Environmental Determinants of Early Childhood Caries: A Narrative Synthesis of Observational Evidence and Implications for Global Policy
Abstract
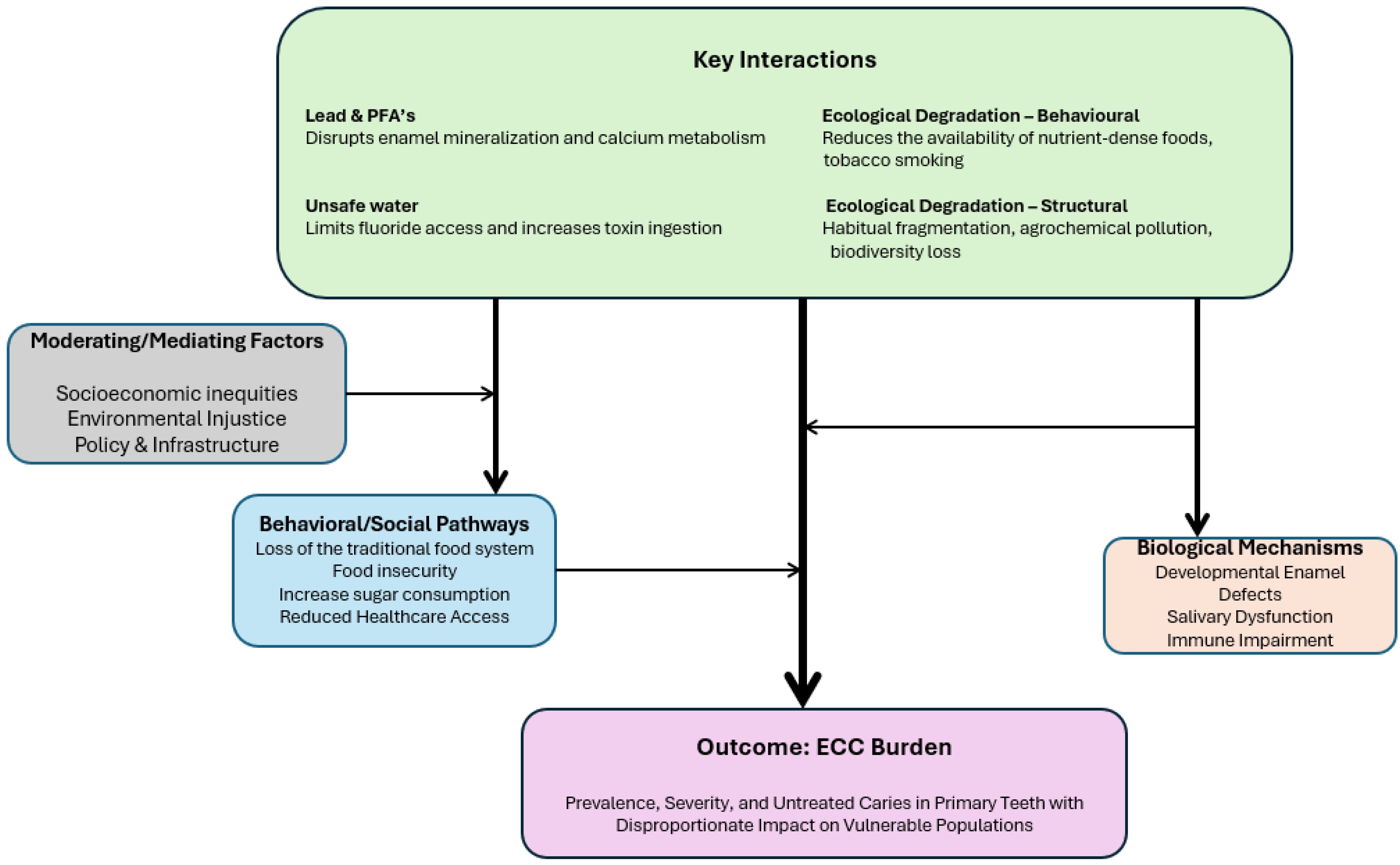
1. Introduction
2. Methods
3. Smoked Tobacco and Early Childhood Caries
4. Lead Poisoning and Early Childhood Caries
5. Unsafe Water and Early Childhood Caries
6. Ecovitality and Early Childhood Caries
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DALYs | Disability-adjusted life years |
| ECC | Early Childhood Caries |
| PFAAs | Perfluoroalkyl acids |
References
- Drury, T.F.; Horowitz, A.M.; Ismail, A.I.; Maertens, M.P.; Rozier, R.G.; Selwitz, R.H. Diagnosing and Reporting Early Childhood Caries for Research Purposes: A Report of a Workshop Sponsored by the National Institute of Dental and Craniofacial Research, the Health Resources and Services Administration, and the Health Care Financing Administration. J. Public Health Dent. 1999, 59, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.B.; Baez, R.J.; Diaz-Guillory, C.; Donly, K.J.; Feldens, C.A.; McGrath, C.; Phantumvanit, P.; Seow, W.K.; Sharkov, N.; Songpaisan, Y.; et al. Early Childhood Caries: IAPD Bangkok Declaration. J. Dent. Child. 2019, 86, 72. [Google Scholar]
- Zou, J.; Du, Q.; Ge, L.; Wang, J.; Wang, X.; Li, Y.; Song, G.; Zhao, W.; Chen, X.; Jiang, B.; et al. Expert consensus on early childhood caries management. Int. J. Oral Sci. 2022, 14, 35. [Google Scholar] [CrossRef]
- Caufield, P.W.; Griffen, A.L. Dental Caries. An infectious and transmissible disease. Pediatr. Clin. N. Am. 2004, 47, 1001–1019. [Google Scholar] [CrossRef]
- Schroth, R.J.; Harrison, R.L.; Moffatt, M.E. Oral Health of Indigenous Children and the Influence of Early Childhood Caries on Childhood Health and Well-being. Pediatr. Clin. N. Am. 2009, 56, 1481–1499. [Google Scholar] [CrossRef]
- GBD 2017 Oral Disorders Collaborators; Bernabé, E.; Marcenes, W.; Hernandez, C.R.; Bailey, J.; Abreu, L.G.; Alipour, V.; Amini, S.; Arabloo, J.; Arefi, Z.; et al. Global, Regional, and National Levels and Trends in Burden of Oral Conditions from 1990 to 2017: A Systematic Analysis for the Global Burden of Disease 2017 Study. J. Dent. Res. 2020, 99, 362–373. [Google Scholar] [CrossRef]
- GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Samani, D.; Ziaei, S.; Musaie, F.; Mokhtari, H.; Valipour, R.; Etemadi, M.; Gharehdaghi, N.; Rezaei, S.F.; Raji, S.; Fazel, T.; et al. Maternal smoking during pregnancy and early childhood dental caries in children: A systematic review and meta-analysis. BMC Oral Health 2024, 24, 781. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.W.; Sun, I.G.; Chan, S.S.C.; Chu, C.H. Secondary Smoking and Early Childhood Caries: A Systematic Review and Meta-Analysis. Int. Dent. J. 2025, 75, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Weuve, J.; Schwartz, J.; Wright, R.O. Association of Environmental Cadmium Exposure with Pediatric Dental Caries. Environ. Health Perspect. 2008, 116, 821–825. [Google Scholar] [CrossRef]
- Silva, M.J.; Kilpatrick, N.M.; Craig, J.M.; Manton, D.J.; Leong, P.; Burgner, D.P.; Scurrah, K.J. Genetic and Early-Life Environmental Influences on Dental Caries Risk: A Twin Study. Pediatrics 2019, 143, e20183499. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, A.L.; Hare, D.J. Tracing Environmental Exposure from Neurodevelopment to Neurodegeneration. Trends Neurosci. 2018, 41, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Folayan, M.O.; El Tantawi, M.; Ramos-Gomez, F.; Sabbah, W. Early childhood caries and its associations with sugar consumption, overweight and exclusive breastfeeding in low, middle and high-income countries: An ecological study. PeerJ 2020, 8, e9413. [Google Scholar] [CrossRef]
- Harris, R.; Nicoll, A.D.; Adair, P.M.; Pine, C.M. Risk factors for dental caries in young children: A systematic review of the literature. Community Dent. Health 2004, 21 (Suppl. S1), 71–85. [Google Scholar] [PubMed]
- Folayan, M.O.; El Tantawi, M.; Oginni, A.B.; Alade, M.; Adeniyi, A.; Finlayson, T.L.; Bourgeois, D. Malnutrition, enamel defects, and early childhood caries in preschool children in a sub-urban Nigeria population. PLoS ONE 2020, 15, e0232998. [Google Scholar] [CrossRef]
- Crystal, Y.O.; Luo, Y.L.; Duangthip, D.; El Tantawi, M.; Benzian, H.; Schroth, R.J.; Feldens, C.A.; Virtanen, J.I.; Al-Batayneh, O.B.; Diaz, A.C.M.; et al. A scoping review of the links between early childhood caries and clean water and sanitation: The Sustainable Development Goal 6. BMC Oral Health 2024, 24, 769. [Google Scholar] [CrossRef]
- Sogi, G.M. Sustainable Development Goals and Oral Health: A Forsaken Link. Contemp. Clin. Dent. 2025, 16, 1–2. [Google Scholar] [CrossRef]
- Foláyan, M.; de Barros Coelho, E.M.R.; Feldens, C.A.; Gaffar, B.; Virtanen, J.I.; Kemoli, A.; Duangthip, D.; Sun, I.G.; Masumo, R.M.; Vukovic, A.; et al. A scoping review on the associations between early childhood caries and sustainable cities and communities using the sustainable development goal 11 framework. BMC Oral Health 2024, 24, 751. [Google Scholar] [CrossRef]
- Foláyan, M.O.; Virtanen, J.I.; Gaffar, B.; Abodunrin, O.; Sun, I.G.; Duangthip, D.; Kemoli, A.; Masumo, R.M.; Vukovic, A.; Al-Batayneh, O.B.; et al. Scoping review on the association between early childhood caries and responsible resource consumption and production: Exploring Sustainable Development Goal 12. BMC Oral Health 2024, 24, 98. [Google Scholar] [CrossRef]
- Foláyan, M.O.; Schroth, R.J.; Abodunrin, O.; Al-Batayneh, O.B.; Arheiam, A.; Mfolo, T.; Virtanen, J.I.; Duangthip, D.; Feldens, C.A.; El Tantawi, M. Early childhood caries, climate change and the sustainable development goal 13: A scoping review. BMC Oral Health 2024, 24, 524. [Google Scholar] [CrossRef]
- Folayan, M.O.; Ayouni, I.; Nguweneza, A.; Al-Batayneh, O.B.; Virtanen, J.I.; Gaffar, B.; Duangthip, D.; Sun, I.G.F.; Onyejaka, N.K.; Daryanavard, H.; et al. A scoping review on the links between sustainable development goal 14 and early childhood caries. BMC Oral Health 2023, 23, 881. [Google Scholar] [CrossRef]
- Foláyan, M.O.; Schroth, R.J.; Duangthip, D.; Al-Batayneh, O.B.; Virtanen, J.I.; Sun, I.G.; Arheiam, A.; Feldens, C.A.; El Tantawi, M.; Ghasemi, H. A scoping review on the association between early childhood caries and life on land: The Sustainable Development Goal 15. PLoS ONE 2024, 19, e0304523. [Google Scholar] [CrossRef]
- Elshout, P.M.F.; van Zelm, R.; van der Velde, M.; Steinmann, Z.; Huijbregts, M.A.J. Global relative species loss due to first-generation biofuel production for the transport sector. Glob. Change Biol. Bioenergy 2019, 11, 763–772. [Google Scholar] [CrossRef]
- Cheesman, O.D. Environmental Impacts of Sugar Production—The Cultivation and Processing of Sugarcane and Sugar Beet; CABI Bioscience UK Centre: Egham, UK, 2004. [Google Scholar]
- Nuruzzaman, M.; Bahar, M.M.; Naidu, R. Diffuse soil pollution from agriculture: Impacts and remediation. Sci. Total. Environ. 2025, 962, 178398. [Google Scholar] [CrossRef] [PubMed]
- Freitas, I.B.F.; Duarte-Neto, P.J.; Sorigotto, L.R.; Yoshii, M.P.C.; Lopes, L.F.d.P.; Pereira, M.M.d.A.; Girotto, L.; Athayde, D.B.; Goulart, B.V.; Montagner, C.C.; et al. Effects of pasture intensification and sugarcane cultivation on non-target species: A realistic evaluation in pesticide-contaminated mesocosms. Sci. Total Environ. 2024, 922, 171425. [Google Scholar] [CrossRef] [PubMed]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- González-Valero, L.; Montiel-Company, J.M.; Bellot-Arcís, C.; Almerich-Torres, T.; Iranzo-Cortés, J.E.; Almerich-Silla, J.M.; Sanchez, Z.M. Association between passive tobacco exposure and caries in children and adolescents. A systematic review and meta-analysis. PLoS ONE 2018, 13, e0202497. [Google Scholar] [CrossRef]
- Aligne, C.A.; Moss, M.E.; Auinger, P.; Weitzman, M. Association of Pediatric Dental Caries with Passive Smoking. JAMA 2003, 289, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Avşar, A.; Darka, O.; Topaloğlu, B.; Bek, Y. Association of passive smoking with caries and related salivary biomarkers in young children. Arch. Oral Biol. 2008, 53, 969–974. [Google Scholar] [CrossRef]
- Hanioka, T.; Ojima, M.; Tanaka, K.; Yamamoto, M. Does Secondhand Smoke Affect the Development of Dental Caries in Children? A Systematic Review. Int. J. Environ. Res. Public Health 2011, 8, 1503–1519. [Google Scholar] [CrossRef]
- Nayani, A.A.; Iqbal, R.; Azam, S.I.; Khan, F.R.; Khan, A.H.; Janjua, N.; Hussain, A. Association between environmental tobacco smoke and dental caries amongst 5–14 years old children in Karachi, Pakistan. J. Pak. Med. Assoc. 2018, 68, 203–209. [Google Scholar] [PubMed]
- Shulman, J. Is There an Association between Low Birth Weight and Caries in the Primary Dentition? Caries Res. 2005, 39, 161–167. [Google Scholar] [CrossRef]
- Bernabé, E.; MacRitchie, H.; Longbottom, C.; Pitts, N.; Sabbah, W. Birth Weight, Breastfeeding, Maternal Smoking and Caries Trajectories. J. Dent. Res. 2016, 96, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Majorana, A.; Cagetti, M.G.; Bardellini, E.; Amadori, F.; Conti, G.; Strohmenger, L.; Campus, G. Feeding and smoking habits as cumulative risk factors for early childhood caries in toddlers, after adjustment for several behavioral determinants: A retrospective study. BMC Pediatr. 2014, 14, 45. [Google Scholar] [CrossRef]
- Nakayama, Y.; Mori, M. Association of environmental tobacco smoke and snacking habits with the risk of early childhood caries among 3-year-old Japanese children. J. Public Health Dent. 2015, 75, 157–162. [Google Scholar] [CrossRef]
- Plonka, K.; Pukallus, M.; Barnett, A.; Holcombe, T.; Walsh, L.; Seow, W. A Longitudinal Case-Control Study of Caries Development from Birth to 36 Months. Caries Res. 2012, 47, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Miyake, Y.; Sasaki, S. The Effect of Maternal Smoking during Pregnancy and Postnatal Household Smoking on Dental Caries in Young Children. J. Pediatr. 2009, 155, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Shinzawa, M.; Tokumasu, H.; Seto, K.; Tanaka, S.; Kawakami, K. Secondhand smoke and incidence of dental caries in deciduous teeth among children in Japan: Population based retrospective cohort study. BMJ 2015, 351, h5397, Erratum in BMJ 2015, 351, h6009. https://doi.org/10.1136/bmj.h6009; Erratum in BMJ 2015, 351, h6425. https://doi.org/10.1136/bmj.h6425. [Google Scholar] [CrossRef]
- Claudia, C.; Ju, X.; Mejia, G.; Jamieson, L. The relationship between maternal smoking during pregnancy and parental-reported experience of dental caries in Indigenous Australian children. Community Dent. Health 2016, 33, 297–302. [Google Scholar] [CrossRef]
- Iida, H.; Auinger, P.; Billings, R.J.; Weitzman, M. Association Between Infant Breastfeeding and Early Childhood Caries in the United States. Pediatrics 2007, 120, e944–e952. [Google Scholar] [CrossRef]
- Schroth, R.J.; Mittermuller, B.-A.; Au, W.; Hai-Santiago, K.; Martin, H.; Martens, P.; Brownell, M. Prenatal, Maternal, and Early Childhood Factors Associated with Dental General Anesthesia to Treat Severe Early Childhood Caries. Pediatr Dent. 2019, 41, 477–485. [Google Scholar] [PubMed]
- World Health Organization. Tobacco Free Initiative Consultative Report: International Consultation on Environmental Tobacco Smoke and Child Health, 1st ed.; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- Slayton, R.L. Exposure to Secondhand Smoke may Cause Dental Caries in Children. J. Evid. Based Dent. Pract. 2012, 12, 8–9. [Google Scholar] [CrossRef]
- Heikkinen, T.; Alvesalo, L.; Osborne, R.H.; Tienari, J. Maternal smoking and tooth formation in the foetus. III. Thin mandibular incisors and delayed motor development at 1 year of age. Early Hum. Dev. 1997, 47, 327–340. [Google Scholar] [CrossRef]
- Chowdhury, I.G.; Bromage, T.G. Effects of fetal exposure to nicotine on dental development of the laboratory rat. Anat Rec. 2000, 258, 397–405. [Google Scholar] [CrossRef]
- Lindemeyer, R.G.; Baum, R.H.; Hsu, S.C.; Going, R.E. In Vitro Effect of Tobacco on the Growth of Oral Cariogenic Streptococci. J. Am. Dent. Assoc. 1981, 103, 719–722. [Google Scholar] [CrossRef]
- Catala-Valentin, A.; Bernard, J.N.; Caldwell, M.; Maxson, J.; Moore, S.D.; Andl, C.D.; Kaspar, J.R. E-Cigarette Aerosol Exposure Favors the Growth and Colonization of Oral Streptococcus mutans Compared to Commensal Streptococci. Microbiol. Spectr. 2022, 10, e0242121. [Google Scholar] [CrossRef] [PubMed]
- Fadus, M.C.; Smith, T.T.; Squeglia, L.M. The rise of e-cigarettes, pod mod devices, and JUUL among youth: Factors influencing use, health implications, and downstream effects. Drug Alcohol Depend. 2019, 201, 85–93. [Google Scholar] [CrossRef]
- Flor, L.S.; Reitsma, M.B.; Gupta, V.; Ng, M.; Gakidou, E. The effects of tobacco control policies on global smoking prevalence. Nat. Med. 2021, 27, 239–243. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Report on the Global Tobacco Epidemic; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Office of the Surgeon General, Public Health Service, Centers for Disease Control, Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion. Reducing Tobacco Use: A Report of the Surgeon General; Office of the Surgeon General, Public Health Service: Atlanta, GA, USA, 2000.
- Jha, P.; Chaloupka, F.J. (Eds.) Tobacco Control in Developing Countries; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Jha, P.; Chaloupka, F.J. Curbing the Epidemic: Governments and the Economics of Tobacco Control; World Bank Publications: Washington, DC, USA, 1999. [Google Scholar]
- Chaloupka, F.J.; Powell, L.M. Health Taxes to Save Lives. Background Materials: Case Studies; Bloomberg Philanthropies: New York, NY, USA, 2019. [Google Scholar]
- International Agency for Research on Cancer. Effectiveness of Tax and Price Policies for Tobacco Control; The World Health Organization: Lyon, France, 2011. [Google Scholar]
- Guindon, G.E.; Paraje, G.R.; Chaloupka, F.J. The impact of prices and taxes on the use of tobacco products in Latin America and the Caribbean. Am. J. Public Health 2015, 105, e9–e19. [Google Scholar] [CrossRef] [PubMed]
- Goodchild, M.; Perucic, A.-M.; Nargis, N. Modelling the impact of raising tobacco taxes on public health and finance. Bull. World Health Organ. 2016, 94, 250–257. [Google Scholar] [CrossRef]
- Levy, D.T.; Tam, J.; Kuo, C.; Fong, G.T.; Chaloupka, F. The Impact of Implementing Tobacco Control Policies: The 2017 Tobacco Control Policy Scorecard. J. Public Health Manag. Pract. 2018, 24, 448–457. [Google Scholar] [CrossRef]
- Pickett, M.S.; Schober, S.E.; Brody, D.J.; Curtin, L.R.; Giovino, G.A. Smoke-free laws and secondhand smoke exposure in US non-smoking adults, 1999–2002. Tob. Control 2006, 15, 302–307. [Google Scholar] [CrossRef]
- Stead, M.; Moodie, C.; Angus, K.; Bauld, L.; McNeill, A.; Thomas, J.; Hastings, G.; Hinds, K.; O’MAra-Eves, A.; Kwan, I.; et al. Is Consumer Response to Plain/Standardised Tobacco Packaging Consistent with Framework Convention on Tobacco Control Guidelines? A Systematic Review of Quantitative Studies. PLoS ONE 2013, 8, e75919. [Google Scholar] [CrossRef]
- Hartmann-Boyce, J.; Chepkin, S.C.; Ye, W.; Bullen, C.; Lancaster, T.; Cochrane Tobacco Addiction Group. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst. Rev. 2018, 5, CD000146. [Google Scholar] [CrossRef] [PubMed]
- Hammond, D.; Wakefield, M.; Durkin, S.; Brennan, E. Tobacco Packaging and Mass Media Campaigns: Research Needs for Articles 11 and 12 of the WHO Framework Convention on Tobacco Control. Nicotine Tob. Res. 2013, 15, 817–831. [Google Scholar] [CrossRef]
- Muggli, M.E.; Zheng, A.; Liberman, J.; Coxon, N.; Candler, L.; Donley, K.; Lambert, P. Tracking the relevance of the WHO Framework Convention on Tobacco Control in legislation and litigation through the online resource, Tobacco Control Laws. Tob. Control 2014, 23, 457–460. [Google Scholar] [CrossRef] [PubMed]
- McCabe Centre for Law and Cancer, Campaign for Tobacco-Free Kids. Report on WHO FCTC in Legislation and Litigation. 2015. Available online: https://fctc.who.int/docs/librariesprovider12/meeting-reports/fctc-in-legislation-and-litigation-mccabe-centre_tfk.pdf (accessed on 4 September 2025).
- Antin, T.M.J.; Lipperman-Kreda, S.; Hunt, G. Tobacco Denormalization as a Public Health Strategy: Implications for Sexual and Gender Minorities. Am. J. Public Health 2015, 105, 2426–2429. [Google Scholar] [CrossRef]
- Farsalinos, K. Electronic cigarettes: An aid in smoking cessation, or a new health hazard? Ther. Adv. Respir. Dis. 2018, 12, 1753465817744960. [Google Scholar] [CrossRef]
- Dai, X.; Gakidou, E.; Lopez, A.D. Evolution of the global smoking epidemic over the past half century: Strengthening the evidence base for policy action. Tob. Control 2022, 31, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Amul, G.G.H.; Tan, G.P.P.; van der Eijk, Y. A Systematic Review of Tobacco Industry Tactics in Southeast Asia: Lessons for Other Low- and Middle Income Regions. Int. J. Health Policy Manag. 2021, 10, 324–337. [Google Scholar] [CrossRef]
- Nguyen-Grozavu, F.T.; Pierce, J.P.; Sakuma, K.-L.K.; Leas, E.C.; McMenamin, S.B.; Kealey, S.; Benmarhnia, T.; Emery, S.L.; White, M.M.; Fagan, P.; et al. Widening disparities in cigarette smoking by race/ethnicity across education level in the United States. Prev. Med. 2020, 139, 106220. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Jia, J.; Li, C.; Zhao, C.; Li, T.; Shi, H.; Zhang, X. Relationship between preterm, low birth weight and early childhood caries: A meta-analysis of the case–control and cross-sectional study. Biosci. Rep. 2020, 40, BSR20200870. [Google Scholar] [CrossRef]
- Stewart, D.J. Teeth as indicators of exposure of children to lead. Arch. Dis. Child. 1974, 49, 895–897. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tapalaga, G.; Stanga, L.; Sîrbu, I. Systematic Review of Lead Exposure and Its Effects on Caries and Aesthetics in Children and Adolescents. Healthcare 2025, 13, 1460. [Google Scholar] [CrossRef]
- Moss, M.E.; Lanphear, B.P.; Auinger, P. Association of Dental Caries and Blood Lead Levels. JAMA 1999, 281, 2294–2298. [Google Scholar] [CrossRef]
- Wiener, R.C.; Long, D.L.; Jurevic, R.J. Blood Levels of the Heavy Metal, Lead, and Caries in Children Aged 24–72 Months: NHANES III. Caries Res. 2015, 49, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.R.; Moss, M.E.; Raubertas, R.F. The association between caries and childhood lead exposure. Environ. Health Perspect. 2000, 108, 1099–1102. [Google Scholar] [CrossRef]
- Youravong, N.; Chongsuvivatwong, V.; Geater, A.F.; Dahlén, G.; Teanpaisan, R. Lead associated caries development in children living in a lead contaminated area, Thailand. Sci. Total Environ. 2006, 361, 88–96. [Google Scholar] [CrossRef]
- Martin, M.D.; Benton, T.; Bernardo, M.; Woods, J.S.; Townes, B.D.; Luis, H.; Leitão, J.; Rosenbaum, G.; Castro-Caldas, A.; Pavão, I.; et al. The association of dental caries with blood lead in children when adjusted for IQ and neurobehavioral performance. Sci. Total Environ. 2007, 377, 159–164. [Google Scholar] [CrossRef]
- Gemmel, A.; Tavares, M.; Alperin, S.; Soncini, J.; Daniel, D.; Dunn, J.; Crawford, S.; Braveman, N.; Clarkson, T.W.; McKinlay, S.; et al. Blood lead level and dental caries in school-age children. Environ. Health Perspect. 2002, 110, A625–A630. [Google Scholar] [CrossRef]
- Youravong, N.; Teanpaisan, R.; Chongsuvivatwong, V. Salivary lead in relation to caries, salivary factors and cariogenic bacteria in children. Int. Dent. J. 2013, 63, 123–129. [Google Scholar] [CrossRef]
- Gomes, V.E.; Wada, R.S.; Cury, J.A.; Sousa, M.L. Lead Level, Enamel Defects and Dental Caries in Deciduous Teeth. Rev. Saude Publica 2004, 38, 716–722. [Google Scholar] [CrossRef]
- Alomary, A.; Al-Momani, I.F.; Obeidat, S.M.; Massadeh, A.M. Levels of lead, cadmium, copper, iron, and zinc in deciduous teeth of children living in Irbid, Jordan by ICP-OES: Some factors affecting their concentrations. Environ. Monit. Assess. 2013, 185, 3283–3295. [Google Scholar] [CrossRef] [PubMed]
- Motevasselian, F.; Abdi, K.; Ghodarati, H.; Shamshiri, A.R.; Lippert, F.; Hessari, H. The role of lead and cadmium in deciduous teeth and saliva on dental caries in children residing in Tehran, Iran. J. Trace Elements Med. Biol. 2023, 79, 127209. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.P.; Hegde, A. Lead Exposure and its Relation to Dental Caries in Children. J. Clin. Pediatr. Dent. 2013, 38, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Ha, M.; Kwon, H.-J.; Kim, H.-Y.; Choi, Y.-H. Association between Low blood lead levels and increased risk of dental caries in children: A cross-sectional study. BMC Oral Health 2017, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- Garot, E.; Vieira, A.R.; Manton, D.J. Molar Incisor Hypomineralisation as a Dental Caries Risk Factor—Is It True? Monogr. Oral Sci. 2024, 32, 166–172. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Bercovitz, K.; Laufer, D. Carious teeth as indicators to lead exposure. Bull. Environ. Contam. Toxicol. 1993, 50, 724–729. [Google Scholar] [CrossRef]
- Tvinnereim, H.M.; Eide, R.; Riise, T. Heavy metals in human primary teeth: Some factors influencing the metal concentrations. Sci. Total. Environ. 2000, 255, 21–27. [Google Scholar] [CrossRef]
- Yepes, J.F.; McCormick-Norris, J.; Vinson, L.A.; Eckert, G.J.; Hu, H.; Wu, Y.; Jansen, E.C.; Peterson, K.E.; Téllez-Rojo, M.M.; Mier, E.A.M. Blood levels of lead and dental caries in permanent teeth. J. Public Health Dent. 2020, 80, 297–303. [Google Scholar] [CrossRef]
- Angrand, R.C.; Collins, G.; Landrigan, P.J.; Thomas, V.M. Relation of blood lead levels and lead in gasoline: An updated systematic review. Environ. Health 2022, 21, 138. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Update on the Global Status of Legal Limits on Lead in Paint, December 2021; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- QIMA. Lead in Toys: Regulations Governing Lead in Children’s Products. 14 March 2025. Available online: https://blog.qima.com/lab-testing/lead-in-toys-regulations-guide (accessed on 17 April 2025).
- Ettinger, A.S.S.; Leonard, M.L.M.; Mason, J. CDC’s Lead Poisoning Prevention Program: A Long-standing Responsibility and Commitment to Protect Children from Lead Exposure. J. Public Health Manag. Pract. 2019, 25 (Suppl. S1), S5–S12. [Google Scholar] [CrossRef]
- Dobrescu, A.-I.; Ebenberger, A.; Harlfinger, J.; Griebler, U.; Klerings, I.; Nußbaumer-Streit, B.; Chapman, A.; Affengruber, L.; Gartlehner, G. Effectiveness of interventions for the remediation of lead-contaminated soil to prevent or reduce lead exposure—A systematic review. Sci. Total. Environ. 2022, 806 Pt 1, 150480. [Google Scholar] [CrossRef]
- Rees, N.; Fuller, R. The Toxic Truth: Children’s Exposure to Lead Pollution Undermines a Generation of Future Potential. 2020. Available online: https://www.unicef.org/reports/toxic-truth-childrens-exposure-to-lead-pollution-2020 (accessed on 14 April 2025).
- GiveWell. Pure Earth—Support for Reducing Lead Exposure in Low- and Middle-Income Countries. December 2022. Available online: https://www.givewell.org/research/incubation-grants/Pure-Earth-lead-exposure-July-2021#:~:text=Summary,the%20effect%20of%20the%20interventions (accessed on 17 April 2025).
- Kordas, K.; Ravenscroft, J.; Cao, Y.; McLean, E.V. Lead Exposure in Low and Middle-Income Countries: Perspectives and Lessons on Patterns, Injustices, Economics, and Politics. Int. J. Environ. Res. Public Health 2018, 15, 2351. [Google Scholar] [CrossRef]
- Larsen, B.; Sánchez-Triana, E. Global health burden and cost of lead exposure in children and adults: A health impact and economic modelling analysis. Lancet Planet. Health 2023, 7, e831–e840. [Google Scholar] [CrossRef]
- Skutlarek, D.; Exner, M.; Färber, H. Perfluorinated Surfactants in Surface and Drinking Waters. Environ. Sci. Pollut. Res. 2006, 13, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Brendel, S.; Fetter, É.; Staude, C.; Vierke, L.; Biegel-Engler, A. Short-chain perfluoroalkyl acids: Environmental concerns and a regulatory strategy under REACH. Environ. Sci. Eur. 2018, 30, 9. [Google Scholar] [CrossRef]
- Harrad, S.; Wemken, N.; Drage, D.S.; Abdallah, M.A.-E.; Coggins, A.-M. Perfluoroalkyl Substances in Drinking Water, Indoor Air and Dust from Ireland: Implications for Human Exposure. Environ. Sci. Technol. 2019, 53, 13449–13457. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Y.; Wen, L.-L.; Su, T.-C.; Chen, P.-C.; Lin, C.-Y. Negative Association Between Serum Perfluorooctane Sulfate Concentration and Bone Mineral Density in US Premenopausal Women: NHANES, 2005–2008. J. Clin. Endocrinol. Metab. 2014, 99, 2173–2180. [Google Scholar] [CrossRef]
- White, S.S.; Fenton, S.E.; Hines, E.P. Endocrine disrupting properties of perfluorooctanoic acid. J. Steroid Biochem. Mol. Biol. 2011, 127, 16–26. [Google Scholar] [CrossRef]
- Bonato, M.; Corrà, F.; Bellio, M.; Guidolin, L.; Tallandini, L.; Irato, P.; Santovito, G. PFAS Environmental Pollution and Antioxidant Responses: An Overview of the Impact on Human Field. Int. J. Environ. Res. Public Health 2020, 17, 8020. [Google Scholar] [CrossRef]
- Vital, S.O.; Gaucher, C.; Bardet, C.; Rowe, P.; George, A.; Linglart, A.; Chaussain, C. Tooth dentin defects reflect genetic disorders affecting bone mineralization. Bone 2012, 50, 989–997. [Google Scholar] [CrossRef]
- Beriashvili, S.; Nikolaishvili, M.; Mantskava, M.; Momtsemlidze, N.; Franchuk, K. Changes in Tooth Hard Tissue Minerali-Zation and Blood Rheology in Healthy Adolescents and Those with Thyroid Dysfunction. Georgian Med. News 2016, 28–34. [Google Scholar] [PubMed]
- Folayan, M.O.; Alade, M.; Adeniyi, A.; El Tantawi, M.; Finlayson, T.L. Association between developmental dental anomalies, early childhood caries and oral hygiene status of 3–5-year-old children in Ile-Ife, Nigeria. BMC Oral Health 2019, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Hekster, F.M.; Laane, R.W.P.M.; de Voogt, P. Environmental and toxicity effects of perfluoroalkylated substances. Rev. Environ. Contam. Toxicol. 2003, 179, 99–121. [Google Scholar] [CrossRef]
- Cunha-Cruz, J.; Scott, J.; Rothen, M.; Mancl, L.; Lawhorn, T.; Brossel, K.; Berg, J. Northwest Practice-based REsearch Collaborative in Evidence-based DENTistry. Salivary characteristics and dental caries: Evidence from general dental practices. J. Am. Dent. Assoc. 2013, 144, e31–e40. [Google Scholar] [CrossRef]
- Ramesh, N.P.; Arora, M.; Braun, J.M. Cross-sectional study of the association between serum perfluorinated alkyl acid concentrations and dental caries among US adolescents (NHANES 1999–2012). BMJ Open 2019, 9, e024189. [Google Scholar] [CrossRef]
- Anamaria, B.; Chibelean, M.; Pacurar, M.; Esian, D.; Bud, E. Correlation between BMI, dental caries, and salivary buffer capacity in a sample of children from Mures County, Romania. Eur. Sci. J. 2015, 11, 38–46. [Google Scholar]
- World Health Organization. Water Factsheet—Africa. 2023. Available online: https://www.afro.who.int/health-topics/water (accessed on 15 November 2023).
- Folayan, M.O.; El Tantawi, M.; Schroth, R.J.; Kemoli, A.M.; Gaffar, B.; Amalia, R.; Feldens, C.A. ECCAG Association Between Environmental Health, Ecosystem Vitality, and Early Childhood Caries. Front. Pediatr. 2020, 8, 196. [Google Scholar] [CrossRef]
- Holling, C.S. Engineering resilience versus ecological resilience. In Engineering Within Ecological Constraints; Shulze, P., Ed.; National Academy Press: Washington, DC, USA, 1996; pp. 31–44. [Google Scholar]
- Owino, V.; Kumwenda, C.; Ekesa, B.; Parker, M.E.; Ewoldt, L.; Roos, N.; Lee, W.T.; Tome, D. The impact of climate change on food systems, diet quality, nutrition, and health outcomes: A narrative review. Front. Clim. 2022, 4, 941842. [Google Scholar] [CrossRef]
- Jenssen, B.M. Endocrine-Disrupting Chemicals and Climate Change: A Worst-Case Combination for Arctic Marine Mammals and Seabirds? Environ. Health Perspect. 2006, 114 (Suppl. S1), 76–80. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Tang, M.; Cai, Y.; Wang, P. Association between exposure to environmental pollutants and increased oral health risks, a comprehensive review. Front. Public Health 2025, 12, 1482991. [Google Scholar] [CrossRef]
- Vo, T.T.T.; Wu, C.-Z.; Lee, I.-T. Potential effects of noxious chemical-containing fine particulate matter on oral health through reactive oxygen species-mediated oxidative stress: Promising clues. Biochem. Pharmacol. 2020, 182, 114286. [Google Scholar] [CrossRef]
- Kemoli, A.M. Paediatric oral health and climate change. Edorium. J. Dent. 2019, 7, 100034D01AK2019. [Google Scholar] [CrossRef]
- Bhardwaj, R.L.; Parashar, A.; Parewa, H.P.; Vyas, L. An Alarming Decline in the Nutritional Quality of Foods: The Biggest Challenge for Future Generations’ Health. Foods 2024, 13, 877. [Google Scholar] [CrossRef]
- El Chami, D.; Daccache, A.; El Moujabber, M. What are the impacts of sugarcane production on ecosystem services and human well-being? A review. Ann. Agric. Sci. 2020, 65, 188–199. [Google Scholar] [CrossRef]
- Davis, M. Impact of Pollution on Marginalized Communities: A Case Study of Omaha, Nebraska. University of Nebras-ka-Lincoln. 2024. Available online: https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1375&context=envstudtheses#:~:text=The%20findings%20reveal%20stark%20disparities,areas%20with%20majority%20white%20communities (accessed on 16 April 2025).
- Northridge, M.E.; Kumar, A.; Kaur, R. Disparities in Access to Oral Health Care. Annu. Rev. Public Health 2020, 41, 513–535. [Google Scholar] [CrossRef] [PubMed]
- Kalra, G.; Nangia, T.; Kumar, Y.; Pal, Y. Assessing the Impact of Climate Change on Early Childhood Caries Within the Framework of Sustainable Developmental Goal 13: A Scoping Review. Cureus 2024, 16, e71872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, A.; Liu, L.; Zeng, Y.; Liu, R.; Ma, Z.; Liu, M.; Bi, J.; Ji, J.S. Assessing the effects of ultraviolet radiation, residential greenness and air pollution on vitamin D levels: A longitudinal cohort study in China. Environ. Int. 2022, 169, 107523. [Google Scholar] [CrossRef]
- Manrique, A.; Clarke, K.; Bisesi, S.; Arosemena, F.A.; Coker, E.S.; Sabo-Attwood, T. The Adverse Health Effects of Air Pollution from Sugarcane Burning: A Scoping Review of Observational and Experimental Evidence. Environ. Health Perspect. 2025, 133, 16002. [Google Scholar] [CrossRef]
- Schroth, R.J.; Levi, J.A.; Sellers, E.A.; Friel, J.; Kliewer, E.; Moffatt, M.E. Vitamin D status of children with severe early childhood caries: A case–control study. BMC Pediatr. 2013, 13, 174. [Google Scholar] [CrossRef]
- Schroth, J.; Jeal, S.; Kliewer, E.; Sellers, A.C. The Relationship Between Vitamin D and Severe Early Childhood Caries: A Pilot Study. Int. J. Vitam. Nutr. Res. 2012, 82, 53–62. [Google Scholar] [CrossRef]
- Durá-Travé, T.; Gallinas-Victoriano, F. Dental caries in children and vitamin D deficiency: A narrative review. Eur. J. Pediatr. 2023, 183, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Hoseinzadeh, E.; Taha, P.; Wei, C.; Godini, H.; Ashraf, G.M.; Taghavi, M.; Miri, M. The impact of air pollutants, UV exposure and geographic location on vitamin D deficiency. Food Chem. Toxicol. 2018, 113, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Lodovici, M.; Bigagli, E. Oxidative Stress and Air Pollution Exposure. J. Toxicol. 2011, 2011, 487074. [Google Scholar] [CrossRef]
- Ji, S.; Zhao, K.; Ma, L.; Chen, X.; Zheng, D.; Lu, Y. The Association Between Vitamin D and Early Childhood Caries: A Systematic Review and Meta-Analysis. Oral Health Prev. Dent. 2024, 22, 63–72. [Google Scholar] [CrossRef]
- Dakó, T.; Lazăr, A.-P.; Lazăr, L.; Stoica, A.-M.; Crișan, A.-S.; Monea, M.; Bica, C.-I. The Role of Oxidative Stress-Related Gene Polymorphisms (SOD2, GPX1) in Severe Early Childhood Caries (S-ECC). Medicina 2025, 61, 432. [Google Scholar] [CrossRef] [PubMed]
- El Tantawi, M.; Folayan, M.O.; Mehaina, M.; Vukovic, A.; Castillo, J.L.; Gaffar, B.O.; Arheiam, A.; Al-Batayneh, O.B.; Kemoli, A.M.; Schroth, R.J.; et al. Prevalence and Data Availability of Early Childhood Caries in 193 United Nations Countries, 2007–2017. Am. J. Public Health 2018, 108, 1066–1072. [Google Scholar] [CrossRef]
- Folayan, M.O.; El Tantawi, M.; Virtanen, J.I.; Feldens, C.A.; Rashwan, M.; Kemoli, A.M.; Villena, R.; Al-Batayneh, O.B.; Amalia, R.; Gaffar, B.; et al. An ecological study on the association between universal health service coverage index, health expenditures, and early childhood caries. BMC Oral Health 2021, 21, 126, Erratum in BMC Oral Health 2021, 21, 278. https://doi.org/10.1186/s12903-021-01624-x. [Google Scholar] [CrossRef]
- Marmot, M.; Friel, S.; Bell, R.; Houweling, T.A.; Taylor, S.; on behalf of the Commission on Social Determinants of Health. Closing the gap in a generation: Health equity through action on the social determinants of health. Lancet 2008, 372, 1661–1669. [Google Scholar] [CrossRef]
- Ramos-Gomez, F. Understanding oral health disparities in the context of social justice, health equity, and children’s human rights. J. Am. Dent. Assoc. 2019, 150, 898–900. [Google Scholar] [CrossRef]
- Folayan, M.O.; El Tantawi, M.; Vukovic, A.; Schroth, R.J.; Alade, M.; Mohebbi, S.Z.; Al-Batayneh, O.B.; Arheiam, A.; Amalia, R.; Gaffar, B.; et al. Governance, maternal well-being and early childhood caries in 3–5-year-old children. BMC Oral Health 2020, 20, 166. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-H. Development and Implementation of the Green Infrastructure Paradigm—Based on the Korean River Restoration Projects. Keynote Paper. In Proceedings of the 2012 World Congress on Advances in Civil, Environmental, and Materials Research (ACEM’ 12), Seoul, Republic of Korea, 26–30 August 2012; Available online: http://www.i-asem.org/publication_conf/acem12/Keynote-01.pdf#:~:text=For%20example%2C%20complementary%20investment%20in%20degrading%20infrastructure,of%20the%20economy%20and%20accelerate%20economic%20recession (accessed on 18 May 2025).
- Alfonzo, L.F.; Bentley, R.; Singh, A. Home ownership, income and oral health of children in Australia—A population-based study. Community Dent. Oral Epidemiol. 2022, 50, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.; Splieth, C.; Matanhire-Zihanzu, C.; Glick, M. Advancing the concept of global oral health to strengthen actions for planetary health and One Health. Int. J. Equity Health 2024, 23, 71. [Google Scholar] [CrossRef]
- Danasekaran, R. One Health: A Holistic Approach to Tackling Global Health Issues. Indian J. Community Med. 2024, 49, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Ladeira, C.; Frazzoli, C.; Orisakwe, O.E. Engaging One Health for Non-Communicable Diseases in Africa: Perspective for Mycotoxins. Front. Public Health 2017, 5, 266. [Google Scholar] [CrossRef]
- Masters, B.; Rohde, K.; Gurner, N.; Reid, D. Reducing the risk of herbicide runoff in sugarcane farming through controlled traffic and early-banded application. Agric. Ecosyst. Environ. 2013, 180, 29–39. [Google Scholar] [CrossRef]
- Bernabe, E.; Marcenes, W.; Abdulkader, R.S.; Abreu, L.G.; Afzal, S.; Alhalaiqa, F.N.; Al-Maweri, S.; Alsharif, U.; Anyasodor, A.E.; Arora, A.; et al. Trends in the global, regional, and national burden of oral conditions from 1990 to 2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2025, 405, 897–910. [Google Scholar] [CrossRef]
- Rappaport, S.M. Implications of the exposome for exposure science. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 5–9. [Google Scholar] [CrossRef]
- Cobbinah, P.B. City in Africa II: Urban environmental health. J. Urban Aff. 2023, 45, 483–487. [Google Scholar] [CrossRef]
- Bellizzi, S.; Lane, C.; Elhakim, M.; Nabeth, P. Health consequences of drought in the WHO Eastern Mediterranean Region: Hotspot areas and needed actions. Environ. Health 2020, 19, 114. [Google Scholar] [CrossRef] [PubMed]
- Maklennan, A.; Borg-Bartolo, R.; Wierichs, R.J.; Esteves-Oliveira, M.; Campus, G. A systematic review and meta-analysis on early-childhood-caries global data. BMC Oral Health 2024, 24, 835. [Google Scholar] [CrossRef] [PubMed]
| Exposure | Direct Biological Pathway | Indirect Social Pathway | Key Supporting Studies |
|---|---|---|---|
| Tobacco smoke | Enamel hypoplasia, decreased saliva | Maternal smoking → increased sugar use | [9,34] |
| Lead | Disrupted Ca2+ metabolism, increased biofilm virulence | Limited access to care in polluted areas | [80,86] |
| PFAAs | Thyroid disruption → enamel defects | Contaminated water → no fluoride |
| Exposure | Evidence Strength | Key Supporting Studies | Major Limitations |
|---|---|---|---|
| Tobacco Smoke | High (consistent meta-analyses) | [9,28,38] | Residual confounding (e.g., socioeconomic status) may inflate risk estimates. |
| Lead | Moderate (dose-response with some mechanistic gaps) | [80,86] | Inconsistent enamel defect data; mechanistic uncertainty |
| PM2.5 Vitamin D | Moderate (epidemiological and biological evidence) | [121,128,129,130] | Confounding by socioeconomic status; difficulty isolating independent effects |
| PFAAs | Low (mechanistic plausibility, limited human data) | [104,110] | Scarcity of longitudinal human studies; reliance on in vitro evidence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foláyan, M.O.; Schroth, R.J.; Olatosi, O.; El Tantawi, M. Environmental Determinants of Early Childhood Caries: A Narrative Synthesis of Observational Evidence and Implications for Global Policy. Dent. J. 2025, 13, 484. https://doi.org/10.3390/dj13110484
Foláyan MO, Schroth RJ, Olatosi O, El Tantawi M. Environmental Determinants of Early Childhood Caries: A Narrative Synthesis of Observational Evidence and Implications for Global Policy. Dentistry Journal. 2025; 13(11):484. https://doi.org/10.3390/dj13110484
Chicago/Turabian StyleFoláyan, Moréniké Oluwátóyìn, Robert J. Schroth, Olubukola Olatosi, and Maha El Tantawi. 2025. "Environmental Determinants of Early Childhood Caries: A Narrative Synthesis of Observational Evidence and Implications for Global Policy" Dentistry Journal 13, no. 11: 484. https://doi.org/10.3390/dj13110484
APA StyleFoláyan, M. O., Schroth, R. J., Olatosi, O., & El Tantawi, M. (2025). Environmental Determinants of Early Childhood Caries: A Narrative Synthesis of Observational Evidence and Implications for Global Policy. Dentistry Journal, 13(11), 484. https://doi.org/10.3390/dj13110484









