Histological Outcomes of Alveolar Ridge Preservation Versus Spontaneous Healing Following Tooth Extraction: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
- Population (P): patients undergoing tooth extraction;
- Intervention (I): alveolar ridge preservation (ARP) using any graft material and/or membrane;
- Comparison (C): spontaneous (unassisted) socket healing;
- Outcome (O): histomorphometric parameters, including percentage of new bone formation, residual graft material, and connective or non-mineralized tissue.
2.2. Eligibility Criteria
2.3. Information Sources
2.4. Search Strategy
2.5. Selection Process
2.6. Data Collection Process
2.7. Data Items
2.8. Risk of Bias Assessment
2.9. Effect Measures
2.10. Synthesis Methods
2.11. Subgroup and Sensitivity Analyses
2.12. Meta-Bias Assessment
2.13. Certainty of Evidence
3. Results
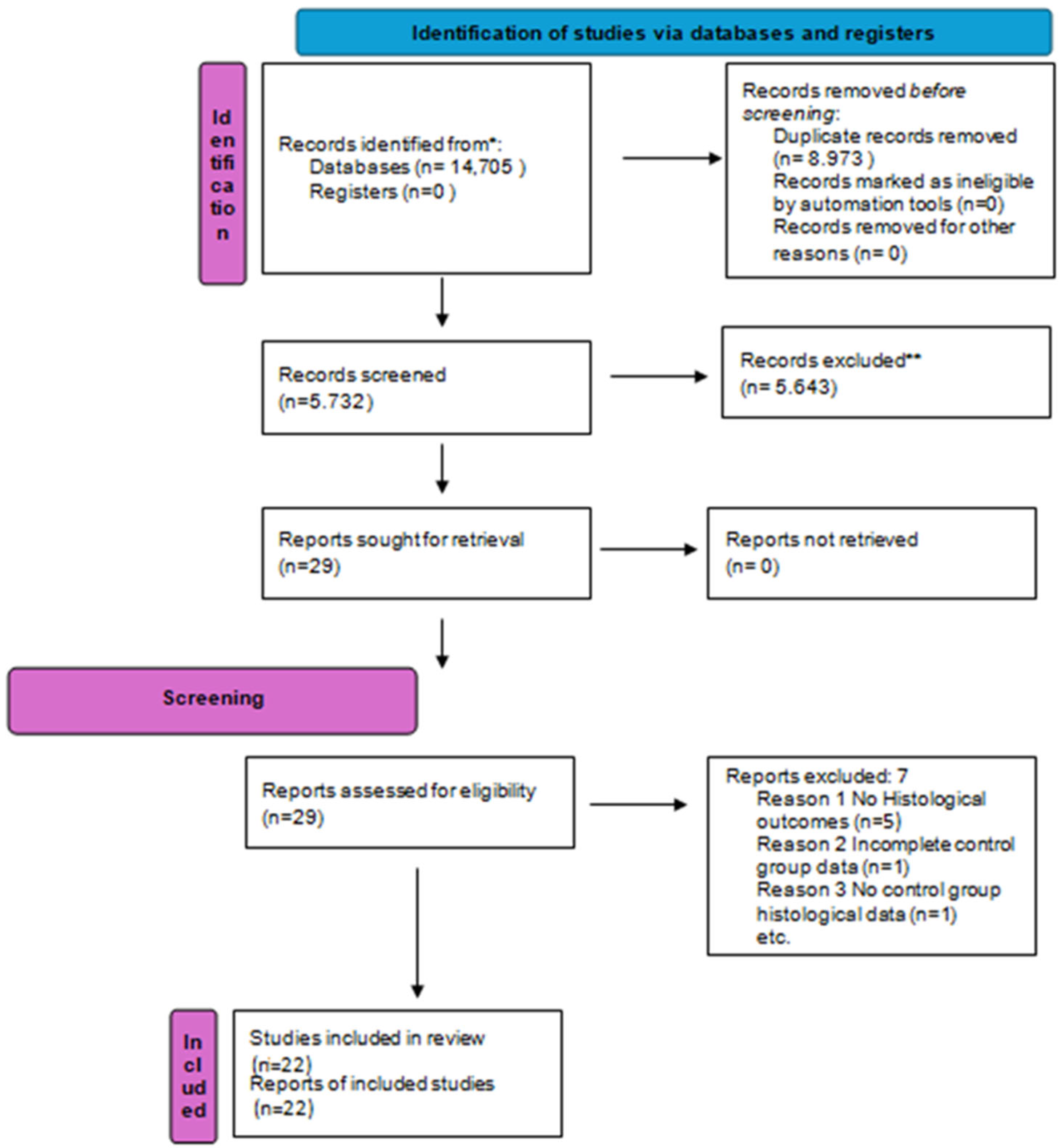
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias Assessment
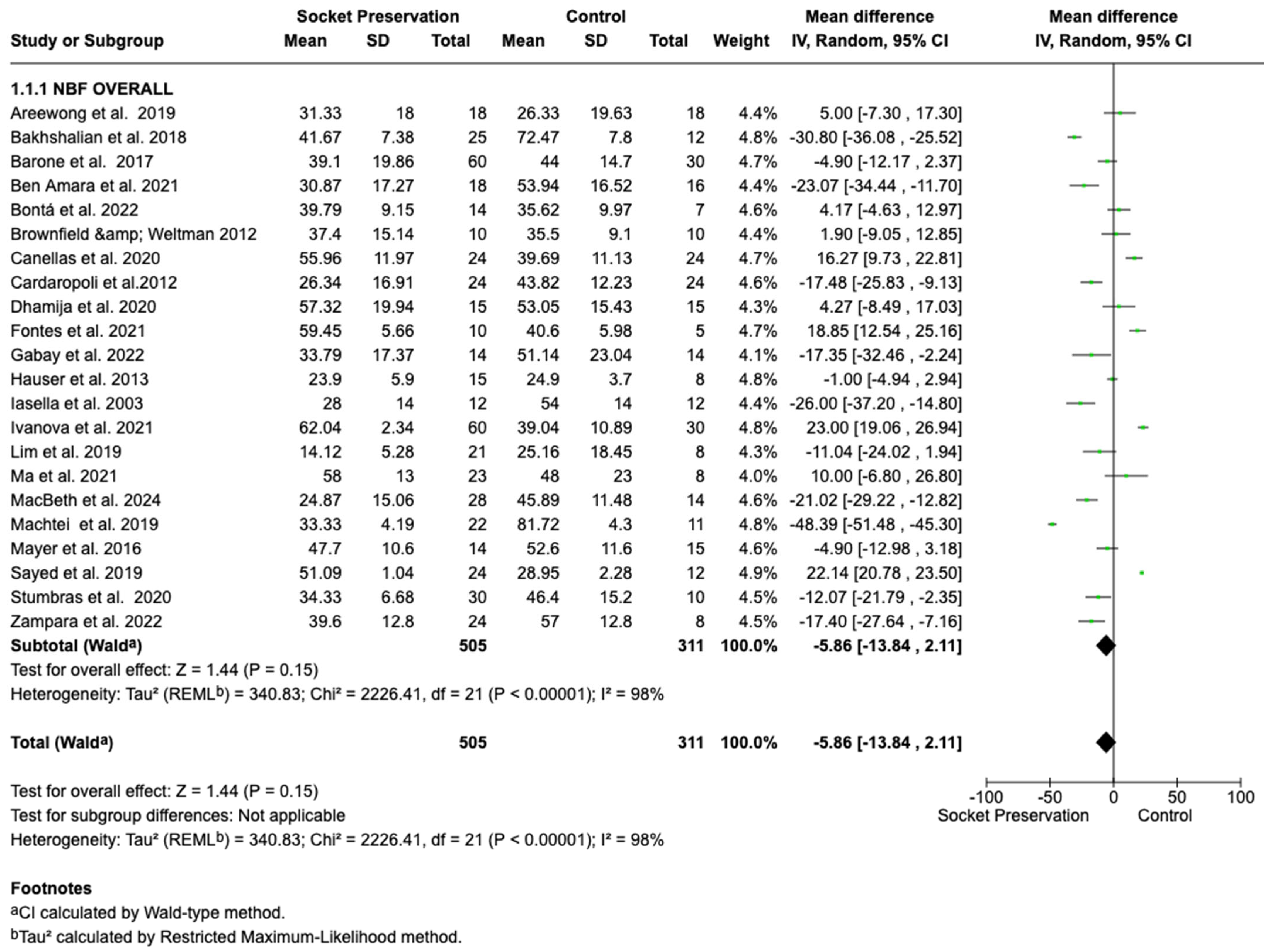
3.4. Primary Outcome: New Bone Formation
3.4.1. Overall Meta-Analysis
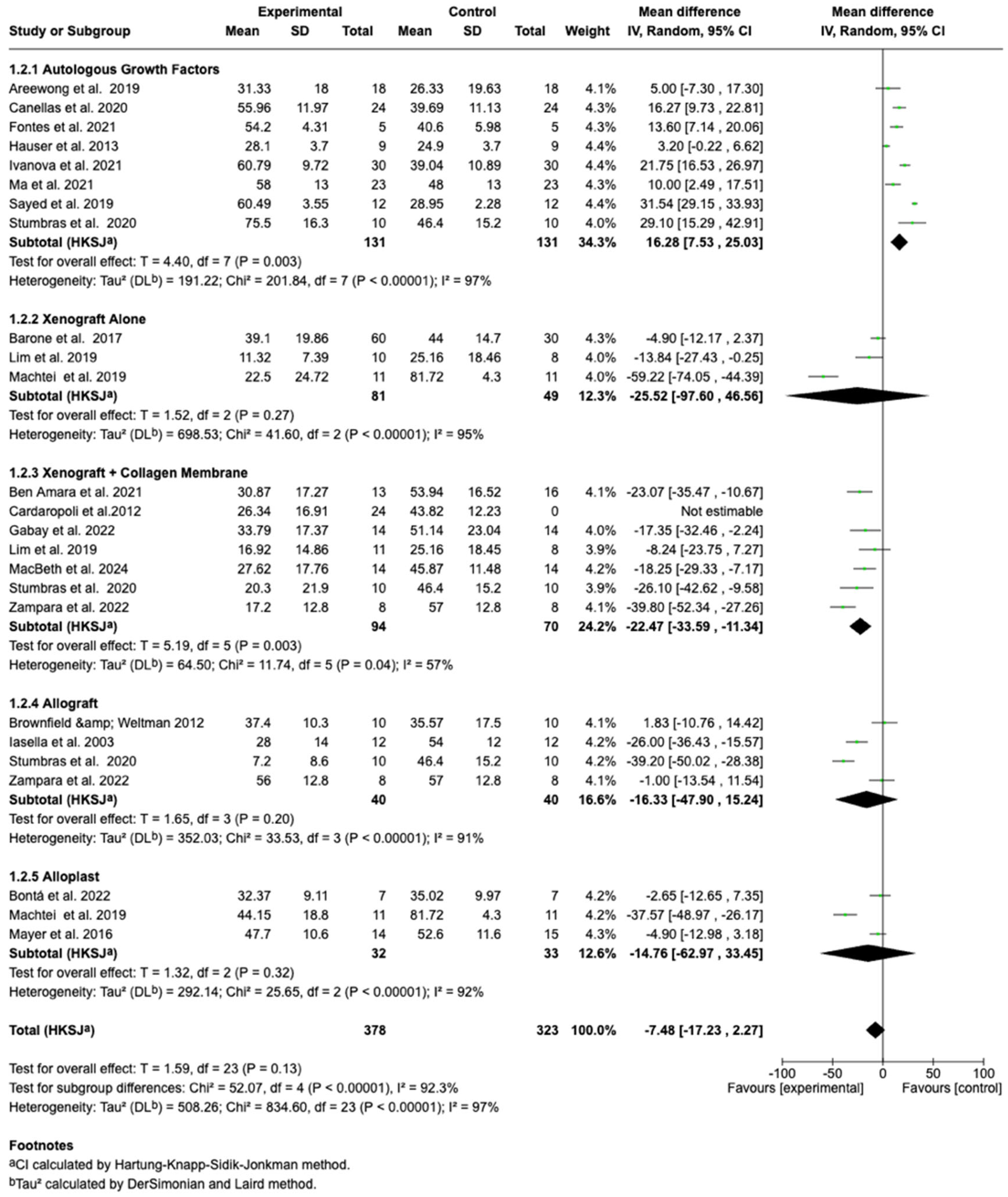
3.4.2. Subgroup Analyses by Graft Material
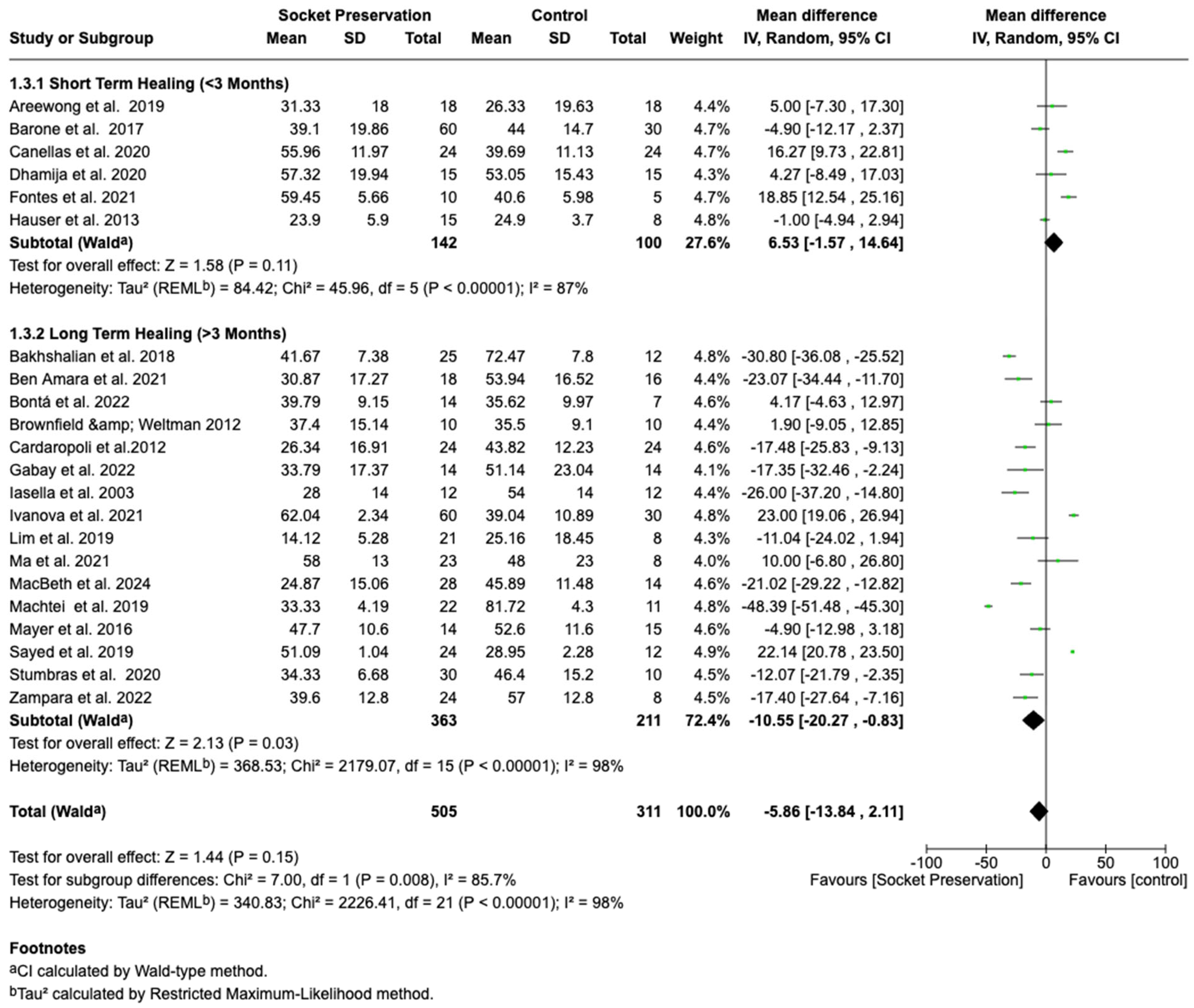
3.4.3. Subgroup Analysis by Healing Duration
3.4.4. Assessment of Publication Bias
3.4.5. Meta-Regression Analysis
3.5. Residual Graft Material
3.5.1. Meta-Analysis of Residual Graft Percentages
3.5.2. Subgroup and Meta-Regression Analysis
3.5.3. Sensitivity Analyses and Influential Studies
3.6. GRADE Assessment: Certainty of Evidence for New Bone Formation
4. Discussion
4.1. Summary of Principal Findings
4.2. Biological and Clinical Interpretation
4.2.1. New Bone Formation
4.2.2. Residual Graft Material
4.2.3. Membrane Use
4.2.4. Healing Time
4.3. Comparison to Previous Literature
4.4. Methodological Strengths
4.5. Limitations
4.6. Clinical Implications
4.7. Recommendations for Future Research
5. Conclusions
6. Registration and Protocol
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Detailed Search Strategies
| Database | Date Searched | Search Terms | Filters Applied |
|---|---|---|---|
| PubMed/MEDLINE | 15 April 2025 | (“Tooth Extraction” [Mesh] OR “tooth extraction”) AND (“Alveolar Ridge Preservation” [Mesh] OR “socket preservation” OR “ridge preservation”) AND (“Bone Substitutes” [Mesh] OR “bone graft” OR “xenograft” OR “allograft” OR “synthetic bone substitute” OR “alloplast” OR “DBBM” OR “PRF” OR “CGF”) AND (“Histological Techniques” [Mesh] OR “histological analysis” OR “histomorphometric” OR “new bone formation”) AND (“Randomized Controlled Trial” [Publication Type] OR “randomized controlled trial” OR “RCT”) | Humans; English; Article type: RCT |
| EMBASE (Elsevier) | 15 April 2025 | (‘tooth extraction’/exp OR ‘tooth extraction’:ti,ab) AND (‘socket preservation’/exp OR ‘ridge preservation’:ti,ab OR ‘extraction socket’:ti,ab) AND (‘bone graft’/exp OR ‘xenograft’:ti,ab OR ‘allograft’:ti,ab OR ‘synthetic bone substitute’:ti,ab OR ‘alloplast’:ti,ab OR ‘dbbm’:ti,ab OR ‘prf’:ti,ab OR ‘cgf’:ti,ab) AND (‘histology’/exp OR ‘histological analysis’:ti,ab OR ‘histomorphometric’:ti,ab OR ‘new bone formation’:ti,ab) AND (‘randomized controlled trial’/exp OR ‘rct’:ti,ab) | Humans; English; RCT |
| Scopus | 15 April 2025 | TITLE-ABS-KEY (“tooth extraction” OR “socket preservation” OR “alveolar ridge preservation” OR “extraction socket”) AND TITLE-ABS-KEY(“bone graft” OR “xenograft” OR “allograft” OR “synthetic bone substitute” OR “alloplast” OR “DBBM” OR “PRF” OR “CGF”) AND TITLE-ABS-KEY(“histological analysis” OR “histomorphometric” OR “new bone formation”) AND TITLE-ABS-KEY(“randomized controlled trial” OR “RCT”) | English; Article; Humans |
| Web of Science | 15 April 2025 | TS = (“tooth extraction” OR “socket preservation” OR “ridge preservation” OR “extraction socket”) AND TS = (“bone graft” OR “xenograft” OR “allograft” OR “synthetic bone substitute” OR “alloplast” OR “DBBM” OR “PRF” OR “CGF”) AND TS = (“histological analysis” OR “histomorphometric” OR “new bone formation”) AND TS = (“randomized controlled trial” OR “RCT”) | Language: English; Document type: Article |
| Cochrane CENTRAL | 15 April 2025 | (“tooth extraction” OR “socket preservation”) AND (“bone graft” OR “xenograft” OR “allograft” OR “synthetic bone” OR “DBBM” OR “PRF” OR “CGF”) AND (“histological” OR “histomorphometric” OR “new bone formation”) AND (“randomized controlled trial” OR “RCT”) | Trials only |
| Manual Search | 15 April 2025 | Reference lists of included articles and relevant systematic reviews were screened | Not applicable |
References
- Araújo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef]
- Van der Weijden, F.; Dell’ACqua, F.; Slot, D.E. Alveolar bone dimensional changes of post-extraction sockets in humans: A systematic review. J. Clin. Periodontol. 2009, 36, 1048–1058. [Google Scholar] [CrossRef]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. Available online: https://www.researchgate.net/publication/10582943 (accessed on 12 November 2025).
- Tan, W.L.; Wong, T.L.T.; Wong, M.C.M.; Lang, N.P. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin. Oral Implant. Res. 2012, 23, 1–21. [Google Scholar] [CrossRef]
- Huynh-Ba, G.; Pjetursson, B.E.; Sanz, M.; Cecchinato, D.; Ferrus, J.; Lindhe, J.; Lang, N.P. Analysis of the socket bone wall dimensions in the upper maxilla in relation to immediate implant placement. Clin. Oral Implant. Res. 2009, 21, 37–42. [Google Scholar] [CrossRef]
- Chappuis, V.; Engel, O.; Reyes, M.; Shahim, K.; Nolte, L.-P.; Buser, D. Ridge alterations post-extraction in the esthetic zone: A 3D analysis with CBCT. J. Dent. Res. 2013, 92, 195S–201S. [Google Scholar] [CrossRef]
- Avila-Ortiz, G.; Elangovan, S.; Kramer, K.; Blanchette, D.; Dawson, D. Effect of alveolar ridge preservation after tooth extraction: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Vignoletti, F.; Matesanz, P.; Rodrigo, D.; Figuero, E.; Martin, C.; Sanz, M. Surgical protocols for ridge preservation after tooth extraction. A systematic review. Clin. Oral Implant. Res. 2012, 23, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Del Fabbro, M.; Khijmatgar, S.; Panda, S.; Ravidà, A.; Tommasato, G.; Sculean, A.; Pesce, P. Dimensional and histomorphometric evaluation of biomaterials used for alveolar ridge preservation: A systematic review and network meta-analysis. Clin. Oral Investig. 2022, 26, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Mardas, N.; Macbeth, N.; Donos, N.; Jung, R.E.; Zuercher, A.N. Is alveolar ridge preservation an overtreatment? Periodontol. 2000 2023, 93, 289–308. [Google Scholar] [CrossRef]
- Iocca, O.; Farcomeni, A.; Lopez, S.P.; Talib, H.S. Alveolar ridge preservation after tooth extraction: A Bayesian Network meta-analysis of grafting materials efficacy on prevention of bone height and width reduction. J. Clin. Periodontol. 2017, 44, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Canellas, J.; Ritto, F.; Figueredo, C.; Fischer, R.; de Oliveira, G.; Thole, A.; Medeiros, P. Histomorphometric evaluation of different grafting materials used for alveolar ridge preservation: A systematic review and network meta-analysis. Int. J. Oral Maxillofac. Surg. 2020, 49, 797–810. [Google Scholar] [CrossRef]
- Barone, A.; Aldini, N.N.; Fini, M.; Giardino, R.; Guirado, J.L.C.; Covani, U. Xenograft Versus Extraction Alone for Ridge Preservation After Tooth Removal: A Clinical and Histomorphometric Study. J. Periodontol. 2008, 79, 1370–1377. [Google Scholar] [CrossRef]
- Temmerman, A.; Vandessel, J.; Castro, A.; Jacobs, R.; Teughels, W.; Pinto, N.; Quirynen, M. The use of leucocyte and platelet-rich fibrin in socket management and ridge preservation: A split-mouth, randomized, controlled clinical trial. J. Clin. Periodontol. 2016, 43, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ortiz, G.; Chambrone, L.; Vignoletti, F. Effect of alveolar ridge preservation interventions following tooth extraction: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 195–223. [Google Scholar] [CrossRef] [PubMed]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Areewong, K.; Chantaramungkorn, M.; Khongkhunthian, P. Platelet-rich fibrin to preserve alveolar bone sockets following tooth extraction: A randomized controlled trial. Clin. Implant Dent. Relat. Res. 2019, 21, 1156–1163. [Google Scholar] [CrossRef]
- Bakhshalian, N.; Abdelhamid, A.; Park, Y.J.; Zadeh, H.H. Histological and histomorphometric response to SocketKAP™ and SocketKAGE™ used for ridge preservation and repair: Results from a randomized controlled clinical trial. J. Oral. Maxillofac. Surg. 2018, 76, 1884–1892. [Google Scholar] [CrossRef]
- Barone, A.; Toti, P.; Quaranta, A.; Alfonsi, F.; Cucchi, A.; Negri, B.; Di Felice, R.; Marchionni, S.; Calvo-Guirado, J.L.; Covani, U.; et al. Clinical and histological changes after ridge preservation with two xenografts: Preliminary results from a multicentre randomized controlled clinical trial. J. Clin. Periodontol. 2017, 44, 204–214. [Google Scholar] [CrossRef]
- Ben Amara, H.; Kim, J.J.; Kim, H.Y.; Lee, J.; Song, H.Y.; Koo, K.T. Is ridge preservation effective in the extraction sockets of periodontally compromised teeth? a randomized controlled trial. J. Clin. Periodontol. 2021, 48, 464–477. [Google Scholar] [CrossRef]
- Bontá, H.; Galli, F.G.; Gualtieri, A.; Renou, S.; Caride, F. The effect of an alloplastic bone substitute and enamel matrix derivative on the preservation of single anterior extraction sockets: A histologic study in humans. Int. J. Periodontics Restor. Dent. 2022, 42, 361–368. [Google Scholar] [CrossRef]
- Brownfield, L.A.; Weltman, R.L. Ridge preservation with or without an osteoinductive allograft: A clinical, radiographic, micro-computed tomography, and histologic study evaluating dimensional changes and new bone formation of the alveolar ridge. J. Periodontol. 2012, 83, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Canellas, J.V.d.S.; da Costa, R.C.; Breves, R.C.; de Oliveira, G.P.; Figueredo, C.M.d.S.; Fischer, R.G.; Thole, A.A.; Medeiros, P.J.D.; Ritto, F.G. Tomographic and histomorphometric evaluation of socket healing after tooth extraction using leukocyte- and platelet-rich fibrin: A randomized, single-blind, controlled clinical trial. J. Craniomaxillofac. Surg. 2020, 48, 24–32. [Google Scholar] [CrossRef]
- Cardaropoli, D.; Tamagnone, L.; Roffredo, A.; Gaveglio, L.; Cardaropoli, G. Socket preservation using bovine bone mineral and collagen membrane: A randomized controlled clinical trial with histologic analysis. Int. J. Periodontics Restor. Dent. 2012, 32, 421–430. [Google Scholar]
- Dhamija, R.; Shetty, V.; Vineeth, K.; Nagaraju, R.; Rao, R.S. Socket preservation with demineralized freeze-dried bone allograft and platelet-rich fibrin for implant site development: A randomized controlled trial. J. Indian Prosthodont. Soc. 2020, 20, 304–311. [Google Scholar] [CrossRef]
- Martins, L.C.F.; de Oliveira, A.L.S.C.; Aloise, A.C.; de Macedo, L.G.S.; Teixeira, M.L.; Moy, P.K.; Pelegrine, A.A. Bone marrow aspirate concentrate and platelet-rich fibrin in fresh extraction sockets: A histomorphometric and immunohistochemical study in humans. J. Craniomaxillofac. Surg. 2021, 49, 283–291. [Google Scholar] [CrossRef]
- Gabay, E.; Katorza, A.; Zigdon-Giladi, H.; Horwitz, J.; Machtei, E.E. Histological and dimensional changes of the alveolar ridge following tooth extraction when using collagen matrix and collagen-embedded xenogenic bone substitute: A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2022, 24, 382–390. [Google Scholar] [CrossRef]
- Hauser, F.; Gaydarov, N.; Badoud, I.; Vazquez, L.; Bernard, J.P.; Ammann, P. Clinical and histological evaluation of postextraction platelet-rich fibrin socket filling: A prospective randomized controlled study. Implant Dent. 2013, 22, 295–303. [Google Scholar] [CrossRef]
- Iasella, J.M.; Greenwell, H.; Miller, R.L.; Hill, M.; Drisko, C.; Bohra, A.A.; Scheetz, J.P. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: A clinical and histologic study in humans. J. Periodontol. 2003, 74, 990–999. [Google Scholar] [CrossRef]
- Ivanova, V.; Chenchev, I.; Zlatev, S.; Mijiritsky, E. Comparison study of the histomorphometric results after socket preservation with PRF and allograft used for socket preservation—Randomized controlled trials. Int. J. Environ. Res. Public Health. 2021, 18, 7451. [Google Scholar] [CrossRef]
- Lim, H.C.; Shin, H.S.; Cho, I.W.; Koo, K.T.; Park, J.C. Ridge preservation in molar extraction sites with an open-healing approach: A randomized controlled clinical trial. J. Clin. Periodontol. 2019, 46, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Lin, Y.; Sun, F.; Jiang, X.; Wei, T. The impact of autologous concentrated growth factors on alveolar ridge preservation after posterior tooth extraction: A prospective, randomized controlled clinical trial. Clin. Implant Dent. Relat. Res. 2021, 23, 579–592. [Google Scholar] [CrossRef]
- MacBeth, N.; Mardas, N.; Davis, G.; Donos, N. Healing patterns of alveolar bone following ridge preservation procedures. Clin. Oral Implant. Res. 2024, 35, 1452–1466. [Google Scholar] [CrossRef]
- Machtei, E.E.; Mayer, Y.; Horwitz, J.; Zigdon-Giladi, H. Prospective randomized controlled clinical trial to compare hard tissue changes following socket preservation using alloplasts, xenografts vs no grafting: Clinical and histological findings. Clin. Implant Dent. Relat. Res. 2019, 21, 14–20. [Google Scholar] [CrossRef]
- Mayer, Y.; Zigdon-Giladi, H.; Machtei, E.E. Ridge preservation using composite alloplastic materials: A randomized control clinical and histological study in humans. Clin. Implant Dent. Relat. Res. 2016, 18, 1163–1170. [Google Scholar] [CrossRef]
- The use of nano graft combined with leukocyte-platelet rich fibrin in ridge preservation (a randomized controlled clinical and histological study). Ain Shams Dent J. 2019, 16, 247–254. [CrossRef]
- Stumbras, A.; Januzis, G.; Gervickas, A.; Kubilius, R.; Juodzbalys, G. Randomized and controlled clinical trial of bone healing after alveolar ridge preservation using xenografts and allografts versus plasma rich in growth factors. J. Oral Implant. 2020, 46, 515–525. [Google Scholar] [CrossRef]
- Zampara, E.; Alshammari, M.; De Bortoli, J.; Mullings, O.; Gkisakis, I.G.; Jalkh, E.B.B.; Tovar, N.; Coelho, P.G.; Witek, L. A histologic and histomorphometric evaluation of an allograft, xenograft, and alloplast graft for alveolar ridge preservation in humans: A randomized controlled clinical trial. J. Oral Implant. 2022, 48, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Heberer, S.; Al-Chawaf, B.; Hildebrand, D.; Nelson, J.J.; Nelson, K. Histomorphometric analysis of extraction sockets augmented with Bio-Oss Collagen after a 6-week healing period: A prospective study. Clin. Oral Implant. Res. 2008, 19, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.J. Is there clinical evidence to support alveolar ridge preservation over extraction alone? A review of recent literature and case reports of late graft failure. Br. Dent. J. 2022, 233, 469–474. [Google Scholar] [CrossRef]
- Ustaoğlu, G.; Bulut, D.G.; Gümüş, K. Evaluation of different platelet-rich concentrates effects on early soft tissue healing and socket preservation after tooth extraction. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Verdugo, F.; Laksmana, T.; D’addona, A.; Uribarri, A. Facial cortical bone regeneration post-extraction in non-grafted sockets allows for early implant placement and long-term functional stability. Arch. Oral Biol. 2020, 112, 104678. [Google Scholar] [CrossRef] [PubMed]
- Ram, A.; Duncan, W.J.; E Coates, D.; Nobakht, S.; Tkatchenko, T.; Milne, T.J. Bone remodelling marker expression in grafted and ungrafted sheep tooth extraction sockets: A comparative randomised study. Arch. Oral Biol. 2023, 153, 105738. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Hu, J.; Zhao, L. Histological analysis of socket preservation using DBBM. A systematic review and meta-analysis. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 729–735. [Google Scholar] [CrossRef]
- Artzi, Z.; Tal, H.; Dayan, D. Porous Bovine Bone Mineral in Healing of Human Extraction Sockets. Part 1: Histomorphometric Evaluations at 9 Months. J. Periodontol. 2000, 71, 1015–1023. [Google Scholar] [CrossRef]
- Pushpanathan, K.V.; Chitrpautirapillay, S.; Pushparaj, R.; Kumar, P. A Systematic Review and Meta-Analysis to Find Out the Efficacy of Socket Preservation Techniques in Adults in Split-Mouth Randomized Controlled Trials. Cureus 2025, 17, e79873. [Google Scholar] [CrossRef]
- Suttapreyasri, S.; Leepong, N. Influence of platelet-rich fibrin on alveolar ridge preservation. J. Craniofacial Surg. 2013, 24, 1088–1094. [Google Scholar] [CrossRef]
- Das, S.; Jhingran, R.; Bains, V.K.; Madan, R.; Srivastava, R.; Rizvi, I. Socket preservation by beta-tri-calcium phosphate with collagen compared to platelet-rich fibrin: A clinico-radiographic study. Eur. J. Dent. 2016, 10, 264–276. [Google Scholar] [CrossRef]
- Jung, R.E.; Hürzeler, M.B.; Thoma, D.S.; Khraisat, A.; Hämmerle, C.H.F. Local tolerance and efficiency of two prototype collagen matrices to increase the width of keratinized tissue. J. Clin. Periodontol. 2011, 38, 173–179. [Google Scholar] [CrossRef]
- Fickl, S.; Schneider, D.; Zuhr, O.; Hinze, M.; Ender, A.; Jung, R.E.; Hürzeler, M.B. Dimensional changes of the ridge contour after socket preservation and buccal overbuilding: An animal study. J. Clin. Periodontol. 2009, 36, 442–448. [Google Scholar] [CrossRef]
| Study (Author, Year) | Country | Study Design | Grafting Material | Control | Sample Size (Intervention/Control) | Follow-Up (Months) | Histological Assessment Method |
|---|---|---|---|---|---|---|---|
| Areewong, 2019 [17] | Thailand | RCT | PRF | Spontaneous healing | 18/18 | 2 | Undecalcified sections; toluidine blue staining; histomorphometry under light microscopy |
| Bakhshalian, 2018 [18] | USA | RCT | SocketKAP/SocketKAP + Xenograft/SocketKAGE + Xenograft + SocketKAP | Spontaneous healing | 6/11/3/2 | 6 | Ground section histomorphometry; specific staining method not reported |
| Barone, 2017 [19] | Italy | RCT | Xenograft | Spontaneous healing | 30/30/30 | 3 | Undecalcified sections; histomorphometry of new bone, residual graft, and connective tissue |
| BenAmara, 2021 [20] | Tunisia | RCT | DBBM-C + collagen membrane | Spontaneous healing | 18/16 | 6 | Undecalcified sections; light microscopy with morphometric analysis |
| Bontá, 2022 [21] | Argentina | RCT | Alloplast/Alloplast + Emdogain | Spontaneous healing | 7/7/7 | 6 | H&E and Masson’s trichrome-stained sections; histomorphometry |
| Brownfield&Weltman, 2012 [22] | USA | RCT | FDBA | Spontaneous healing | 10/10 | 2.5–3 | Undecalcified histological sections; staining method not specified |
| Canellas, 2020 [23] | Brazil | RCT | PRF | Spontaneous healing | 2184/24 | 3 | Resin-embedded undecalcified sections; toluidine blue; image analysis |
| Cardaropoli,2012 [24] | Italy | RCT | Bovine bone mineral (Bio-Oss Collagen) + Porcine collagen membrane (Bio-Gide) | Spontaneous healing | 24/24 (48 sockets from 41 patients) | 4 | Undecalcified resin-embedded sections; Azure II and pararosaniline staining; histomorphometric analysis under light microscopy) |
| Dhamija, 2020 [25] | India | RCT | FDBA + PRF | Spontaneous healing | 15/15 | 3–4 | Toluidine blue-stained undecalcified sections; histomorphometry |
| FontesMartins, 2020 [26] | Brazil | RCT | PRF/PRF + BMAC | Spontaneous healing | 5/5/5 | 6 | Undecalcified histological sections; method of staining not described |
| Gabay, 2022 [27] | Israel | RCT | Xenograft (DBBM-C) + collagen membrane | Spontaneous healing | 14/14 | 6 | Masson’s trichrome staining of undecalcified sections; quantitative light microscopy |
| Hauser, 2013 [28] | Switzerland | RCT | PRF/PRF-Flap | Spontaneous healing | 9/6/8 | 2 | Semithin ground sections stained with toluidine blue; light microscopy |
| Iasella, 2003 [29] | USA | RCT | FDBA + collagen membrane | Spontaneous healing | 12/12 | 6 | Undecalcified histological sections; morphometric software analysis |
| Ivanova, 2021 [30] | Bulgaria | RCT | PRF + Allograft/PRF | Spontaneous healing | 30/30/30 | 4 | Histomorphometry; specific details of staining not reported |
| Lim, 2019 [31] | South Korea | RCT | DBBM-C + collagen membrane/DBBM-C | Spontaneous healing | 11/10/8 | 4 | Undecalcified sectioning; digital image analysis |
| Ma, 2021 [32] | China | RCT | CGFs (Concentrated Growth Factors) | Spontaneous healing | 23/23 | 3.5 | Histology and histomorphometry of new bone vs. residual graft; image analysis tools |
| MacBeth, 2024 [33] | UK | RCT | Xenograft | Spontaneous healing | 30/30 | 4 | Resin-embedded sections; toluidine blue staining; quantitative histomorphometry |
| Machtei, 2019 [34] | Israel | RCT | DBBM vs. Alloplast | Spontaneous healing | 11/11/11 | 4–4.5 | Undecalcified ground sections; standardized light microscopy |
| Mayer, 2016 [35] | USA | RCT | Alloplast | Spontaneous healing | 14/15 | 5 | H&E-stained undecalcified sections; point-counting histomorphometry |
| Sayed, 2019 [36] | Egypt | RCT | PRF/PRF + Alloplast | Spontaneous healing | 12/12/12 | 3 | Plastic-embedded sections; toluidine blue; histomorphometric measurement |
| Stumbras, 2019 [37] | Lithuania | RCT | Xenograft/Allograft/PRGF | Spontaneous healing | 10/10/10/10 | 3 | Undecalcified resin blocks; toluidine blue staining; light microscopy |
| Zampara, 2022 [38] | Greece | RCT | Allograft/Xenograft/Alloplast | Spontaneous healing | 8/8/8/8 | 6 | Light microscopy on stained undecalcified sections; histomorphometry |
| Study (Author, Year) | Domain 1: Randomization | Domain 2: Deviations from Intervention | Domain 3: Missing Data | Domain 4: Outcome Measurement | Domain 5: Selective Reporting | Overall Risk of Bias |
|---|---|---|---|---|---|---|
| Areewong, 2019 [17] | Low | Low | Low | Low | Some concerns | Low |
| Bakhshalian, 2018 [18] | Low | Low | Some concerns | Low | Low | Low |
| Barone, 2017 [19] | Low | Low | Low | Low | Low | Low |
| Ben Amara, 2021 [20] | Low | Low | Some concerns | Low | Low | Low |
| Bontá, 2022 [21] | Some concerns | Low | Low | Some concerns | Low | Some concerns |
| Brownfield&Weltman, 2012 [22] | Low | Low | Low | Low | Low | Low |
| Canellas,2020 [23] | Low | Low | Low | Low | Low | Low |
| Cardaropoli,2012 [24] | Low | Low | Low | Low | Low | Low |
| Dhamija, 2020 [25] | Some concerns | Low | Low | Low | Some concerns | Some concerns |
| Fontes Martins, 2021 [26] | Low | Low | Low | Low | Some concerns | Low |
| Gabay, 2022 [27] | High | Some concerns | Low | Some concerns | High | High |
| Hauser, 2013 [28] | High | Low | Some concerns | Low | Some concerns | High |
| Iasella, 2003 [29] | Some concerns | High | Some concerns | High | High | High |
| Ivanova, 2021 [30] | High | Low | Low | Low | Some concerns | High |
| Lim, 2019 [31] | Low | Low | Low | Low | Low | Low |
| Ma, 2021 [32] | Low | Low | Low | Low | Low | Low |
| MacBeth, 2024 [33] | Low | Low | Low | Low | Low | Low |
| Machtei, 2019 [34] | Low | Low | Low | Low | Low | Low |
| Mayer, 2016 [35] | Some concerns | Low | Low | Low | Some concerns | Some concerns |
| Sayed, 2019 [36] | Some concerns | Some concerns | Low | Some concerns | Low | Some concerns |
| Stumbras, 2020 [37] | Low | Low | Some concerns | Low | Some concerns | Some concerns |
| Zampara, 2022 [38] | Low | Low | Low | Low | Low | Low |
| Study (Author, Year) | Socket Preservation Mean (%) | Socket Preservation SD (%) | Socket Preservation N | Control Mean (%) | Control SD (%) | Control N |
|---|---|---|---|---|---|---|
| Areewong et al., 2019 [17] | 31.33 | 18 | 18 | 26.33 | 19.63 | 18 |
| Bakhshalian et al., 2018 [18] | 41.67 | 7.38 | 12 | 27.47 | 7.8 | 12 |
| Barone et al., 2017 [19] | 41.64 | 19.86 | 44 | 35.9 | 14.7 | 30 |
| Ben Amaral et al., 2021 [20] | 30.87 | 17.27 | 16 | 15.62 | 15.62 | 16 |
| Bontà et al., 2022 [21] | 39.79 | 9.97 | 14 | 35.62 | 9.97 | 14 |
| Cardaropoli et al., 2012 [24] | 26.34 | 16.91 | 24 | 43.82 | 12.23 | 24 |
| Brownfield & Weltman, 2012 [22] | 47.14 | 9.1 | 10 | 35.9 | 9.1 | 10 |
| Canellas et al., 2020 [23] | 55.66 | 11.13 | 24 | 39.89 | 11.13 | 24 |
| Dhamija et al., 2020 [25] | 57.32 | 9.34 | 15 | 15.43 | 15.43 | 15 |
| Fontes et al., 2021 [26] | 49.5 | 5.96 | 8 | 5.98 | 5.98 | 8 |
| Gaby et al., 2022 [27] | 39.7 | 17.37 | 14 | 23.04 | 23.04 | 14 |
| Hauser et al., 2013 [28] | 23.9 | 5.7 | 15 | 24.9 | 3.7 | 15 |
| Iasella et al., 2003 [29] | 62.04 | 2.34 | 30 | 39.04 | 10.89 | 30 |
| Ivanova et al., 2021 [30] | 39.6 | 9.34 | 18 | 10.89 | 10.89 | 18 |
| Lim et al., 2019 [31] | 14.12 | 1.9 | 18 | 18.45 | 18.45 | 18 |
| Ma et al., 2021 [32] | 5.8 | 13 | 23 | 48 | 23 | 23 |
| Macbeth et al., 2024 [33] | 24.87 | 15.06 | 22 | 45.89 | 11.48 | 23 |
| Machetti et al., 2019 [34] | 33.33 | 4.36 | 26 | 81.72 | 4.3 | 26 |
| Mayer et al., 2018 [35] | 37.6 | 6.47 | 14 | 29.85 | 2.28 | 12 |
| Sayed et al., 2018 [36] | 51.0 | 9.04 | 10 | 29.85 | 2.28 | 12 |
| Stumbras et al., 2020 [37] | 34.33 | 5.88 | 40 | 48.4 | 15.2 | 40 |
| Zamparra et al., 2022 [38] | 39.6 | 12.8 | 8 | 57.0 | 12.8 | 8 |
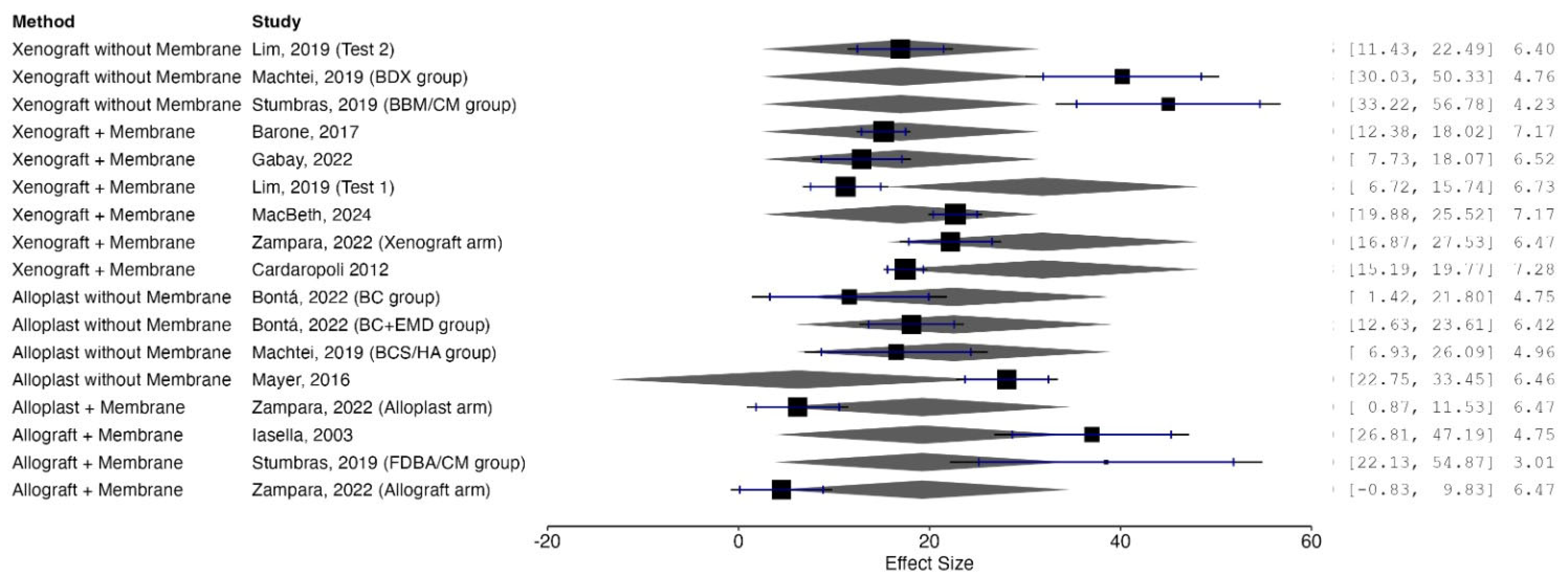
| Category | Study (Author, Year) | Graft Material | Membrane | Healing | Residual Graft (%) < br > (Mean ± SD) | Sample Size (n) |
|---|---|---|---|---|---|---|
| Xenograft + Membrane | Barone, 2017 [19] | Porcine xenograft | Yes | Open | 15.2% ± 7.87% | 30 |
| Cardaropoli, 2012 [24] | DBBM-C | Yes | Open | 18.46 ± 11.18% | 24 | |
| Gabay, 2022 [27] | DBBM-C | Yes | Open | 12.9% ± 9.88% | 14 | |
| Lim, 2019 (Test 1) [31] | DBBM-C + NBCM | Yes | Open | 11.23% ± 7.64% | 11 | |
| MacBeth, 2024 [33] | DBBM-C | Yes | Open | 22.7% ± 7.9% | 30 | |
| Zampara, 2022 (Xenograft arm) [38] | DBBM | Yes | Open | 22.2% ± 7.7% | 8 | |
| Xenograft without Membrane | Lim, 2019 (Test 2) [31] | DBBM-C | No | Open | 16.96% ± 8.93% | 10 |
| Machtei, 2019 (BDX group) [33] | Bio-Oss | No | Open | 40.18% ± 17.2% | 11 | |
| Stumbras, 2019 (BBM/CM group) [37] | BBM + Collagen membrane | No (exposed) | Open | 45.0% ± 19.0% | 10 | |
| Allograft + Membrane | Iasella, 2003 [29] | FDBA | Yes | Open | 37% ± 18% | 12 |
| Stumbras, 2019 (FDBA/CM group) [37] | FDBA + Collagen membrane | Yes | Open | 38.5% ± 26.4% | 10 | |
| Zampara, 2022 (Allograft arm) [38] | Human cancellous allograft | Yes | Open | 4.5% ± 7.7% | 8 | |
| Allograft without Membrane | Dhamija, 2020 [25] | DFDBA + PRF | No | Primary closure | 1.5% (SD not reported) | 15 |
| Alloplast + Membrane | Zampara, 2022 (Alloplast arm) [38] | Alloplast (Bondbone) | Yes | Open | 6.2% ± 7.7% | 8 |
| Alloplast without Membrane | Bontá, 2022 (BC group) [21] | Alloplast | No | Open | 11.61% ± 13.75% | 7 |
| Bontá, 2022 (BC + EMD group) [21] | Alloplast + EMD | No | Open | 18.12% ± 7.42% | 7 | |
| Machtei, 2019 (BCS/HA group) [34] | BCS/HA | No | Open | 16.51% ± 16.2% | 11 | |
| Mayer, 2016 [35] | Alloplast | No | Open | 28.1% ± 10.2% | 14 |
| Grade Domain | Judgment | Explanation |
| Risk of bias | ⬤⬤◯◯Serious | Several RCTs had high or unclear risk in randomization, allocation concealment, or assessor blinding. |
| Inconsistency | ⬤⬤◯◯Serious | Very high heterogeneity (I2 = 98%) across studies, even after subgroup analysis. |
| Indirectness | ⬤⬤⬤⬤None | Direct evidence from human studies assessing histological new bone formation following ARP vs. spontaneous healing. |
| Imprecision | ⬤⬤⬤◯Moderate | Confidence intervals crossed the null and included both clinically relevant benefit and no effect. |
| Publication bias | ⬤⬤⬤⬤None | Egger’s test (p = 0.576) and funnel plot inspection did not suggest reporting bias. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benekou, I.; Fragkioudakis, I.; Chatzopoulos, G.S.; Vouros, I. Histological Outcomes of Alveolar Ridge Preservation Versus Spontaneous Healing Following Tooth Extraction: A Systematic Review and Meta-Analysis. Dent. J. 2025, 13, 556. https://doi.org/10.3390/dj13120556
Benekou I, Fragkioudakis I, Chatzopoulos GS, Vouros I. Histological Outcomes of Alveolar Ridge Preservation Versus Spontaneous Healing Following Tooth Extraction: A Systematic Review and Meta-Analysis. Dentistry Journal. 2025; 13(12):556. https://doi.org/10.3390/dj13120556
Chicago/Turabian StyleBenekou, Ioanna, Ioannis Fragkioudakis, Georgios S. Chatzopoulos, and Ioannis Vouros. 2025. "Histological Outcomes of Alveolar Ridge Preservation Versus Spontaneous Healing Following Tooth Extraction: A Systematic Review and Meta-Analysis" Dentistry Journal 13, no. 12: 556. https://doi.org/10.3390/dj13120556
APA StyleBenekou, I., Fragkioudakis, I., Chatzopoulos, G. S., & Vouros, I. (2025). Histological Outcomes of Alveolar Ridge Preservation Versus Spontaneous Healing Following Tooth Extraction: A Systematic Review and Meta-Analysis. Dentistry Journal, 13(12), 556. https://doi.org/10.3390/dj13120556









