Abstract
Background/Objectives: Autofluorescence (AF) is a widely used adjunctive tool in the detection of oral potentially malignant disorders (OPMDs) and malignant lesions, but its performance can be influenced by clinicians’ experiences. This study aimed to examine how AF influences diagnostic decision-making and performances of a novice clinician compared with those of an experienced examiner. Methods: A total of 80 patients with oral lesions participated in this cross-sectional study. Each underwent a standard oral examination (OE) followed by an assessment with the VELscope® System Vx (LED Medical Diagnostics Inc., Burnaby, BC, Canada), independently conducted by an expert clinician (E) and a postgraduate dentist (PD), both blinded to each other’s results. Biopsy and histopathological analysis provided the reference diagnosis. After every examination, lesions were categorized as either “Risk of Malignancy” (RM) or “No Risk of Malignancy” (NRM). Results: Based on OE, PD identified 39 RM lesions, while E 29. AF with VELscope® identified an additional 12 RM lesions for the PD and 7 for the E that were not suspected on OE alone. Combining OE with VELscope® improved sensitivity (PD: 90.9%; E: 95.4%) and negative predictive value (PD: 91.7%; E: 97.6%), while decreasing specificity (PD: 37.9%; E: 70.7%) and positive predictive value (PD: 35.7%; E: 55.3%) compared with OE alone. Conclusions: AF increases diagnostic sensitivity, particularly for less experienced clinicians, while offering moderate advantages for experts. Nevertheless, the corresponding decline in specificity emphasizes the need for cautious interpretation. AF should be incorporated as a complementary tool within structured diagnostic pathways, accompanied by adequate training, and cannot replace histopathological confirmation or clinical expertise.
1. Introduction
Oral cancer is a major public health concern, ranking as the sixteenth most common malignancy worldwide and the fifteenth in cancer-related mortality (Global Cancer Observatory, 2022) [1]. This is also related to the fact that, unfortunately, nearly half of oral cancers are detected at an advanced stage (III–IV). However, most of them develop from oral potentially malignant disorders (OPMDs) that may persist for several years before progressing [2]. Therefore, early recognition of OPMDs is crucial to improve prognosis, reduce mortality, and minimize treatment-related morbidity [3].
Among the factors contributing to diagnostic delay, professional delay plays a key role. It is influenced by clinicians’ expertise in interpreting patients’ signs and symptoms and by their level of clinical experience [4,5,6,7]. Evidence from studies carried out on students or general practitioners shows a clear link between insufficient knowledge and the inability to apply standardized preventive and diagnostic measures [8,9,10].
Studies have suggested that improved knowledge and continuous training, beginning at the undergraduate level and extending into professional practice, are essential for minimizing diagnostic delays [11,12,13,14]; the visual Oral Examination (OE) is the primary screening method in dental practice employed by both general dental practitioners and Expert clinicians (E) [15,16], and biopsy followed by histological exam remains the gold standard to distinguish OPMDs and malignant lesions from benign ones [17]. What is also important is the development of supportive diagnostic tools that can assist general dentists and specialists in assessing persistent lesions of uncertain biological behavior [18,19,20]. A number of adjunctive diagnostic techniques to support clinical decision-making have been introduced alongside OE to improve early detection of OPMDs [17,21], including vital staining, oral cytology, light-based technique, evaluation of mRNA and protein biomarkers, and emerging artificial intelligence-based systems [17,22,23].
Among light-based tools, autofluorescence (AF) has gained particular interest because it provides non-invasive, real-time visualization of mucosal changes. It is based on the interaction of a high-intensity blue light source (typically 400–600 nm) with endogenous fluorophores of oral mucosal lesions (such as, collagen, NADH, FAD, elastin, keratin, and hemoglobin), which produce tissue-specific fluorescence emission patterns [24,25]. Healthy oral mucosa displays a uniform pale green fluorescence under AF devices, whereas dysplastic or malignant changes often show disrupted stromal collagen and altered fluorophore concentration, leading to a Loss of Autofluorescence (LAF) or to an Alteration of Autofluorescence (AAF), with a darker appearance [26]. However, inflammatory or certain benign conditions can also produce AF patterns due to vascular or metabolic changes, potentially generating false positive (FP) results. A recent systematic review on AF methods has demonstrated a sensitivity for AF > 70%, with an AUC ranging from 0.80 to 0.90, suggesting good diagnostic accuracy [27].
The Visually Enhanced Lesion Scope (VELscope®), developed by LED Medical Diagnostics Inc. in collaboration with the British Columbia Cancer Agency, is one of the most widely used chairside AF devices. It consists of a handheld scope emitting 400–460 nm wavelength light to enable direct visualization of oral mucosa under fluorescence. Owing to its handling properties, this device has been extensively adopted in clinical settings, and its diagnostic performances have been widely evaluated in the literature. A systematic review of 25 studies reported an average sensitivity of 70.19% (range: 22–100%) and average specificity of 65.95% (range: 8.4–100%), along with a high number of FP results. Therefore, although VELscope® represents an excellent tool for the detection of oral mucosal lesions, it lacks the ability to differentiate between benign, malignant or inflamed areas. Consequently, its effective use requires the clinical experience and judgment of a trained clinician [28].
Despite this, relatively few studies have addressed the role of clinician experience in diagnostic accuracy and biopsy decision-making when AF is used in conjunction with OE [29,30]. It remains unclear to what extent AF may mitigate the limitations of clinical inexperience in recognizing high-risk lesions or in guiding biopsy site selection. Clarifying these dynamics is important to define training requirements, optimize referral pathways, and balance the risks of unnecessary biopsies against the danger of missing high-grade lesions.
Accordingly, the present study aims to assess the influence of clinician experience (postgraduate dentist vs. expert) on diagnostic parameters in detecting OPMDs and malignancies using OE alone, AF alone, and a combined approach. A secondary objective is to evaluate how AF influences biopsy decision-making—specifically, whether AF-guided selection of biopsy sites differs between generalists and experts. By enrolling both clinically suspicious and benign lesions, we also evaluate each clinician’s ability and VELscope®’s diagnostic capacity to distinguish across a broad lesion spectrum. Based on the previous literature, we hypothesize that AF improves sensitivity in both groups, with a more pronounced reduction in specificity among less-experienced clinicians, while experts can integrate AF findings more effectively in guiding biopsy decisions.
2. Materials and Methods
This cross-sectional study was conducted at the Dentistry Department of the University “Magna Graecia” of Catanzaro between March 2019 and March 2024. The methodology conformed to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [31]. This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Regional Ethical Review Board of Central Calabria (reference for the Magna Graecia University of Catanzaro) (17 January 2019 Nr. 24/2019).
2.1. Study Sample
A total of 80 patients presenting consecutively as first visits for oral lesions were enrolled in the study (Figure 1). All patients were informed about the purpose of the study and provided written informed consent.
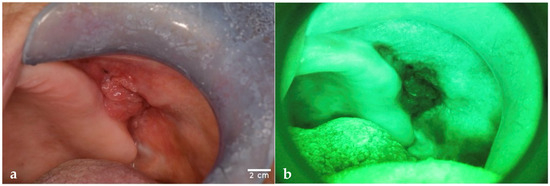
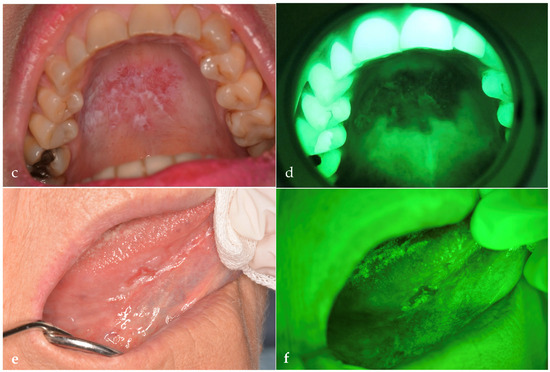
Figure 1.
The images show lesions observed with OE and with VELscope® (AF). (a) OSCC observed on OE and (b) on AF. (c) Erosive OLP observed on OE and (d) on AF. (e) Capillary subepithelial hemangioma observed on OE and (f) on AF. This lesion was misdiagnosed as an erythroplakia by both PD and E. Images were obtained with a Nikon D5200 and AF Micro-Nikkor 105 mm lens (1:1 magnification). Scale bar = 2 cm is the same for each picture and has been drawn with ImageJ v0.6.0.
Subjects included in the study were aged 18 years or older and presented at least one lesion in the oral cavity persistent for more than 14 days.
The exclusion criteria were as follows:
- Pregnant women;
- Patients with pre-treated lesions (laser treatment, medical treatment, surgery);
- Patients with bioptic examination already performed.
2.2. Data Collection
Socio-demographic data, including age, gender, smoking and drinking habits, systemic comorbidities, and current medication usage, were recorded.
Every patient on the same day underwent a standardized diagnostic protocol conducted independently by both a postgraduate dentist (PD) and an expert (E). The PD had 3 years of experience after graduating but never completed a postgraduate training course in oral medicine and pathology; the E was, instead, an expert in the field of oral medicine and oral surgery.
First, every patient underwent a conventional visual and manual OE under incandescent operative white light. For each lesion, clinical details—including margins, color, location, surface appearance, and phenotype—were recorded. Standardized photographic documentation was obtained (Figure 1) using a Nikon D5200 camera (AF Micro Nikkor 105 mm, Nikon, Tokyo, Japan) set with the following parameters for intra-oral photographing: manual program mode, shutter speed 1/125 s, fixed focus F32, and magnification ratio 1:1.
After, both PD and E, blinded to each other’s evaluations, independently classified each lesion as either Non-Risk of Malignancy (NRM) or Risk of Malignancy (RM) [2,32,33]. This assessment was based on predefined clinical indicators: non-homogeneous appearance (e.g., mixed erythematous and keratotic patterns such as speckled or erosive lesions), non-uniform morphology, large size (>200 mm2), and verrucous phenotype—all considered strong predictors of malignancy risk [33,34,35]. Each examiner also recorded biopsy recommendations, proposed biopsy sites, and provided a provisional clinical diagnosis.
Immediately following OE, the same clinicians carried out an AF-guided examination under dimmed ambient lighting, utilizing the VELscope® System Vx (LED Medical Diagnostics Inc., Burnaby, BC, Canada) device, which emits light in the 400–460 nm wavelength range. Patients wore protective eyewear during the procedure. During this evaluation, updated information on lesion features was recorded, with particular attention to fluorescence changes: AAF for modifications in fluorescence patterns and LAF for completely darkened areas suggestive of mucosal alteration. AF findings were documented photographically using the Nikon D5200 camera fitted with a VELscope® adapter (Figure 1). After AF assessment, both clinicians, still blinded to each other, reassessed malignancy risk, biopsy need and location, and diagnostic hypotheses based on fluorescence outcomes.
At the end, all patients underwent either scalpel incisional or excisional biopsy, performed by an experienced oral and maxillofacial surgeon. Excisional biopsy was reserved for small lesions with a benign appearance, according to E’s opinion. In all other cases, an incisional biopsy was preferred [36,37]. Biopsies were taken from the most representative areas, focusing on sites with heterogeneous clinical features or the most marked AF alterations. In cases of multifocal lesions, the most diagnostically relevant site was selected. Specimens were immediately fixed in 10% buffered formalin and submitted for histopathological examination by a pathologist, who was blinded to the AF findings and the clinical assessments of both examiners.
2.3. Statistical Analysis
All statistical analyses were performed using R (version 4.3; R Core Team; Vienna, Austria).
A preliminary pilot study on 10 patients was conducted to estimate the required sample size needed for comparing two proportions. Based on the expected difference in RM detection after AF assessment (P1 = 0.9; P2 = 0.7), with significance level α = 0.05 and statistical power β = 0.8, a total of 72 participants was calculated as necessary.
Descriptive statistics were computed for all socio-demographic variables of patients and clinical variables of lesions after OE and AF. Categorical variables, including RM assessments, were reported as absolute frequencies and percentages.
Inter-examiner agreement between E and PD was quantified using Cohen’s kappa coefficient (κ) with 95% confidence intervals (CI). Kappa values were interpreted according to Landis and Koch criteria (κ < 0.00, poor; 0.00–0.20, slight; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, substantial; 0.81–1.00, almost perfect) [38].
Diagnostic performance was evaluated by calculating sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy for each examiner (PD and E) under both examination conditions (OE and AF). As both modalities were independently applied to the same lesions, tests were analyzed in parallel.
Differences in diagnostic performances between AF and OE on the same lesions were assessed using McNemar’s test for paired nominal data. The continuity-corrected χ2 statistic was used, with a two-tailed p-value < 0.05 considered statistically significant.
A multivariable logistic regression was constructed to explore associations between clinical predictors of the lesions (dishomogeneous phenotype and color categories: keratotic, erythema, keratotic + erythema, pigmented) and the assessed RM, separately for each operator (PD and E) and after each diagnostic modality (OE and AF). Odds ratios (ORs) with 95% CI and p-values were calculated. A two-tailed α-level of 0.05 was considered statistically significant.
3. Results
3.1. Descriptive Analysis
Table 1 presents the sociodemographic characteristics of the study population.

Table 1.
Demographic variables of patients included in the study.
The majority of patients were female (52.5%, n = 42), with a mean age of 54.0 ± 14.6 years.
A total of 51.2% of patients (n = 41) were current smokers (more than 10 cigarettes/day).
A total of 104 oral lesions were identified. As detailed in Table 2, the buccal mucosa was the most common site (48.1%, n = 50), followed by the tongue (25%; n = 26).

Table 2.
Site and characteristics of oral lesions included in the study.
More than half of the lesions displayed a non-homogeneous phenotype (50.9%, n = 53), with a mixed color appearance (45.2%, n = 47) and a flat morphology (84.6%, n = 88).
Out of 104 lesions, 80 underwent scalpel biopsy.
As reported in Table 3, histopathological analysis of the 80 biopsied lesions revealed that 22 (27.5%) presented malignant histopathological features. These included 5 Oral Squamous Cell Carcinomas (OSCCs) (6.2%), 2 carcinomas in situ (2.5%), 1 adenosquamous carcinoma (1.2%), and 13 OPMDs (16.2%). The most frequent OPMD was erosive Oral Lichen Planus (OLP) (11.2%, n = 9), which showed moderate-to-severe dysplasia [39,40,41,42]

Table 3.
Histological diagnosis of 80 lesions that underwent biopsy and relative malignancy risk assessment after OE and AF for both PD and E.
Among the 58 non-malignant lesions, the most common were traumatic ulcer (17.5%; n = 14) and keratotic OLP (16.2%, n = 13), which showed no histological evidence of dysplasia.
3.2. Malignancy Risk Assessment and Biopsy Indications After OE
After OE alone, both the PD and E independently assessed RM, as summarized in Table 3.
The PD classified 39 lesions (48.7%) as RM, of which 16 were True Positives (TP) and 23 FP. Among the 58 non-malignant lesions, 35 were correctly identified as True Negatives (TN) (Figure 2).
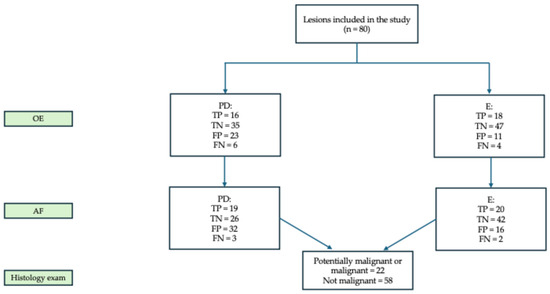
Figure 2.
Assessment of RM for each examiner after each examination. Abbreviations: OE = oral examination; PD = postgraduate dentist; E = expert; AF = autofluorescence examination; TP = True Positive; TN = True Negative; FP = false positive; FN = False Negative.
As reported in Table S1, PD recommended biopsy in 70 cases (87.5%), based either on overtly suspicious features, diagnostic uncertainty or on the presence of small benign lesions, such as fibromas. However, the PD did not suggest biopsy for one erosive OLP that later revealed moderate–severe dysplasia, five keratotic OLPs, two traumatic ulcers, and one traumatic keratosis, all of which had been classified as NRM.
The E evaluated 29 lesions (36.2%) as RM, with 18 TP and 11 FP (Figure 1). Biopsy was recommended in 69 cases (86.2%), including for vesciculo-bullous lesions suspected for pemphigus vulgaris, small benign lesions, and cases of diagnostic doubt (Table S1).
Notably, four traumatic lesions (two ulcers and two keratotic lesions) that were considered biopsy-worthy by the general dental practitioner (PD) were recommended for a “wait-and-see” approach (Table S1). Conversely, the E advised biopsy for five keratotic OLP lesions, whereas the PD did not, in order to obtain histological confirmation.
Among malignant cases missed by PD after OE, one adenosquamous carcinoma was misinterpreted as a traumatic ulcer and one mucoepidermoid carcinoma as a pleomorphic adenoma. Four erosive OLPs with moderate dysplasia were underestimated by both examiners.
3.3. Malignancy Risk Assessment and Biopsy Indications After AF
With AF examination, the PD identified 45 lesions (56.2%) showing LAF, and an additional 27 with AFA, as illustrated in Table 4. Based on these findings, 51 lesions were classified as RM, adding 12 cases compared to OE alone (Table 3), and 29 lesions as NRM, increasing the FP to 32. Following AF, PD recommended biopsy in 78 cases (97.5%), which turned out to be adenosquamous carcinoma (n = 1), keratotic OLP (n = 5), and traumatic ulcers (n = 2). In 11 cases, AF led to a change in the chosen biopsy site compared with OE alone. These included carcinoma in situ (n = 1), adenosquamous carcinoma (n = 1), erosive OLP (n = 1), and keratotic OLPs (n = 2).

Table 4.
AF patterns under VELscope® of lesions that underwent biopsy as described by PD and E.
The E identified 41 lesions (51.2%) with LAF and 15 additional lesions (53.8%) with AAF (Table 4). After AF assessment, 36 lesions (45%) were classified as RM, adding 7 cases more than with OE (Table 3), with 16 FP in total. Biopsy was recommended in 49 cases (61.2%). AF also influenced biopsy site selection in two instances: one carcinoma in situ and one keratotic OLP, both showing higher levels of AAF.
3.4. Changes in Diagnostic Concordance Between PD and E
As shown in Table S2, the initial clinical diagnostic hypothesis proposed by PD was confirmed by histopathology analysis in 54 cases (67.5%), while E’s diagnostic impressions were validated in 71 cases (88.7%).
The PD misdiagnosed an adenosquamous carcinoma as a traumatic ulcer, and a mucoepidermoid carcinoma as a pleomorphic adenoma; however, both cases were correctly classified as RM following AF assessment. Both examiners misclassified one of the two in situ carcinomas as leukoerythroplakia. Similarly, two erosive OLP lesions were mistaken for leukoerythroplakia due to their plaque-like appearance. A subepithelial capillary hemangioma, presenting as a firm, erythematous lesion with well-defined margins, was misinterpreted as erythroplakia by both examiners (Figure 1e,f). Conversely, a fibroma with surface ulceration and irregularity was erroneously classified as OSCC by both PD and E. All cases of Peripheral Giant Cell Granuloma (PGCG) were falsely considered RM by PD while the E misclassified two such cases, mainly due to a complete LAF pattern. One keratotic OLP, initially assessed as NRM during OE, was reclassified as RM after AF examination by both clinicians because of marked AAF. Another keratotic OLP was misdiagnosed by PD as a melanoma owing to its pigmented features. A melanocytic nevus was incorrectly identified by PD as smoker’s melanosis. Notably, pemphigus vulgaris had the highest rate of diagnostic error by the PD, being variously misdiagnosed as erythroplakia (n = 2), traumatic ulcer (n = 1), and carcinoma in situ (n = 1). Traumatic ulcers were also frequently misclassified: the PD diagnosed one as leukoerythroplakia, one as carcinoma in situ, and three as OSCC; the E misclassified one case as leukoerythroplakia. For traumatic keratosis, the PD misdiagnosed one lesion as carcinoma in situ and two as leukoerythroplakia, with one of these errors shared by the E.
3.5. Clinical Predictors of RM Assessment
Table 5 presents the multivariable logistic models predicting RM assessment for each examiner and examination modality. For EO performed by PD, non-homogeneous lesions were significantly more likely to be assessed as malignant or potentially malignant (OR = 3.06; 95% CI 1.17–8.03; p = 0.0231). Although erythematous lesions showed increased odds of being classified as malignant (OR = 1.14), this finding did not reach statistical significance. Under AF evaluation by PD, all the clinical variables showed predictive trends, even if without statistical significance. In the case of the E, non-homogeneous color emerged as the strongest predictor of RM, both during OE (OR = 4.3; 95% CI 1.51–12.2; p = 0.006) and, to a lesser extent, AF assessment (OR = 2.77; 95% CI 1.02–7.51; p = 0.046).

Table 5.
Multivariable logistic regression of clinical predictors for RM assessment for every evaluation and for each operator.
3.6. Inter-Operator Agreement for OE and AF
Table 6 presents the inter-rater reliability between PD and E for both OE and AF.

Table 6.
Inter-observer concordance (Cohen’s κ) for OE and AF.
Moderate inter-rater agreement was observed for OE (κ = 0.494), whereas agreement was lower for AF (κ = 0.284), reflecting greater variability in the interpretation of AF findings.
3.7. Sensitivity, Specificity, PPV, NPV, and Accuracy
The clinicians’ RM assessment after OE and AF were compared with histopathological findings.
AF increased sensitivity for both clinicians (PD: 86.4%, E: 95.4%), corresponding to gains of 18.2% and 13.6%, respectively, as well as NPV (PD: 89.3%, E: 97.7%). Conversely, specificity decreased when AF was used alone (PD: 43.1%, E: 74.1%). When OE and AF were combined, sensitivity improved further (PD: 90.9%, E: 95.4%) but at the cost of a more pronounced reduction in specificity (PD: 37.9%, E: 70.7%). Diagnostic accuracy was highest for OE alone, both for the PD (62.5% vs. 55%) and the expert (81.25% vs. 80%) (Table 7). According to McNemar’s test with continuity correction, the difference between OE and AF was statistically significant for the PD (χ2 = 10.240; p = 0.001) but not for the expert (χ2 = 1.778; p = 0.182) (Table 8).

Table 7.
Sensitivity, specificity, PPV, NPV, and accuracy for OE and AF examinations for both PD and E.

Table 8.
Differences in diagnostic performance between OE and AF in both PD and E.
4. Discussion
This study aimed to determine how clinician experience influences detection of OPMDs when AF is used as an adjunct to conventional OE. By including both lesions clinically suspicious for OPMDs and clearly benign lesions, we evaluated AF’s discriminatory value and compared examiners’ diagnostic abilities across a broad spectrum of oral conditions.
Leuci and Coppola et al. (2020) [29] conducted a comparable study in dentists who followed an oral medicine training program versus general dentists and observed that experienced clinicians benefitted more from VELscope® use than general dentists, suggesting that AF’s value for less-experienced practitioners depends on structured training.
Instead, Simonato et al. (2017) [30] showed that fluorescence visualization improved the sensitivity and overall accuracy of inexperienced examiners to a level comparable to that of experts using OE alone.
In our cohort, AF increased sensitivity and NPV for both clinicians, but this gain was accompanied by a reduction in specificity and PPV. The difference between OE and AF reached statistical significance only for the PD (McNemar p = 0.001 **), but not for the E, suggesting that less experienced clinicians may rely more heavily on AF findings, sometimes overestimating malignancy risk. From this perspective, our findings partially align with Leuci and Coppola [29], emphasizing the importance of structured training to maximize AF’s utility. At the same time, they slightly diverge from Simonato et al. (2017) [30], who showed an overall diagnostic accuracy for PD using AF to levels comparable to experts using OE alone. While our data also show improved sensitivity for the less-experienced clinician, the overall accuracy of the less experienced-clinician did not fully reach that of the expert, indicating that while AF can enhance sensitivity, it cannot entirely replace clinical judgment.
Dentists play a fundamental role in early recognition and diagnosis of OPMDs and oral cancer, especially in countries where regular dental attendance is common, as opportunistic screening may reduce diagnostic delay [43,44]. Current guidelines designate visual and tactile OE and relative biopsy as the gold standard for early detection of OPMDs [45]. However, multiple studies highlight persistent knowledge gaps among general dental practitioners in recognizing mucosal pathology [46,47,48,49,50,51]. These gaps support the potential role of adjunctive diagnostic tools during OE in clinical decision-making [52,53] but highlight that any adjunct must be interpreted within a clinical context and accompanied by education.
Among light-based tests, like chemiluminescence and AF, the latter has displayed superior accuracy levels in identifying premalignant lesions and early neoplastic changes [54]. Multiple AF devices have been developed through the years (VELscope®, approved for oral use in 2008, IllumiScan® [55], GOCCLES® [56,57]).
Systematic reviews, however, have consistently observed limited specificity for AF. Nagi et al. [27] concluded that VELscope® can assist trained clinicians in detecting precursor lesions but cannot reliably differentiate dysplasia from inflammatory conditions. This pattern is reflected in our findings: AF increased FP, particularly for the PD, producing a 15.5% drop in specificity. Many benign entities (e.g., capillary subepithelial hemangioma, PGCG, keratotic OLP, melanocytic nevus, traumatic ulcers) exhibited LAF or AAF and were therefore classified as RM by AF despite being correctly excluded on OE. This phenomenon is attributable to AF alterations caused by non-neoplastic conditions: inflammation, trauma, and vascular changes often increase submucosal hemoglobin content or alter fluorophore concentrations (collagen, NADH, FAD, porphyrins, elastin, keratin), producing AF patterns that mimic dysplasia or carcinoma [26,58].
Our findings mirror meta-analyses reporting pooled AF specificity around 60% [59], which is generally lower than that of clinical examinations and other tools such as toluidine blue staining. AF does not seem reliable for distinguishing benign from dysplastic or malignant lesions [60], as LAF also occurs across benign lesions [61]. Consequently, AF may increase the number of unnecessary biopsies if interpreted in isolation—an effect observed in our study, where the PD recommended biopsy for seven lesions later proven benign, while the E avoided such procedures, highlighting the operator-dependent nature of AF interpretation. The E’s avoidance of unnecessary biopsies indicates that this limitation could potentially be mitigated through structured training, which may help less experienced clinicians to interpret AF findings more accurately and reduce overestimation of malignancy risk.
Several authors have previously warned about AF’s interpretative limitations in primary care. Bhatia et al. [62] noted that the predominance of benign lesions and the frequent LAF of inflammatory conditions may lead to over-referral and harm. Farah et al. [63] emphasized that clinical judgment—not LAF alone—should guide management, and McNamara et al. [64] argued that low AF specificity constrains its routine use in general practice. Our findings corroborate these concerns: AF reduced inter-observer agreement (OE κ = 0.544 to AF κ = 0.284), illustrating substantial operator dependence in fluorescence interpretation. In conclusion, the tendency of AF to generate FP emphasizes the need for careful contextual interpretation, especially by inexperienced clinicians, despite the larger gain in sensitivity.
Despite these limitations, AF demonstrated clinically useful advantages. In fact, it has been demonstrated that NPV ranges from 81.1% [65] to approximately 94% [59], implicating that most AF-negative patients do not harbor high-risk lesions after examination, which is comforting for both the dentist and the patient [66].
Across systematic reviews, AF-based screening sensitivities range from 74% to 82%, specificity from 52% to 62%, NPV from 79% to 81% [59,65,67], and AUC around 0.815, suggesting reasonable diagnostic accuracy [59] but limited specificity. It was also demonstrated that for overt neoplastic lesions, AF adds little beyond OE, as also in our study both PD and E detected most frank carcinomas on OE; however, when OE is negative for neoplasm, AF may uncover occult or asymptomatic lesions [68].
In our cohort, AF enabled the PD to detect malignant or high-grade lesions missed on OE (with sensitivity gain of 13.7%), including an infiltrating adenosquamous carcinoma, a mucoepidermoid carcinoma, and several erosive OLPs later shown to harbor moderate-to-severe dysplasia. The E also identified additional dysplastic OLP cases with AF.
AF influenced biopsy site selection—11 changes for the PD and 2 for the E—leading to identification of carcinoma in situ and higher-grade dysplasia that might otherwise have been missed. These observations support AF’s role in guiding selection of the most representative biopsy area and in delineating lesion margins: AF often revealed irregular boundaries beyond those apparent on white light, and AF-defined margins have been reported to improve molecular clearance relative to white-light margins, with potential surgical benefits for OSCC management and recurrence reduction [68,69,70]. AF may also assist in balancing excision of at-risk mucosa against preservation of healthy tissue in conditions such as OLP and in early detection of molecular recurrence [70].
Multivariable analysis in our sample identified non-homogeneous appearance as the most consistent clinical predictor of perceived malignancy across both examiners and modalities—reinforcing the clinical value of heterogeneous texture and color as red flags.
Interestingly, we observed a diagnostic concordance in 54 cases (67.5%) for PD and in 71 cases (88.7%) for E. The greatest diagnostic challenge for the inexperienced examiner was pemphigus vulgaris, which was variably misinterpreted as erythroplakia, traumatic ulcer or carcinoma in situ; AF changes did not resolve this ambiguity and in some cases reinforced the diagnostic dilemma. Such diagnostic delays for pemphigus are documented in the literature and reflect the relative under-recognition of mucosal versus cutaneous presentations [71,72,73].
Our findings underscore that AF is a useful tool, as it increases sensitivity, but it yields more false positives in less experienced clinicians, who may lack the specific training to distinguish benign AF alterations (e.g., due to inflammation, pigmentation, denture effects, prior treatments) from true premalignant signals [58]. Therefore, while AF can serve as a useful adjunct, especially in biopsy site decision-making, it cannot replace histopathological examination (which remains the gold standard) and cannot be used as a unique diagnostic device. Clinicians—particularly those with less experience—should interpret AF findings with caution, integrating them with clinical context and lesion history.
This study presents several limitations that need to be acknowledged. This single-center, cross-sectional study involved just one PD and one E examiner. This limited sample of examiners substantially restricts the generalizability of the findings and precludes any robust assessment of interobserver variability. Given that the diagnostic performance of the VELscope® is known to be influenced by the examiner’s clinical experience, as also discussed in this paper, the inclusion of multiple examiners from each professional category would be essential to validate these observations and strengthen external validity. Moreover, the study design did not address long-term outcomes or cost-effectiveness. Larger, multicenter studies with multiple examiners and more heterogeneous populations are necessary to validate these findings and to define optimal training pathways for identification and treatment of oral mucosal pathology.
5. Conclusions
AF can improve sensitivity and be a useful adjunct in the detection of OPMDs and oral cancer. However, its limited specificity and operator-dependent interpretation preclude its use as a substitute for OE and histopathology, which remain the diagnostic gold standard. Our results emphasize AF’s value as an adjunctive tool rather than a replacement, highlighting the importance of targeted training in oral medicine and structured AF use.
Strengthening education in oral medicine (including improved training in conventional oral examination) and developing standardized protocols for AF use will help exploit AF’s high NPV while reducing unnecessary biopsies and referrals. Future research should focus on refining AF-based strategies, evaluating combined diagnostic approaches (e.g., AF plus molecular markers or AI), and validating training programs to optimize early detection and management of OPMDs and oral cancers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/dj13110512/s1: Table S1: Biopsy indications after OE and AF for both PD and comparison of biopsy indication site between OE and AF for both PD and E.; Table S2: Comparison of diagnostic concordance between PD and E after the histological evaluation.
Author Contributions
Conceptualization, A.A.; methodology, A.A. and C.D.; software, C.D. and S.B.; validation, R.F., A.M., V.G. and V.C.; formal analysis, C.D., A.M. and S.B.; investigation, A.A., R.F. and A.G.; resources, A.M.B. and V.G.; data curation, A.M.B., V.C. and F.B. (Flavia Biamonte); writing—original draft preparation, A.A. and C.D.; writing—review and editing, S.B., F.B. (Francesco Bennardo) and A.G.; visualization, A.M.B. and F.B. (Flavia Biamonte); super-vision, F.B. (Francesco Bennardo) and F.B. (Flavia Biamonte); project administration, A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Regional Ethical Review Board of Central Calabria (reference for the Magna Graecia University of Catanzaro) (17 January 2019 Nr. 24/2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Graphical abstract created with BioRender.com. © 2025 BioRender.com (Science Suite Inc. dba BioRender), licensed to Cristina D’Antonio under the BioRender Academic Publication License (Agreement No. DL28XQ7BCP). Accessed on 30 October 2025.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AAF | Alterations of Autofluorescence |
| AF | Autofluorescence |
| CI | Confidence Intervals |
| E | Expert |
| FAD | Flavin Adenine Dinucleotide |
| FN | False Negative |
| FP | False Positive |
| LAF | Loss of Autofluorescence |
| NADH | Nicotinamide Adenine Dinucleotide |
| NPV | Negative Predictive Value |
| NRM | Non-Risk of Malignancy |
| OE | Oral Examination |
| OLP | Oral Lichen Planus |
| OPMDs | Oral Potentially Malignant Disorders |
| OSCC | Oral Squamous Cell Carcinoma |
| PD | Postgraduate Dentist |
| PGCG | Peripheral Giant Cell Granuloma |
| PPV | Positive Predictive Value |
| RM | Risk of Malignancy |
| SD | Standard Deviation |
| TN | True Negative |
| TP | True Positive |
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today (Version 1.1); International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.who.int/today (accessed on 24 June 2025).
- Iocca, O.; Sollecito, T.P.; Alawi, F.; Weinstein, G.S.; Newman, J.G.; De Virgilio, A.; Di Maio, P.; Spriano, G.; Pardiñas López, S.; Shanti, R.M. Potentially Malignant Disorders of the Oral Cavity and Oral Dysplasia: A Systematic Review and Meta-Analysis of Malignant Transformation Rate by Subtype. Head Neck 2020, 42, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Abati, S.; Bramati, C.; Bondi, S.; Lissoni, A.; Trimarchi, M. Oral Cancer and Precancer: A Narrative Review on the Relevance of Early Diagnosis. Int. J. Environ. Res. Public Health 2020, 17, 9160. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.M.; Ogden, G.R. Oral Cancer Awareness of General Medical and General Dental Practitioners. Br. Dent. J. 2007, 203, E10. [Google Scholar] [CrossRef] [PubMed]
- González-Moles, M.Á.; Aguilar-Ruiz, M.; Ramos-García, P. Challenges in the Early Diagnosis of Oral Cancer, Evidence Gaps and Strategies for Improvement: A Scoping Review of Systematic Reviews. Cancers 2022, 14, 4967. [Google Scholar] [CrossRef]
- Güneri, P.; Epstein, J.B. Late Stage Diagnosis of Oral Cancer: Components and Possible Solutions. Oral Oncol. 2014, 50, 1131–1136. [Google Scholar] [CrossRef]
- Saka-Herrán, C.; Jané-Salas, E.; Mari-Roig, A.; Estrugo-Devesa, A.; López-López, J. Time-to-Treatment in Oral Cancer: Causes and Implications for Survival. Cancers 2021, 13, 1321. [Google Scholar] [CrossRef]
- Poudel, P.; Srii, R.; Marla, V. Oral Cancer Awareness among Undergraduate Dental Students and Dental Surgeons: A Descriptive Cross-Sectional Study. J. Nepal Med. Assoc. 2020, 58, 102–107. [Google Scholar] [CrossRef]
- Greenwood, M.; Lowry, R.J. Primary Care Clinicians’ Knowledge of Oral Cancer: A Study of Dentists and Doctors in the North East of England. Br. Dent. J. 2001, 191, 510–512. [Google Scholar] [CrossRef]
- Strey, J.R.; Roxo-Gonçalves, M.; Guzenski, B.D.; Martins, M.A.T.; Romanini, J.; de Figueiredo, M.A.Z.; D’Ávila, O.P.; Gonçalves, M.R.; Umpierre, R.N.; Harzheim, E.; et al. Oral Medicine Experience and Attitudes Toward Oral Cancer: An Evaluation of Dentists Working in Primary Health Care. J. Cancer Educ. 2022, 37, 1621–1628. [Google Scholar] [CrossRef]
- Shubayr, M.A.; Moaleem, M.M.A.; Hakami, S.A.; Khalufi, K.N.; Daghriri, R.M.; Bokhari, A.M.; Alhazmi, A.S.; Farsi, A.H.; Adawi, M.A.; Nahari, H.H.; et al. Factors Influencing Engagement in Oral Cancer Prevention Activities among Dental Students and Professionals in Saudi Arabia. BMC Oral Health 2024, 24, 1465. [Google Scholar] [CrossRef]
- Pentenero, M.; Chiecchio, A.; Gandolfo, S. Impact of Academic and Continuing Education on Oral Cancer Knowledge, Attitude and Practice among Dentists in North-Western Italy. J. Cancer Educ. 2014, 29, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Shadid, R.M.; Abu Ali, M.A.; Kujan, O. Knowledge, Attitudes, and Practices of Oral Cancer Prevention among Dental Students and Interns: An Online Cross-sectional Questionnaire in Palestine. BMC Oral Health 2022, 22, 381. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.G.; Duffy, N.G.; Walters, K.C.; Day, T.A. Oral Cancer Knowledge and Experience: A Survey of South Carolina Medical Students in 2002. J. Cancer Educ. 2005, 20, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.F.A.; Oliveira, M.C.M.; Leite, A.C.; Bruzinga, F.F.B.; Mendes, P.A.; Grossmann, S.D.M.C.; De Araújo, V.E.; Souto, G.R. Assessment of Screening Programs as a Strategy for Early Detection of Oral Cancer: A Systematic Review. Oral Oncol. 2022, 130, 105936. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Kerr, A.R. Oral Cancer Screening: Past, Present, and Future. J. Dent. Res. 2021, 100, 1313–1320. [Google Scholar] [CrossRef]
- Su, Y.-F.; Chen, Y.-J.; Tsai, F.-T.; Li, W.-C.; Hsu, M.-L.; Wang, D.-H.; Yang, C.-C. Current Insights into Oral Cancer Diagnostics. Diagnostics 2021, 11, 1287. [Google Scholar] [CrossRef]
- Giovannacci, I.; Vescovi, P.; Manfredi, M.; Meleti, M. Non-Invasive Visual Tools for Diagnosis of Oral Cancer and Dysplasia: A Systematic Review. Med. Oral Patol. Oral Bucal 2016, 21, e305–e315. [Google Scholar] [CrossRef]
- Cheng, Y.-S.L.; Jordan, L.; Rees, T.; Chen, H.-S.; Oxford, L.; Brinkmann, O.; Wong, D. Levels of Potential Oral Cancer Salivary mRNA Biomarkers in Oral Cancer Patients in Remission and Oral Lichen Planus Patients. Clin. Oral Investig. 2014, 18, 985–993. [Google Scholar] [CrossRef]
- Warin, K.; Suebnukarn, S. Deep Learning in Oral Cancer- a Systematic Review. BMC Oral Health 2024, 24, 212. [Google Scholar] [CrossRef]
- Lingen, M.W.; Kalmar, J.R.; Karrison, T.; Speight, P.M. Critical Evaluation of Diagnostic Aids for the Detection of Oral Cancer. Oral Oncol. 2008, 44, 10–22. [Google Scholar] [CrossRef]
- Antonelli, A.; Battaglia, A.M.; Sacco, A.; Petriaggi, L.; Giorgio, E.; Barone, S.; Biamonte, F.; Giudice, A. Ferroptosis and Oral Squamous Cell Carcinoma: Connecting the Dots to Move Forward. Front. Oral Health 2024, 5, 1461022. [Google Scholar] [CrossRef]
- Sacco, A.; Battaglia, A.M.; Santamaria, G.; Buffone, C.; Barone, S.; Procopio, A.; Lavecchia, A.M.; Aversa, I.; Giorgio, E.; Petriaggi, L.; et al. SOX2 Promotes a Cancer Stem Cell-like Phenotype and Local Spreading in Oral Squamous Cell Carcinoma. PLoS ONE 2023, 18, e0293475. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Warnakulasuriya, S. The Use of Light-Based (Optical) Detection Systems as Adjuncts in the Detection of Oral Cancer and Oral Potentially Malignant Disorders: A Systematic Review. J. Oral Pathol. Med. 2015, 44, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Petriaggi, L.; Giorgio, E.; Bulotta, S.; Antonelli, A.; Bonacci, S.; Frisina, M.; Procopio, A.; Prestagiacomo, L.E.; Giuliano, A.; Gaspari, M.; et al. Acute Exposure to Cadmium Triggers NCOA4-Mediated Ferritinophagy and Ferroptosis in Never-Smokers Oral Cancer Cells. Int. J. Biol. Sci. 2025, 21, 4131–4152. [Google Scholar] [CrossRef] [PubMed]
- Giovannacci, I.; Magnoni, C.; Vescovi, P.; Painelli, A.; Tarentini, E.; Meleti, M. Which Are the Main Fluorophores in Skin and Oral Mucosa? A Review with Emphasis on Clinical Applications of Tissue Autofluorescence. Arch. Oral Biol. 2019, 105, 89–98. [Google Scholar] [CrossRef]
- Nagi, R.; Reddy-Kantharaj, Y.-B.; Rakesh, N.; Janardhan-Reddy, S.; Sahu, S. Efficacy of Light Based Detection Systems for Early Detection of Oral Cancer and Oral Potentially Malignant Disorders: Systematic Review. Med. Oral Patol. Oral Bucal 2016, 21, e447–e455. [Google Scholar] [CrossRef]
- Cicciù, M.; Cervino, G.; Fiorillo, L.; D’Amico, C.; Oteri, G.; Troiano, G.; Zhurakivska, K.; Lo Muzio, L.; Herford, A.S.; Crimi, S.; et al. Early Diagnosis on Oral and Potentially Oral Malignant Lesions: A Systematic Review on the VELscope® Fluorescence Method. Dent. J. 2019, 7, 93. [Google Scholar] [CrossRef]
- Leuci, S.; Coppola, N.; Turkina, A.; Bizzoca, M.E.; Favia, G.; Spagnuolo, G.; Mignogna, M.D. May VelScope Be Deemed an Opportunistic Oral Cancer Screening by General Dentists? A Pilot Study. J. Clin. Med. 2020, 9, 1754. [Google Scholar] [CrossRef]
- Simonato, L.E.; Tomo, S.; Miyahara, G.I.; Navarro, R.S.; Villaverde, A.G.J.B. Fluorescence Visualization Efficacy for Detecting Oral Lesions More Prone to Be Dysplastic and Potentially Malignant Disorders: A Pilot Study. Photodiagn. Photodyn. Ther. 2017, 17, 1–4. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Kujan, O.; Oliver, R.J.; Khattab, A.; Roberts, S.A.; Thakker, N.; Sloan, P. Evaluation of a New Binary System of Grading Oral Epithelial Dysplasia for Prediction of Malignant Transformation. Oral Oncol. 2006, 42, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Reibel, J.; Bouquot, J.; Dabelsteen, E. Oral Epithelial Dysplasia Classification Systems: Predictive Value, Utility, Weaknesses and Scope for Improvement. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2008, 37, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Speight, P.M.; Khurram, S.A.; Kujan, O. Oral Potentially Malignant Disorders: Risk of Progression to Malignancy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 612–627. [Google Scholar] [CrossRef] [PubMed]
- Dost, F.; Lê Cao, K.-A.; Ford, P.J.; Farah, C.S. A Retrospective Analysis of Clinical Features of Oral Malignant and Potentially Malignant Disorders with and without Oral Epithelial Dysplasia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 725–733. [Google Scholar] [CrossRef]
- Jeng, P.-Y.; Chang, M.-C.; Chiang, C.-P.; Lee, C.-F.; Chen, C.-F.; Jeng, J.-H. Oral Soft Tissue Biopsy Surgery: Current Principles and Key Tissue Stabilization Techniques. J. Dent. Sci. 2024, 19, 11–20. [Google Scholar] [CrossRef]
- Shanti, R.M.; Tanaka, T.; Stanton, D.C. Oral Biopsy Techniques. Dermatol. Clin. 2020, 38, 421–427. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Ramos-García, P. An Evidence-Based Update on the Potential for Malignancy of Oral Lichen Planus and Related Conditions: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 608. [Google Scholar] [CrossRef]
- Roberts, S.L.; Bhamra, R.; Ilankovan, V. Malignant Transformation Rate of Erosive Oral Lichen Planus: A Retrospective Study. Br. J. Oral Maxillofac. Surg. 2024, 62, 788–793. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral Potentially Malignant Disorders: A Consensus Report from an International Seminar on Nomenclature and Classification, Convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Ruiz-Ávila, I.; González-Ruiz, L.; Ayén, Á.; Gil-Montoya, J.A.; Ramos-García, P. Malignant Transformation Risk of Oral Lichen Planus: A Systematic Review and Comprehensive Meta-Analysis. Oral Oncol. 2019, 96, 121–130. [Google Scholar] [CrossRef]
- Lim, K.; Moles, D.R.; Downer, M.C.; Speight, P.M. Opportunistic Screening for Oral Cancer and Precancer in General Dental Practice: Results of a Demonstration Study. Br. Dent. J. 2003, 194, 497–502; discussion 493. [Google Scholar] [CrossRef]
- Lingen, M.W.; Abt, E.; Agrawal, N.; Chaturvedi, A.K.; Cohen, E.; D’Souza, G.; Gurenlian, J.; Kalmar, J.R.; Kerr, A.R.; Lambert, P.M.; et al. Evidence-Based Clinical Practice Guideline for the Evaluation of Potentially Malignant Disorders in the Oral Cavity: A Report of the American Dental Association. J. Am. Dent. Assoc. 1939 2017, 148, 712–727.e10. [Google Scholar] [CrossRef]
- Awadallah, M.; Idle, M.; Patel, K.; Kademani, D. Management Update of Potentially Premalignant Oral Epithelial Lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Tarakji, B. Dentists’ Perception of Oral Potentially Malignant Disorders. Int. Dent. J. 2022, 72, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Dissanayaka, D.W.V.N.; Wijeratne, K.M.S.L.; Amarasinghe, K.A.D.K.D.; Jayasinghe, R.D.; Jayasooriya, P.R.; Mendis, B.R.R.N.; Lombardi, T. A Preliminary Study on Early Detection of Oral Cancer with Opportunistic Screening: Insights from Dental Surgeons in Sri Lanka. Cancers 2023, 15, 5511. [Google Scholar] [CrossRef] [PubMed]
- Idrees, M.; Halimi, R.; Gadiraju, S.; Frydrych, A.M.; Kujan, O. Clinical Competency of Dental Health Professionals and Students in Diagnosing Oral Mucosal Lesions. Oral Dis. 2024, 30, 3108–3116. [Google Scholar] [CrossRef]
- Sari, E.F.; Hidayat, W.; Dewi, T.S.; Rezeki, S.; Krimadi, R.; McCullough, M.J.; Cirillo, N. General Dentists’ Knowledge, Perceptions, and Practices Regarding Oral Potentially Malignant Disorders and Oral Cancer in Indonesia. Clin. Exp. Dent. Res. 2024, 10, e807. [Google Scholar] [CrossRef]
- Najim, A.M.; Bruers, J.J.M.; De Visscher, J.G. Knowledge, Attitude, and Practice of Dutch Dentists on Oral Leukoplakia and Their Possible Role in Its Follow-Up. Int. Dent. J. 2025, 75, 1029–1035. [Google Scholar] [CrossRef]
- Colella, G.; Gaeta, G.M.; Moscariello, A.; Angelillo, I.F. Oral Cancer and Dentists: Knowledge, Attitudes, and Practices in Italy. Oral Oncol. 2008, 44, 393–399. [Google Scholar] [CrossRef]
- Chaurasia, A.; Alam, S.I.; Singh, N. Oral Cancer Diagnostics: An Overview. Natl. J. Maxillofac. Surg. 2021, 12, 324–332. [Google Scholar] [CrossRef]
- Jitender, S.; Sarika, G.; Varada, H.R.; Omprakash, Y.; Mohsin, K. Screening for Oral Cancer. J. Exp. Ther. Oncol. 2016, 11, 303–307. [Google Scholar] [PubMed]
- Buenahora, M.R.; Peraza-L, A.; Díaz-Báez, D.; Bustillo, J.; Santacruz, I.; Trujillo, T.G.; Lafaurie, G.I.; Chambrone, L. Diagnostic Accuracy of Clinical Visualization and Light-Based Tests in Precancerous and Cancerous Lesions of the Oral Cavity and Oropharynx: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2021, 25, 4145–4159. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, Y.; Toratani, S.; Matsui, K.; Hayashi, S.; Eboshida, N.; Hamada, A.; Ito, N.; Obayashi, F.; Kimura, N.; Yanamoto, S. Evaluation of Oral Mucosal Lesions Using the IllumiScan® Fluorescence Visualisation Device: Distinguishing Squamous Cell Carcinoma. Int. J. Environ. Res. Public Health 2022, 19, 10414. [Google Scholar] [CrossRef]
- Moro, A.; De Waure, C.; Di Nardo, F.; Spadari, F.; Mignogna, M.D.; Giuliani, M.; Califano, L.; Giannì, A.B.; Cardarelli, L.; Celentano, A.; et al. The GOCCLES® Medical Device Is Effective in Detecting Oral Cancer and Dysplasia in Dental Clinical Setting. Results from a Multicentre Clinical Trial. Acta Otorhinolaryngol. Ital. 2015, 35, 449–454. [Google Scholar] [CrossRef]
- Lajolo, C.; Tranfa, M.; Patini, R.; Fiorino, A.; Musarra, T.; Boniello, R.; Moro, A. Clinical Evaluation of the Optical Filter for Autofluorescence Glasses for Oral Cancer Curing Light Exposed (GOCCLES®) in the Management of Potentially Premalignant Disorders: A Retrospective Study. Int. J. Environ. Res. Public Health 2022, 19, 5579. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, V.; Sullivan, M.; Merzianu, M.; Rigual, N.R.; Loree, T.R.; Popat, S.R.; Moysich, K.B.; Ramananda, S.; Johnson, T.; Marshall, J.R.; et al. Autofluorescence-Guided Surveillance for Oral Cancer. Cancer Prev. Res. 2009, 2, 966–974. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.W.; Hwang, S.H. Autofluorescence Imaging to Identify Oral Malignant or Premalignant Lesions: Systematic Review and Meta-Analysis. Head Neck 2020, 42, 3735–3743. [Google Scholar] [CrossRef]
- Awan, K.H.; Morgan, P.R.; Warnakulasuriya, S. Evaluation of an Autofluorescence Based Imaging System (VELscopeTM) in the Detection of Oral Potentially Malignant Disorders and Benign Keratoses. Oral Oncol. 2011, 47, 274–277. [Google Scholar] [CrossRef]
- Ganga, R.S.; Gundre, D.; Bansal, S.; Shirsat, P.M.; Prasad, P.; Desai, R.S. Evaluation of the Diagnostic Efficacy and Spectrum of Autofluorescence of Benign, Dysplastic and Malignant Lesions of the Oral Cavity Using VELscope. Oral Oncol. 2017, 75, 67–74. [Google Scholar] [CrossRef]
- Bhatia, N.; Lalla, Y.; Vu, A.N.; Farah, C.S. Advances in Optical Adjunctive Aids for Visualisation and Detection of Oral Malignant and Potentially Malignant Lesions. Int. J. Dent. 2013, 2013, 194029. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.S.; McIntosh, L.; Georgiou, A.; McCullough, M.J. Efficacy of Tissue Autofluorescence Imaging (Velscope) in the Visualization of Oral Mucosal Lesions. Head Neck 2012, 34, 856–862. [Google Scholar] [CrossRef] [PubMed]
- McNamara, K.K.; Martin, B.D.; Evans, E.W.; Kalmar, J.R. The Role of Direct Visual Fluorescent Examination (VELscope) in Routine Screening for Potentially Malignant Oral Mucosal Lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Moffa, A.; Giorgi, L.; Costantino, A.; De Benedetto, L.; Cassano, M.; Spriano, G.; Mercante, G.; De Virgilio, A.; Casale, M. Accuracy of Autofluorescence and Chemiluminescence in the Diagnosis of Oral Dysplasia and Carcinoma: A Systematic Review and Meta-Analysis. Oral Oncol. 2021, 121, 105482. [Google Scholar] [CrossRef]
- Shi, L.; Li, C.; Shen, X.; Zhou, Z.; Liu, W.; Tang, G. Potential Role of Autofluorescence Imaging in Determining Biopsy of Oral Potentially Malignant Disorders: A Large Prospective Diagnostic Study. Oral Oncol. 2019, 98, 176–179. [Google Scholar] [CrossRef]
- Flores Dos Santos, L.C.; Fernandes, J.R.; Lima, I.F.P.; Bittencourt, L.D.S.; Martins, M.D.; Lamers, M.L. Applicability of Autofluorescence and Fluorescent Probes in Early Detection of Oral Potentially Malignant Disorders: A Systematic Review and Meta-Data Analysis. Photodiagn. Photodyn. Ther. 2022, 38, 102764. [Google Scholar] [CrossRef]
- Poh, C.F.; Ng, S.P.; Williams, P.M.; Zhang, L.; Laronde, D.M.; Lane, P.; Macaulay, C.; Rosin, M.P. Direct Fluorescence Visualization of Clinically Occult High-Risk Oral Premalignant Disease Using a Simple Hand-Held Device. Head Neck 2007, 29, 71–76. [Google Scholar] [CrossRef]
- Giudice, A.; Bennardo, F.; Barone, S.; Antonelli, A.; Figliuzzi, M.M.; Fortunato, L. Can Autofluorescence Guide Surgeons in the Treatment of Medication-Related Osteonecrosis of the Jaw? A Prospective Feasibility Study. J. Oral Maxillofac. Surg. 2018, 76, 982–995. [Google Scholar] [CrossRef]
- Biamonte, F.; Buffone, C.; Santamaria, G.; Battaglia, A.M.; Mignogna, C.; Fortunato, L.; Costanzo, F.S.; Giudice, A. Gene Expression Analysis of Autofluorescence Margins in Leukoplakia and Oral Carcinoma: A Pilot Study. Oral Dis. 2021, 27, 193–203. [Google Scholar] [CrossRef]
- Petruzzi, M.; Della Vella, F.; Squicciarini, N.; Lilli, D.; Campus, G.; Piazzolla, G.; Lucchese, A.; van der Waal, I. Diagnostic Delay in Autoimmune Oral Diseases. Oral Dis. 2023, 29, 2614–2623. [Google Scholar] [CrossRef]
- Villa, A.; Stock, S.; Aboalela, A.; Lerman, M.A.; Woo, S.-B.; Sonis, S.T.; Treister, N.S. Oral Medicine Referrals at a Hospital-Based Practice in the United States. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 119, 423–429. [Google Scholar] [CrossRef]
- Arduino, P.G.; Broccoletti, R.; Carbone, M.; Gambino, A.; Sciannameo, V.; Conrotto, D.; Cabras, M.; Sciascia, S.; Ricceri, F.; Baldovino, S.; et al. Long-term Evaluation of Pemphigus Vulgaris: A Retrospective Consideration of 98 Patients Treated in an Oral Medicine Unit in North-west Italy. J. Oral Pathol. Med. 2019, 48, 406–412. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).