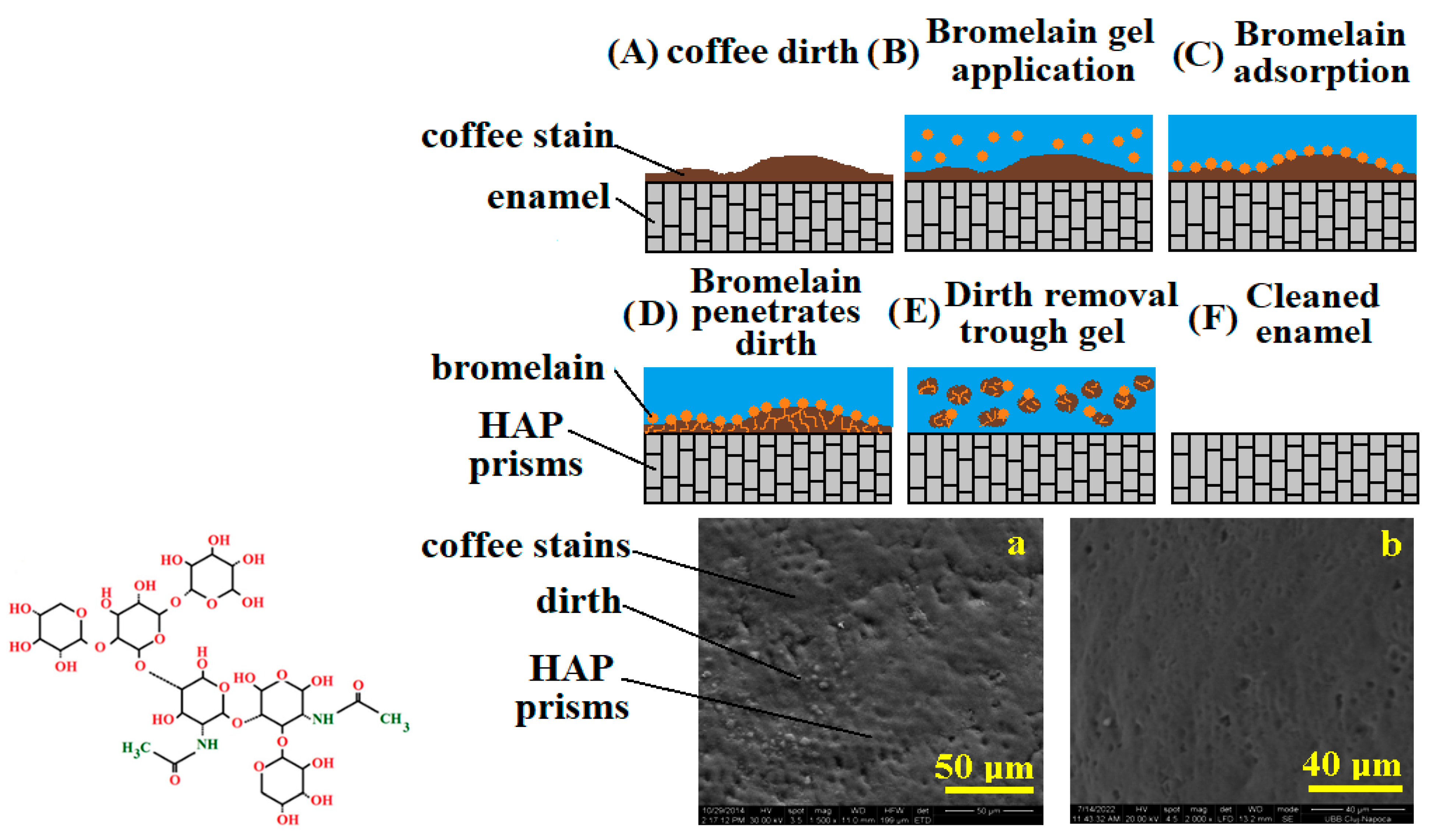
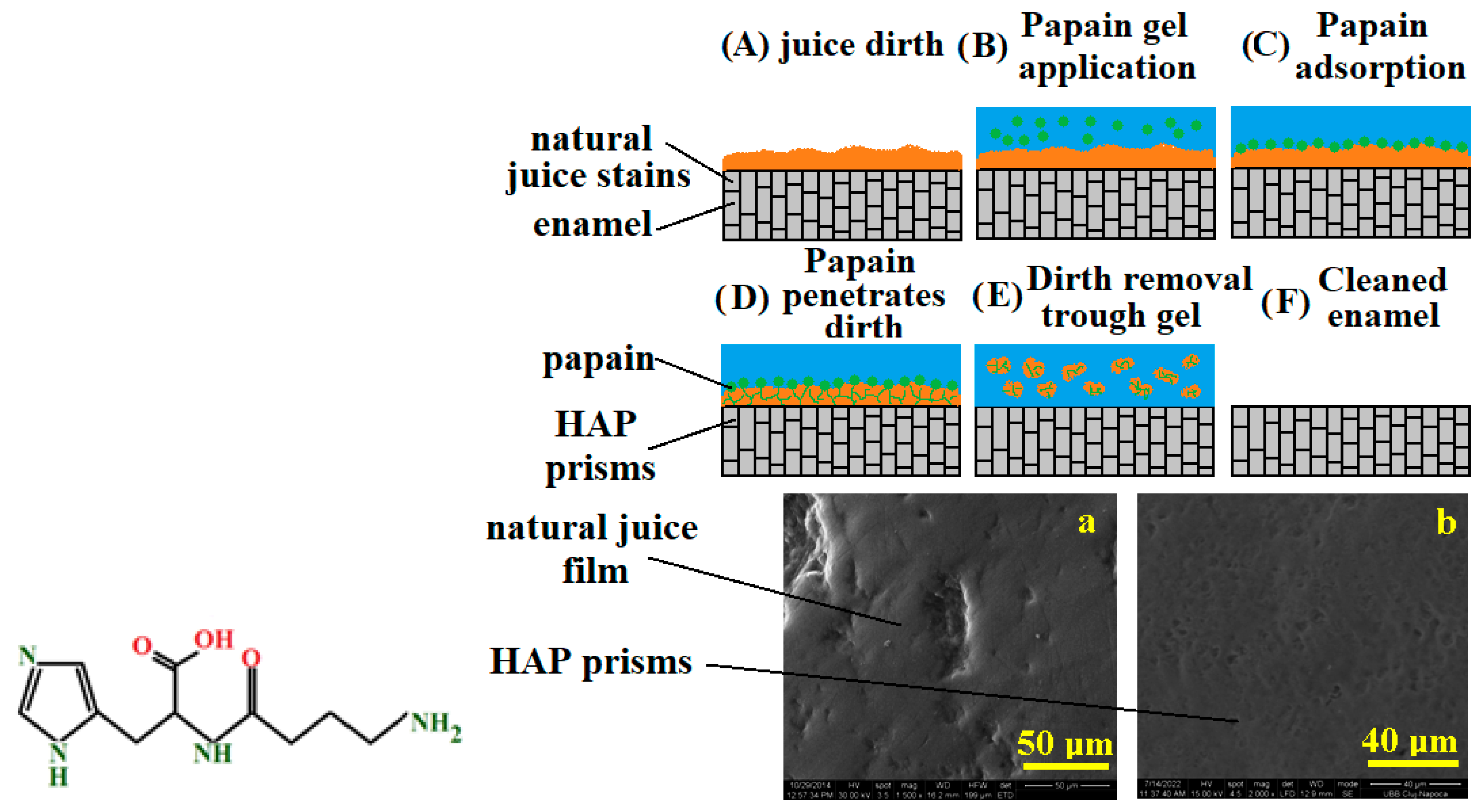
3.1. Bromelain and Papain Effects in Tooth Whitening
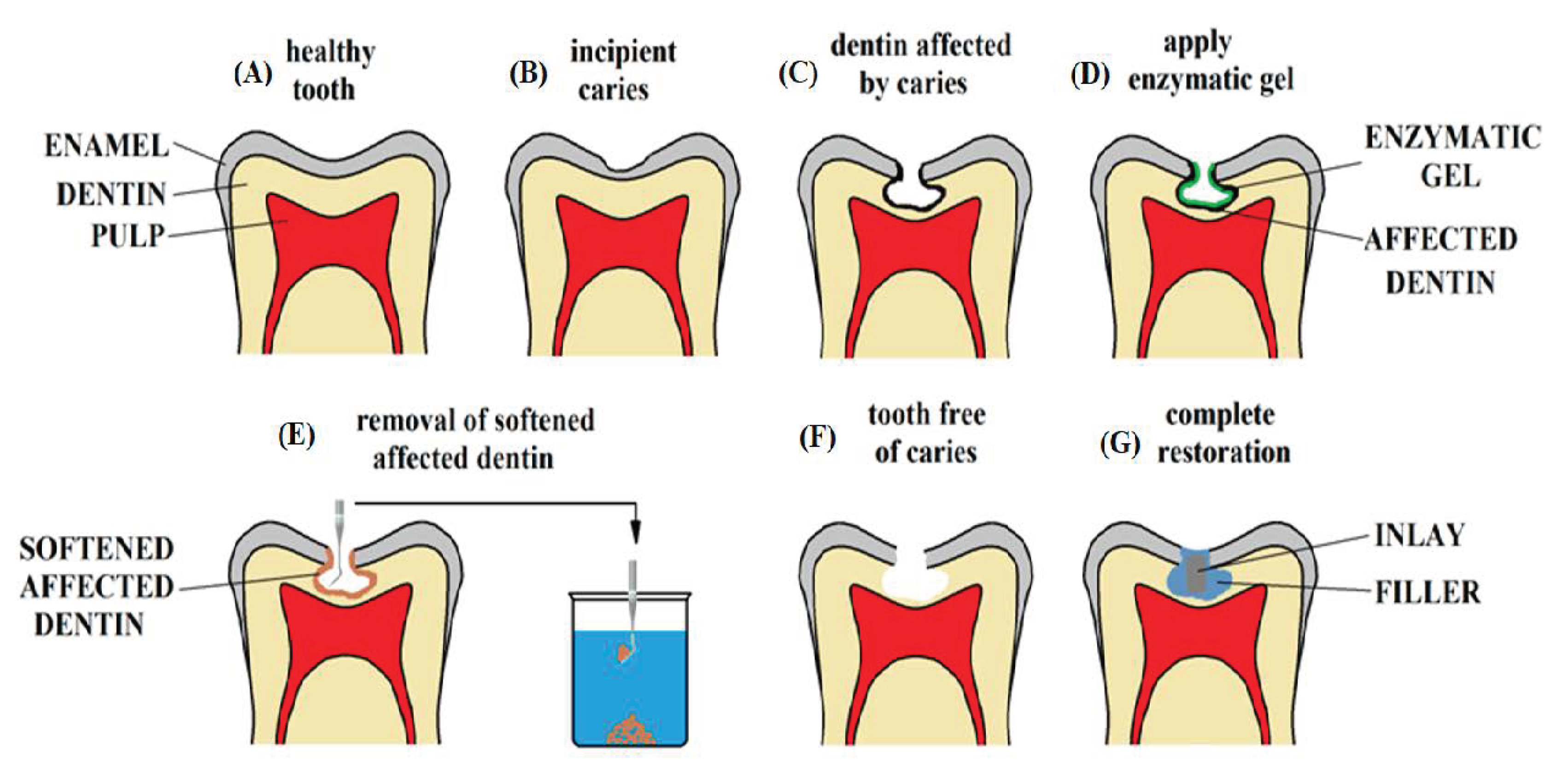
3.2. Chemo-Mechanical/Atraumatic Removal of the Damaged Dental Tissues
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Schwarzbold, C.G.; Cuevas-Suárez, C.E.; Pacheco, R.R.; Ribeiro, J.S.; Carreño, N.L.V.; Lund, R.G.; Piva, E. In vitro efficacy of commercial and experimental proteolytic enzyme-based whitening dentifrices on enamel whitening and superficial roughness. J. Esthet. Restor. Dent. 2021, 33, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, S.; Wang, J.; Xiao, X.; Song, Z.; Liu, S. Tooth whitening: Current status and prospects. Odontology 2024, 112, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Medina-Zamora, N.; Busch, M.; Palmer, J. Bromelain: A Natural Approach to Whitening Teeth Effectively. Bachelor’s Thesis, Texas A&M University, Texas, USA, 2021. Available online: https://oaktrust.library.tamu.edu/server/api/core/bitstreams/4ac17610-3da9-4b13-9262-ef7c277b4258/content (accessed on 10 December 2024).
- Essam, M. Natural dental bleaching agents. In Natural Consevative Dentistry: An Alternativ Approach to Solve Restorative Problems; Bentham Science Publishers: Potomac, MD, USA, 2023; Volume 22, pp. 169–190. Available online: https://www.eurekaselect.com/chapter/22246 (accessed on 10 December 2024).
- Patil, P.A.; Ankola, A.V.; Hebbal, M.I.; Patil, A.C. Comparison of effectiveness of abrasive and enzymatic action of whitening toothpastes in removal of extrinsic stains—A clinical trial. Int. J. Dent. Hyg. 2015, 13, 25–29. [Google Scholar] [CrossRef]
- Mishra, D.; Kamath, D.G.; Alagla, M.; Rahman, S.A.; Amin, R.; Ahmed, H.; Singh, G.; Singh, D.K.; Renugalakshmi, A. Evaluation of stain removal efficacy and color stability of three different dentifrices on artificially stained enamel surface—An in vitro study. J. Contemp. Dent. Pract. 2024, 25, 68–71. [Google Scholar] [PubMed]
- Hinman, R.L.; Lang, J. Peroxidase-catalyzed oxidation of Indole-3-acetic acid. Biochemistry 1965, 4, 144–158. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Castañeda-Valbuena, D.; Tavano, O.; Abellanas, P.; de Andrades, D.; Santiz-Gómez, J.A.; Berenguer-Murcia, A.; Fernandez-Lafuente, R. A review on the immobilization of bromelain. Int. J. Biol. Macromol. 2024, 273, 133089. [Google Scholar] [CrossRef]
- Aparecida-Pereira, A.; de Carvalho-Freitas, I.; Souza de Mendonça, S.M. A utilização do gel de papaína na remoção de lesões cariosas dentinárias. Rev. Odontol. Univ. Cid. (São Paulo) 2013, 25, 68–76. [Google Scholar] [CrossRef]
- Al-Badri, H.; Al-Taee, L.A.; Banerjee, A.; Al-Shammaree, S.A. An in-vitro evaluation of residual dentin retained after using novel enzymatic-based chemomechanical caries removal agents. Sci. Rep. 2024, 14, 19223. [Google Scholar] [CrossRef]
- Martins, B.C.; Rescolino, R.; Coelho, D.D.F.; Espindola, F.; Zanchetta, B.; Tambourgi, E.B.; Silveira, E. Characterization of bromelain from Ananas comosus agroindustrial residues purified by ethanol factional precipitation. Chem. Eng. Trans. 2014, 37, 781–786. [Google Scholar] [CrossRef]
- Hikisz, P.; Bernasinska-Slomczewska, J. Beneficial properties of bromelain. Nutrients 2021, 13, 4313. [Google Scholar] [CrossRef]
- Taussig, S.J.; Batkin, S. Bromelain, the enzyme complex of pineapple (Annanus comosus) and its clinical application: An update. J. Ethnopharmacol. 1988, 22, 191–203. [Google Scholar] [CrossRef]
- Balls, A.K.; Thompson, R.R.; Kies, M.W. Bromelin. Properties and commercial production. Ind. Eng. Chem. 1941, 33, 950–953. [Google Scholar] [CrossRef]
- Kelly, G.S. Bromelain: A literature review and discussion of its therapeutic applications. Altern. Med. Rev. 1996, 1, 243–257. [Google Scholar]
- Gautam, S.S.; Mishra, S.K.; Dash, V.; Goyal, A.K.; Rath, G. Comparative study of extraction, purification and estimation of bromelain from stem and fruit of pineapple plant. Thai J. Pharm. Sci. 2010, 34, 67–76. [Google Scholar] [CrossRef]
- Rowan, A.D.; Buttle, D.J.; Barett, A.J. The cysteine proteinases of the pineapple plant. Biochem. J. 1990, 266, 869–875. [Google Scholar] [PubMed]
- Hebbar, H.U.; Sumana, B.; Raghavarao, K.S.M.S. Use of reverse micellar systems for the extraction and purification of bromelain from pineapple wastes. Bioresour. Technol. 2008, 99, 4896–4902. [Google Scholar] [CrossRef]
- Tanaka, K.; Hilary, Z.D.; Ishizaki, A. Investigation of the utility of pineapple juice and pineapple waste material as low costs substrate for ethanol fermentation by Zymomonasmobilis. J. Biosci. Bioeng. 1999, 87, 642–646. [Google Scholar] [CrossRef]
- Bhattacharyya, B.K. Bromelain: An overview. Nat. Prod. Radiance 2008, 7, 359–363. [Google Scholar]
- Cooreman, W.M., VIII. Bromelain. In Pharmaceutical Enzymes-Properties and Assay Methods; Lauwers, A., Ruyssen, R., Eds.; Story-Scientia Scientific Publishing Co.: Gent, Belgium, 1978; pp. 107–121. [Google Scholar]
- Napper, A.D.; Bennett, S.P.; Borowski, M.; Holdridge, M.B.; Leonard, M.J.C.; Rogers, E.E.; Duan, Y.; Laursen, R.A.; Reinhold, B.; Shames, S.L. Purification and characterization of multiple forms of the pineapple stem-derived cysteine proteinases ananain and comosain. Biochem. J. 1994, 301, 727–735. [Google Scholar] [CrossRef]
- Upadhyay, A.; Lama, J.P.; Tawata, S. Utilization of pineapple waste: A review. J. Food Sci. Technol. Nepal 2010, 6, 10–18. [Google Scholar] [CrossRef]
- Mohamed Thameemul Ansari, K.A.; Adimulapu, H.S. Comparison between the effect of commercially available chemical teeth whitening paste and teeth whitening paste containing bromelain on human enamel. J. Surv. Fish. Sci. 2023, 10, 162–167. Available online: https://sifisheriessciences.com/journal/index.php/journal/article/view/159/153 (accessed on 10 December 2024).
- Larocca, M.; Rossano, R.; Santamaria, M.; Riccio, P. Analysis of pineapple [Ananascomosus comosus (L.) Merr.] fruit proteinases by 2-D zymography and direct identification of the major zymographic spots by mass spectrometry. Food Chem. 2010, 123, 1334–1342. [Google Scholar] [CrossRef]
- Oliver-Simancas, R.; Labrador-Fernández, L.; Abellán-Diéguez, C.; García-Villegas, A.; Del Caro, A.; Leyva-Jimenez, F.J.; Alañón, M.E. Valorization applications of pineapple and papaya byproducts in food industry. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13359. [Google Scholar] [CrossRef] [PubMed]
- Nanda, R.F.; Bahar, R.; Syukri, D.; Thu, N.N.A.; Kasim, A. A review: Application of bromelain enzymes in animal food products. Andalasian Int. J. Agric. Nat. Sci. (AIJANS) 2020, 1, 33–44. [Google Scholar] [CrossRef]
- Jan, B.; Abass, S.; Ahmad, S. Application of Microbial Enzymes in Food Industry. In Microbial Biotechnology in the Food Industry; Ahmad, F., Mohammad, Z.H., Ibrahim, S.A., Zaidi, S., Eds.; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Ketnawa, S.; Sai-Ut, S.; Theppakorn, T.; Chaiwut, P.; Rawdkuen, S. Partitioning of bromelain from pineapple peel (Nang Lae cultv.) by aqueous two-phase system. Asian J. Food Agro-Ind. 2009, 2, 457–468. [Google Scholar]
- Kumar, V.; Mangla, B.; Javed, S.; Ahsan, W.; Kumar, P.; Garga, V.; Dureja, H. Bromelain: A review of its mechanisms, pharmacological effects and potential applications. Food Funct. 2023, 18, 8101–8128. [Google Scholar] [CrossRef] [PubMed]
- Rojek, M. Enzyme Nutrition Therapy Beyond a Raw Food Diet. Nexus Mag. 2004, 11, 21–26. [Google Scholar]
- de Lencastre Novaes, L.C.; Jozala, A.F.; Lopes, A.M.; de Carvalho Santos-Ebinuma, V.; Mazzola, P.G.; Pessoa Junior, A. Stability, purification, and applications of bromelain: A review. Biotechnol. Prog. 2016, 32, 5–13. [Google Scholar] [CrossRef]
- Agrawal, P.; Nikhade, P.; Patel, A.; Mankar, N.; Sedani, S. Bromelain: A Potent Phytomedicine. Cureus 2022, 14, e27876. [Google Scholar] [CrossRef]
- Abdeldaiem, A.M.; Ekram, H.E.-B.; Abbas, F.; Faisal, M.A. Effect of some Factors on the Proteolytic Activities of Bromelain, Cichorium and Papain Extracts. Ismailia J. Dairy Sci. Technol. 2019, 6, 1–7. [Google Scholar]
- Mohapatra, A.; Rao, V.M.; Ranjan, M. Comparative study of the increased production and characterization of Bromelain from the peel, pulp and stem pineapple (Anannus commas). Int. J. Adv. Res. Technol. 2013, 2, 249–277. [Google Scholar]
- Colletti, A.; Li, S.; Marengo, M.; Adinolfi, S.; Cravotto, G. Recent advances and insights into bromelain processing, pharmacokinetics and therapeutic uses. Appl. Sci. 2021, 11, 8428. [Google Scholar] [CrossRef]
- Varilla, C.; Marcone, M.; Paiva, L.; Baptista, J. Bromelain, a group of pineapple proteolytic complex enzymes (Ananas comosus) and their possible therapeutic and clinical effects. A summary. Foods 2021, 10, 2249. [Google Scholar] [CrossRef]
- Babalola, B.A.; Akinwande, A.I.; Otunba, A.A.; Adebami, G.E.; Babalola, O.; Nwufo, C. Therapeutic benefits of Carica papaya: A review on its pharmacological activities and characterization of papain. Arab. J. Chem. 2024, 17, 105369. [Google Scholar] [CrossRef]
- Nadzri, F.A.; Tawalbeh, D.; Sarbon, N.M. Physicochemical properties and antioxidant activity of enzymatic hydrolysed chickpea (Cicer arietinum L.) protein as influence by alcalase and papain enzyme. Biocatal. Agric. Biotechnol. 2021, 36, 102131. [Google Scholar] [CrossRef]
- Sagadevan, P.; Selvakumar, S.; Raghunath, M.; Megala, R.; Janarthanan, P.; Vinitha, E.C.; Senthil, K.V. Medicinal properties of Carica papaya Linn: Review. Madridge J. Nov. Drug Res. 2019, 3, 120–125. [Google Scholar] [CrossRef]
- Choudhary, R.; Kaushik, R.; Chawla, P.; Manna, S. Exploring the extraction, functional properties, and industrial applications of papain from Carica papaya. J. Sci. Food Agric. 2025, 105, 1533–1545. [Google Scholar] [CrossRef]
- Pandey, S.; Cabot, P.J.; Shaw, P.N.; Hewavitharana, A.K. Anti-inflammatory and immunomodulatory properties of Carica papaya. J. Immunotoxicol. 2016, 13, 590–602. [Google Scholar] [CrossRef]
- Nariya, A.; Jhala, D. Pharmacognostic study of Carica papaya leaf extract as inhibitors of reactive oxygen species. Int. Res. J. Pharm. 2017, 8, 13–17. [Google Scholar] [CrossRef]
- Sharma, N.; Mishra, K.P.; Chanda, S.; Bhardwaj, V.; Tanwar, H.; Ganju, L.; Kumar, B.; Singh, S.B. Evaluation of anti-dengue activity of Carica papaya aqueous leaf extract and its role in platelet augmentation. Arch. Virol. 2019, 164, 1095–1110. [Google Scholar] [CrossRef]
- Charan, J.; Saxena, D.; Goyal, J.P.; Yasobant, S. Efficacy and safety of Carica papaya leaf extract in the dengue: A systematic review and meta-analysis. Int. J. Appl. Basic Med. Res. 2016, 6, 249–254. [Google Scholar] [CrossRef]
- Tan, B.; Zhang, X.; Ansari, A.; Jadhav, P.; Tan, H.; Li, K.; Chopra, A.; Ford, A.; Chi, X.; Ruiz, F.X.; et al. Design of a SARS-CoV-2 papain-like protease inhibitor with antiviral efficacy in a mouse model. Science 2024, 383, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.H.; Marques, F.L.; Real, C.C.; Thipe, V.C.; Freitas, L.F.; Lima, C.S.; de Souza, L.E.; Junqueira, M.S.; Faria, D.P.; Varca, G.H.C.; et al. Green Nanotechnology Through Papain Nanoparticles: Preclinical in vitro and in vivo Evaluation of Imaging Triple-Negative Breast Tumors. Nanotechnol. Sci. Appl. 2024, 17, 211–226. [Google Scholar] [CrossRef]
- Hakim, R.F.; Fakhrurrazi; Dinni. Effect of Carica papaya Extract toward incised wound healing process in mice (Mus musculus) clinically and histologically. Evid. Based Complement. Altern. Med. 2019, 8306519. [Google Scholar] [CrossRef] [PubMed]
- Vilhena, K.F.B.; Nogueira, B.C.L.; Fagundes, N.C.F.; Loretto, S.C.; Angelica, R.S.; Lima, R.R.; Souza, M.H.S., Jr. Dental enamel bleached for a prolonged and excessive time: Morphological changes. PLoS ONE 2019, 14, e0214948. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Barboza, A.S.; Cuevas-Suárez, C.E.; da Silva, A.F.; Piva, E.; Lund, R.G. Novel in-office peroxide-free teeth-whitening gels: Bleaching effectiveness, enamel surface alterations, and cell viability. Sci. Rep. 2020, 10, 10016. [Google Scholar] [CrossRef]
- Müller-Heupt, L.K.; Wiesmann-Imilowski, N.; Kaya, S.; Schumann, S.; Steiger, M.; Bjelopavlovic, M.; Lehmann, K.M. Effectiveness and safety of over-the-counter tooth-whitening agents compared to hydrogen peroxide in vitro. Int. J. Mol. Sci. 2023, 24, 1956. [Google Scholar] [CrossRef] [PubMed]
- Lilaj, B.; Dauti, R.; Agis, H.; Schmid-Schwap, M.; Franz, A.; Kanz, F.; Moritz, A.; Schedle, A.; Cvikl, B. Comparison of bleaching products with up to 6% and with more than 6% hydrogen peroxide: Whitening efficacy using BI and WID and side effects—An in vitro study. Front. Physiol. 2019, 10, 919. [Google Scholar] [CrossRef] [PubMed]
- Al-Badri, H.; Al-Badri, H.; Al-Shammaree, S.A.; Banerjee, A.; Al-Taee, L.A. The in-vitro development of novel enzyme-based chemo-mechanical caries removal agents. J. Dent. 2023, 138, 104714. [Google Scholar] [CrossRef]
- Barbosa, L.M.; Carneiro, T.S.; Favoreto, M.W.; Borges, C.P.; Reis, A.; Loguercio, A.D.; Meireles, S.S. Effect of whitening toothpastes with different hydrogen peroxide concentrations: Penetration into the pulp chamber and color change. J. Dent. 2024, 144, 104951. [Google Scholar] [CrossRef]
- Jurema, A.L.B.; de Souza, M.Y.; Torres, C.R.G.; Borges, A.B.; Caneppele, T.M.F. Effect of pH on whitening efficacy of 35% hydrogen peroxide and enamel microhardness. J. Esthet. Restor. Dent. 2018, 30, E39–E44. [Google Scholar] [CrossRef]
- Mazilu, A.; Popescu, V.; Sarosi, C.; Silaghi Dumitrescu, R.; Chisnoiu, A.M.; Moldovan, M.; Silaghi Dumitrescu, L.; Prodan, D.; Carpa, R.; Gheorghe, G.F.; et al. Preparation and in vitro characterization of gels based on bromelain, whey and quince extract. Gels 2021, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Pasril, Y.; Nurfalah, I. The effectiveness of pineapple extract (Ananas comosus) as a natural tooth whitening. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2024; Volume 3127, p. 020009. [Google Scholar] [CrossRef]
- Vekaash, C.J.V.; Reddy, T.V.K.; Venkatesh, K.V. Effect of vital bleaching with solutions containing different concentrations of hydrogen peroxide and pineapple extract as an additive on human enamel using reflectance spectrophotometer: An in vitro study. J. Conserv. Dent. 2017, 20, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Solanki, M.N.; Attur, K.M.; Vachhani, K.A.; Patel, N.A.; Shah, M.A.; Doshi, D.M. Phytochemicals in vital tooth bleaching: Spectrophotometric evaluation of efficacy with papaya, pineapple, or kiwi extracts and 30% hydrogen peroxide. J. Conserv. Dent. Endod. 2024, 27, 760–764. [Google Scholar] [CrossRef]
- Moldovan, M.A.; Cuc, S.; Gasparik, C.; Sarosi, C.; Moldovan, M.; Ilie, N.; Petean, I.; Rusu, L.M.; Ionescu, A.; Pastrav, M. Effect of experimental bleaching gels with enzymes on composite and enamel. Int. Dent. J. 2024. [Google Scholar] [CrossRef]
- Shobana, G.; Muthu Karuppaiah, R.; Garla, B.K.; Taranath, M.; Palanivel Pandian, R. Effect of Whitening Toothpastes on Extrinsic Dental Stains. J. Adv. Oral Res. 2019, 10, 19–23. [Google Scholar] [CrossRef]
- Munchow, E.A.; Hamann, H.J.; Carvajal, M.T.; Pinal, R.; Bottino, M.C. Stain removal effect of novel papain- and bromelain gels applied to enamel. Clin. Oral Investig. 2016, 20, 2315–2320. [Google Scholar] [CrossRef]
- Ananthakrishna, S.; Raghu, T.N.; Shankar, S.; Saumya Shree, B.V. Tooth whitening efficacy of a dentifrice containing papain and bromelain extracts: An in vivo clinical study. RRJDS 2014, 2, 86–92. [Google Scholar]
- Yoshikawa, Y.; Teramoto, A.; Nishida, A.; Okamoto, E.; Kinosada, H.; Sugimoto, W.; Hirose, T.; Shimaoka, T.; Kamada, A.; Domae, E.; et al. Characterization of the mechanism by which papain suppresses tooth discoloration. Nano Biomed. 2017, 9, 83–88. [Google Scholar] [CrossRef]
- Chakravarthy, P.K.; Yeturu, S.K. Role of proteolytic enzymes in dental care. In Natural Oral Care in Dental Therapy; Wiley: Hoboken, NJ, USA, 2020; pp. 153–170. [Google Scholar] [CrossRef]
- Chhabile, S.; Vishwakarma, P.; Agrawal, A.; Pundkar, S.R.; Mali, G.; Patil, S.; Gupta, S. Effectiveness of Papain-Based Organic Dentifrices Versus Commercial Whitening Dentifrice on Tea-Induced Tooth Stains: An In Vitro Study. Cureus 2024, 16, e69225. [Google Scholar] [CrossRef]
- Large, J.F.; Madigan, C.; Pradeilles, R.; Markey, O.; Boxer, B.; Rousham, E.K. Impact of unhealthy food and beverage consumption on children’s risk of dental caries: A systematic review. Nutr. Rev. 2024, 82, 1539–1555. [Google Scholar] [CrossRef]
- Spatafora, G.; Li, Y.; He, X.; Cowan, A.; Tanner, A.C. The evolving microbiome of dental caries. Microorganisms 2024, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Çelik, Ö.M.; Karacil, E.M.Ş.; Duran, S.; Şahin, E.M. Evaluation of the Relationship between Oral Care Practices, Food Consumption, and Dental Caries in Young Adults. Balikesir Health Sci. J. 2023, 12, 516. [Google Scholar] [CrossRef]
- Barani-Sveçla, M.; Buleshkaj, S. Etiopathogenesis of Dental Caries. In Enamel and Dental-Pulp Complex; Ardelean, L.C., Ed.; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar] [CrossRef]
- Drevnitska, R.; Boykiv, A.; Avdeev, O. Modern scientific trends in the treatment and prevention of dental caries. East. Ukr. Med. J. 2024, 12, 212–220. [Google Scholar] [CrossRef]
- Warreth, A. Dental caries and its management. Int. J. Dent. 2023, 1, 9365845. [Google Scholar] [CrossRef]
- Nyvad, B. Diagnosis versus detection of caries. Caries Res. 2004, 38, 192–198. [Google Scholar] [CrossRef]
- Kara, E.; İpek, B. Dental caries from the past to the future: Is it possible to reduce caries prevalence? Anatol. Curr. Med. J. 2024, 6, 240–247. [Google Scholar] [CrossRef]
- Lian, L.; Zhu, T.; Zhu, F.; Zhu, H. Deep learning for caries detection and classification. Diagnostics 2021, 11, 1672. [Google Scholar] [CrossRef]
- Jameel, R.A.; Zaidi, S.J.A.; Siddiqui, S.; Rehman, A.; Gul, J.; Saquib, M.; Rahim, Z.A. The effects of beverage erosion on enamel: Evaluating surface characteristics and loss of calcium and phosphate ions. Discov. Appl. Sci. 2024, 6, 439. [Google Scholar] [CrossRef]
- Zero, D.T.; Fontana, M.; Martinez-Mier, E.A.; Ferreira-Zandona, A.; Ando, M.; Gonzalez-Cabezas, C.; Bayne, S. The biology, prevention, diagnosis and treatment of dental caries: Scientific advances in the United States. J. Am. Dent. Assoc. 2009, 140, 25S–34S. [Google Scholar] [CrossRef]
- Koontongkaew, S.; Utispan, K.; Chawhuaveang, D.D.; Yu, O.Y.; Worawongvasu, R. Enamel and Its Interaction with the Oral Environment. In Enamel and Dental-Pulp Complex; Ardelean, L.C., Ed.; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Memis, I.; Dionysopoulos, D.; Papadopoulos, C.; Mourouzis, P.; Davidopoulou, S.; Tolidis, K. Effect of air-abrasion pretreatment with three desensitizing agents on efficacy of in-office tooth bleaching. J. Esthet. Restor. Dent. 2024, 36, 1426–1436. [Google Scholar] [CrossRef]
- Eram, A.; Kr, R.V.; Chethan, K.N.; Keni, L.G.; Shetty, D.D.; Zuber, M.; Pradeep, S. Air-Abrasion in Dentistry: A Short Review of the Materials and Performance Parameters. J. Biomed. Phys. Eng. 2024, 14, 99. [Google Scholar] [CrossRef]
- Wong, Y.J. Caries removal using lasers. Evid. Based Dent. 2018, 19, 45. [Google Scholar] [CrossRef] [PubMed]
- Al-Sagheer, R.M.; Addie, A.J.; Al-Taee, L.A. An in vitro assessment of the residual dentin after using three minimally invasive caries removal techniques. Sci. Rep. 2024, 14, 7087. [Google Scholar] [CrossRef]
- Schwendicke, F.; Paris, S.; Tu, Y.K. Effects of using different criteria for caries removal: A systematic review and network meta-analysis. J. Dent. 2015, 43, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kathuria, V.; Ankola, A.V.; Hebbal, M.; Mocherla, M. Carisolv-an innovative method of caries removal. J. Clin. Diagn. Res. JCDR 2013, 7, 3111. [Google Scholar] [CrossRef]
- Hamama, H.; Yiu, C.; Burrow, M. Current update of chemomechanical caries removal methods. Aust. Dent. J. 2014, 59, 446–456. [Google Scholar] [CrossRef]
- Kulkarni, G.; Rane, D.C.; Mishra, V.K. Comparison of the Efficacy of Chemomechanical Caries Removal (Papacarie-A Papain Gel) and Conventional Excavation in Reducing Cariogenic Flora: An: In Vivo: Study. J. Int. Oral Health 2016, 8, 564–568. [Google Scholar] [CrossRef]
- Mancini, L.; Pisaneschi, A.; Mancini, V.; Ginoble, M.; Quinzi, V.; Marchetti, E.; Marzo, G. BRIX3000 papain gel for cavity treament in the adult patient. Case Rep. Dent. 2021, 2021, 6624825. [Google Scholar] [CrossRef]
- Bertassoni, L.E.; Marshall, G.W. Papain-gel degrades intact nonmineralized type I collagen fibrils. Scanning 2009, 31, 253–258. [Google Scholar] [CrossRef]
- Lopes, M.C.; Mascarini, R.C.; Da Silva, B.M.C.G.; Flório, F.M.; Basting, R.T. Effect of a papain-based gel for chemomechanical caries removal on dentin shear bond strength. J. Dent. Child. (Chic) 2007, 74, 93–97. [Google Scholar] [PubMed]
- Kumar, J.; Nayak, M.; Prasad, K.L.; Gupta, N. A comparative study of the clinical efficacy of chemomechanical caries removal using Carisolv and Papacarie—A papain gel. Indian J. Dent. Res. 2012, 23, 697. [Google Scholar] [CrossRef] [PubMed]
- AlHumaid, J. Efficacy and efficiency of papacarie versus conventional method in caries removal in primary teeth: An SEM study. Saudi J. Med. Med. Sci. 2020, 8, 41–45. [Google Scholar] [CrossRef]
- Chittem, J.; Sajjan, G.S.; Varma, K.M. Comparative evaluation of microshear bond strength of the caries-affected dentinal surface treated with conventional method and chemomechanical method (papain). J. Conserv. Dent. Endod. 2015, 18, 369–373. [Google Scholar] [CrossRef]
- Bussadori, S.K.; Leal de Godoy, C.H.; Alfaya, T.A.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A.; Motta, L.J. Chemo-mechanical caries removal with PapacarieTM: Case series with 84 reports and 12 months of follow up. J. Contemp. Dent. Pract. 2014, 15, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Silva, Z.S., Jr.; Botta, S.B.; Ana, P.A.; França, C.M.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A.; Deana, A.; Bussadori, S.K. Effect of papain-based gel on type I collagen—Spectroscopy applied for microstructural analysis. Sci. Rep. 2015, 5, 11448. [Google Scholar] [CrossRef]
- Coirrêa, F.N.P.; de Oliveira Rocha, R.; Filho, L.E.R.; Muench, A.; Rodrigues, C.R.M.D. Chemical versus conventional caries removal techniques in primary teeth: A microhardness study. J. Clin. Pediatr. Dent. 2007, 31, 187–192. [Google Scholar] [CrossRef]
- Ozsoy, M.; Gungor, O.E. Management of severity lesions of hypomineralized molars (MIH) with different treatment alternatives: 9-month results of a clinical trial. J. Clin. Pediatr. Dent. 2024, 48, 68–75. [Google Scholar] [CrossRef]
- Khaleh, A.A.; Elkateb, M.A.; Abdel Aziz, W.E.; Tantawi, M.E.l. Effect of Papacarie and alternative restorative treatment on pain reaction during caries removal among children: A randomized controlled clinical trial. J. Clin. Pediatr. Dent. 2017, 41, 219–224. [Google Scholar] [CrossRef]
- Jawa, D.; Singh, S.; Somani, R.; Jaidka, S.; Sirkar, K.; Jaidka, R. Comparative evaluation of the efficacy of chemomechanical caries removal agent (Papacarie) and conventional method of caries removal: An in vitro study. J. Indian Soc. Pedod. Prev. Dent. 2010, 28, 73–77. [Google Scholar] [CrossRef]
- Ardani, I.G.A.W.; Nilam, M.; Puspita, H.A.; Narmada, I.B. Effectiveness of toothpaste containing pyrophosphate and papain to inhibit calculus formation in patient using fixed orthodontic appliance. Res. J. Pharm. Technol. 2019, 12, 3797–3801. [Google Scholar] [CrossRef]
- Hong, J.H.; Kim, M.R.; Lee, B.N.; Oh, W.M.; Min, K.S.; Im, Y.G.; Hwang, Y.C. Anti-inflammatory and mineralization effects of bromelain on Lipopolysaccharide-induced inflammation of human dental pulp cells. Medicina 2021, 57, 591. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.S.; Nagar, P.; Reddy, S.; Ragulakollu, R.; Tirupathi, S.P.; Ravi, R.; Purumadla, U. Bromelain vs papain gel for caries removal in primary teeth. J. Contemp. Dent. Pract. 2019, 20, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Mameli, A.; Natoli, V.; Casu, C. Bromelain: An overview of applications in medicine and dentistry. Biointerface Res. Appl. Chem. 2021, 11, 8165–8170. [Google Scholar] [CrossRef]

| Articles | Results | References |
|---|---|---|
| Vilhena K.F.B. et al. Ribeiro J.S. et al. Müller-Heupt L. K. et al. Lilaj B. et al. Al-Badri H. et al. Barbosa L.M. et al. | Hydrogen peroxide side effects:
| [49,50,51,52,53,54] |
| Medina-Zamora N. et al. Mohamed Thameemul Ansari K.A. et al. Hikisz, P. Ribeiro J.S. et al. Jurema A.L.B. et al. Mazilu A. et al. Parlis Y. et al. | Bromelain effects:
| [3,24,34,50,55,56,57] |
| Vekaash C. et al. | Hydrogen peroxide + bromelain (different concentrations):
| [58] |
| Solanki M.N. et al. Mazilu M.A. et al. | Hydrogen peroxide + papain:
| [59,60] |
| Patil P. A. et al. Shobana G. et al. Munchow E. et al. Ananthakrishna S. et al. | Bromelain + Papain toothpaste effects:
| [5,61,62,63] |
| Yoshikawa Y. et al. Chakravarthy P.K. et al. Chhabile S. et al. | Papain effects:
| [64,65,66] |
| Articles | Results | References |
|---|---|---|
| Kathuria V. et al. | Atraumatic treatment:
| [84] |
| Hamama H. et al. | Chemo-mechanical methods using sodium hypochlorite-based gels (Caridex and Carisolv) and enzyme-based gels could be used to reduce the treatment stress in caries therapy. | [85] |
| Kulkarni G. et al. Mancini L. et al. Bertassoni L.E. et al. Lopes M.C. et al. | Papacarie (Papain gel):
| [86,87,88,89] |
| Kumar J. et al. | Carisolv and Papacarie:
| [90] |
| AlHumaid J. et al. Chittem J. et al. Bussadori S.K. et al. Silva Z.S. Jr et al. Coirêa F.N.P. et al. Ozsoy S. et al. | Papacarie gel:
| [91,92,93,94,95,96] |
| Khaleh A.A. Jawa D. et al. | Papacarie:
| [97,98] |
| Ardani I.G.A.W. et al. | Papain toothpaste:
| [99] |
| Hong J.H. et al. Reddy V.S. et al. Maneli A. et al. | Bromelain:
| [100,101,102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuc, S.; Moldovan, A.; Moldovan, M.; Sarosi, C.; Buduru, S.; Bacali, C.; Prodan, D.; Lazar, V.; Man, S.C. Effects of Bromelain and Papain in Tooth Whitening and Caries Removal: A Literature Review. Dent. J. 2025, 13, 132. https://doi.org/10.3390/dj13030132
Cuc S, Moldovan A, Moldovan M, Sarosi C, Buduru S, Bacali C, Prodan D, Lazar V, Man SC. Effects of Bromelain and Papain in Tooth Whitening and Caries Removal: A Literature Review. Dentistry Journal. 2025; 13(3):132. https://doi.org/10.3390/dj13030132
Chicago/Turabian StyleCuc, Stanca, Amalia Moldovan, Marioara Moldovan, Codruta Sarosi, Smaranda Buduru, Cecilia Bacali, Doina Prodan, Viorica Lazar, and Sorin Claudiu Man. 2025. "Effects of Bromelain and Papain in Tooth Whitening and Caries Removal: A Literature Review" Dentistry Journal 13, no. 3: 132. https://doi.org/10.3390/dj13030132
APA StyleCuc, S., Moldovan, A., Moldovan, M., Sarosi, C., Buduru, S., Bacali, C., Prodan, D., Lazar, V., & Man, S. C. (2025). Effects of Bromelain and Papain in Tooth Whitening and Caries Removal: A Literature Review. Dentistry Journal, 13(3), 132. https://doi.org/10.3390/dj13030132











