From Break-Even Point to Dynamic Regenerative Balance: A Conceptual and Quantitative Framework Based on Preclinical Rabbit Sinus Lift Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of the Articles
2.2. Biomaterials Evaluated
2.3. Break-Even Point Calculation
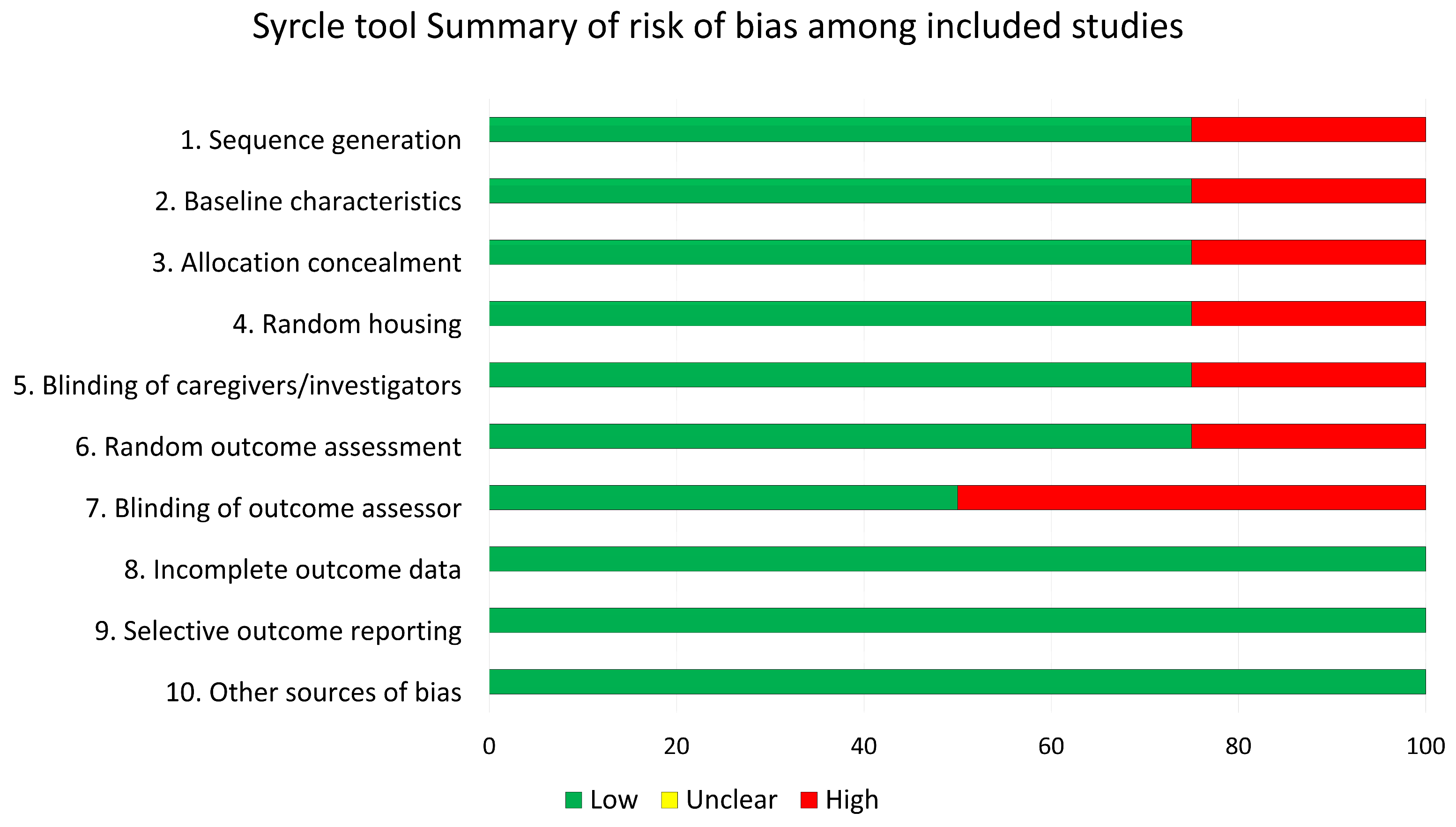
2.4. Risk of Bias Assessment
3. Results
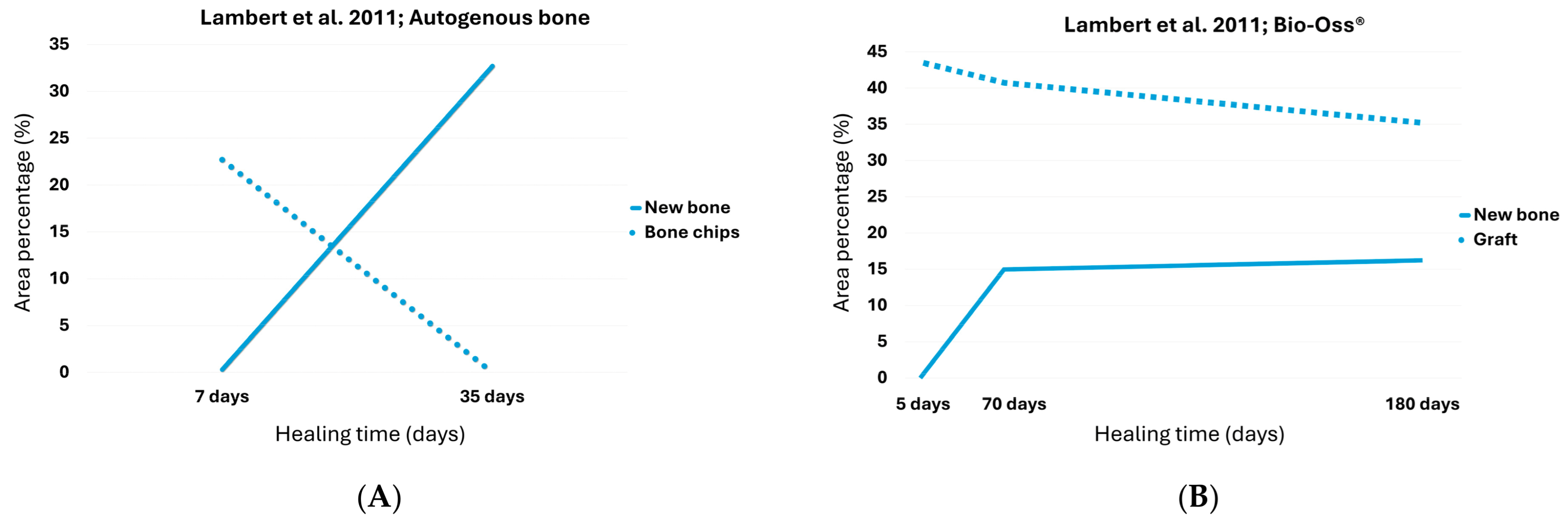
3.1. Autogenous Bone Chips
3.2. Bio-Oss®
3.3. Bio-Oss Collagen®
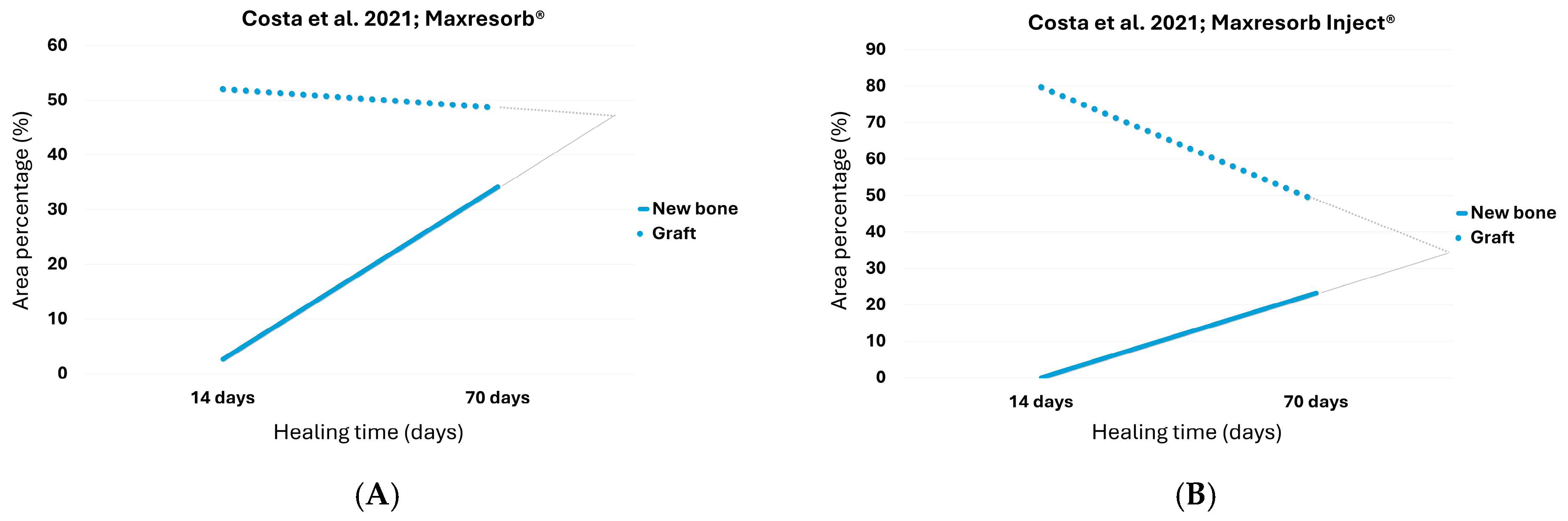
3.4. Maxresorb®
3.5. Maxresorb® Inject
3.6. Gen-Os®
3.7. Risk of Bias Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brånemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindström, J.; Hallén, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surg. Suppl. 1977, 16, 1–132. [Google Scholar]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10 (Suppl. S2), S96–S101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Telleman, G.; Albrektsson, T.; Hoffman, M.; Johansson, C.B.; Vissink, A.; Meijer, H.J.; Raghoebar, G.M. Peri-implant endosseous healing properties of dual acid-etched mini-implants with a nanometer-sized deposition of CaP: A histological and histomorphometric human study. Clin. Implant Dent. Relat. Res. 2010, 12, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Donos, N.; Hamlet, S.; Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Bosshardt, D.D.; Ivanovski, S. Gene expression profile of osseointegration of a hydrophilic compared with a hydrophobic microrough implant surface. Clin. Oral Implant. Res. 2011, 22, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Sul, Y.T.; Kang, B.S.; Johansson, C.; Um, H.S.; Park, C.J.; Albrektsson, T. The roles of surface chemistry and topography in the strength and rate of osseointegration of titanium implants in bone. J. Biomed. Mater. Res. A 2009, 89, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Chrcanovic, B.; Mölne, J.; Wennerberg, A. Foreign body reactions, marginal bone loss and allergies in relation to titanium implants. Eur. J. Oral Implantol. 2018, 11 (Suppl. S1), S37–S46. [Google Scholar] [PubMed]
- Trindade, R.; Albrektsson, T.; Galli, S.; Prgomet, Z.; Tengvall, P.; Wennerberg, A. Osseointegration and foreign body reaction: Titanium implants activate the immune system and suppress bone resorption during the first 4 weeks after implantation. Clin. Implant Dent. Relat. Res. 2018, 20, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Trindade, R.; Albrektsson, T.; Wennerberg, A. Current concepts for the biological basis of dental implants: Foreign body equilibrium and osseointegration dynamics. Oral Maxillofac. Surg. Clin. N. Am. 2015, 27, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Trindade, R.; Albrektsson, T.; Tengvall, P.; Wennerberg, A. Foreign Body Reaction to Biomaterials: On Mechanisms for Buildup and Breakdown of Osseointegration. Clin. Implant Dent. Relat. Res. 2016, 18, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20 (Suppl. S4), 172–184. [Google Scholar] [CrossRef] [PubMed]
- Saini, P.; Sood, S.; Chahal, G.S.; Jain, A. Evaluation of bone apposition on surface modified titanium implant in experimental animal model: A systematic review and meta-analysis. J. Indian Soc. Periodontol. 2024, 28, 43–74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berglundh, T.; Abrahamsson, I.; Lang, N.P.; Lindhe, J. De novo alveolar bone formation adjacent to endosseous implants. Clin. Oral Implant. Res. 2003, 14, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, I.; Berglundh, T.; Linder, E.; Lang, N.P.; Lindhe, J. Early bone formation adjacent to rough and turned endosseous implant surfaces. An experimental study in the dog. Clin. Oral Implant. Res. 2004, 15, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Lang, N.P. The role of bone debris in early healing adjacent to hydrophilic and hydrophobic implant surfaces in man. Clin. Oral Implant. Res. 2011, 22, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin. Oral Implant. Res. 2011, 22, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Galletti, F.; D’Angelo, T.; Fiorillo, L.; Lo Giudice, P.; Irrera, N.; Rizzo, G.; Cervino, G. Micro-CT Structure Analysis on Dental Implants: Preliminary In Vitro Trial. Prosthesis 2024, 6, 1437–1447. [Google Scholar] [CrossRef]
- Stocchero, M.; Toia, M.; Cecchinato, D.; Becktor, J.P.; Coelho, P.G.; Jimbo, R. Biomechanical, Biologic, and Clinical Outcomes of Undersized Implant Surgical Preparation: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2016, 31, 1247–1263. [Google Scholar] [CrossRef] [PubMed]
- Rues, S.; Schmitter, M.; Kappel, S.; Sonntag, R.; Kretzer, J.P.; Nadorf, J. Effect of bone quality and quantity on the primary stability of dental implants in a simulated bicortical placement. Clin. Oral Investig. 2021, 25, 1265–1272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pesce, P.; Del Fabbro, M.; Modenese, L.; Sandron, S.; Francetti, L.; Isola, G.; Canullo, L.; Menini, M. Influence of implant diameter on implant survival rate and clinical outcomes in the posterior area: A systematic review and meta-analysis. BMC Oral Health 2023, 23, 235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raghavendra, S.; Wood, M.C.; Taylor, T.D. Early wound healing around endosseous implants: A review of the literature. Int. J. Oral Maxillofac. Implant. 2005, 20, 425–431. [Google Scholar] [PubMed]
- Pieralli, S.; Kohal, R.J.; Lopez Hernandez, E.; Doerken, S.; Spies, B.C. Osseointegration of zirconia dental implants in animal investigations: A systematic review and meta-analysis. Dent. Mater. 2018, 34, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.E.; Dietsh-Misch, F.; Hoar, J.; Beck, G.; Hazen, R.; Misch, C.M. A bone quality-based implant system: First year of prosthetic loading. J. Oral Implantol. 1999, 25, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Mistretta, F.; Magnini, A.; Cinci, L.; Zanobini, P.; Pisano, M.; Barcali, E.; Bocchi, L.; Nardi, C. A systematic review and meta-analysis on the concept of bone quality in dento-maxillofacial Cone Beam Computed Tomography. Radiol. Med. 2025, 130, 1193–1206. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Fujita, H.; Kanou, M.; Takahashi-Nakagawa, Y.; Nakajima, Y.; Sunano, A.; Kimura, Y.; Ueno, T. Rapid and Easy Histological Evaluation of Alveolar Human Bone Quality at Dental Implant Sites Using a Nondecalcified Frozen Cryofilm Section Technique: A Technical Report. Implant Dent. 2015, 24, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, D.; Lang, N.P. Dynamics of osseointegration in various human and animal models—A comparative analysis. Clin. Oral Implant. Res. 2017, 28, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Amari, Y.; Ohnishi, H.; Seo, H.; Chi, Y.C.; Botticelli, D.; Xavier, S.P.; Baba, S. Application of the break-even point to express the bone dynamics around implants. Oral Maxillofac. Surg. 2024, 28, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Caneva, M.; Lang, N.P.; Garcia Rangel, I.J.; Ferreira, S.; Caneva, M.; De Santis, E.; Botticelli, D. Sinus mucosa elevation using Bio-Oss® or Gingistat® collagen sponge: An experimental study in rabbits. Clin. Oral Implant. Res. 2017, 28, e21–e30. [Google Scholar] [CrossRef] [PubMed]
- Lambert, F.; Léonard, A.; Drion, P.; Sourice, S.; Layrolle, P.; Rompen, E. Influence of space-filling materials in subantral bone augmentation: Blood clot vs. autogenous bone chips vs. bovine hydroxyapatite. Clin. Oral Implant. Res. 2011, 22, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Yamada, R.; Xavier, S.P.; Nakajima, Y.; Silva, E.R.; Botticelli, D.; Teranishi, Y.; Baba, S. Impact of Collagenated and Non-Collagenated Deproteinized Bovine Bone Mineral on Schneiderian Membrane Integrity in Rabbits. Dent. J. 2025, 13, 19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Costa, M.M.; Botticelli, D.; Moses, O.; Omori, Y.; Fujiwara, S.; Silva, E.R.; Xavier, S.P. Maxillary Sinus Augmentation Using Ceramic Alloplastic Granules or Paste: An Experimental Study in Rabbits. Dent. J. 2021, 9, 65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iida, T.; Carneiro Martins Neto, E.; Botticelli, D.; Apaza Alccayhuaman, K.A.; Lang, N.P.; Xavier, S.P. Influence of a collagen membrane positioned subjacent the sinus mucosa following the elevation of the maxillary sinus. A histomorphometric study in rabbits. Clin. Oral Implant. Res. 2017, 28, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.; Henriques, J.; Martins, G.; Guerra, F.; Judas, F.; Figueiredo, H. Physicochemical characterization of biomaterials commonly used in dentistry as bone substitutes--comparison with human bone. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.G.; Awartani, F.A.; Niazy, A.A.; Jansen, J.A.; Alghamdi, H.S. A Combination of Biphasic Calcium Phosphate (Maxresorb®) and Hyaluronic Acid Gel (Hyadent®) for Repairing Osseous Defects in a Rat Model. Appl. Sci. 2020, 10, 1651. [Google Scholar] [CrossRef]
- Busenlechner, D.; Huber, C.D.; Vasak, C.; Dobsak, A.; Gruber, R.; Watzek, G. Sinus augmentation analysis revised: The gradient of graft consolidation. Clin. Oral Implant. Res. 2009, 20, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Čandrlić, M.; Tomas, M.; Karl, M.; Malešić, L.; Včev, A.; Perić Kačarević, Ž.; Matijević, M. Comparison of Injectable Biphasic Calcium Phosphate and a Bovine Xenograft in Socket Preservation: Qualitative and Quantitative Histologic Study in Humans. Int. J. Mol. Sci. 2022, 23, 2539. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Friedmann, A.; Dard, M.; Kleber, B.M.; Bernimoulin, J.P.; Bosshardt, D.D. Ridge augmentation and maxillary sinus grafting with a biphasic calcium phosphate: Histologic and histomorphometric observations. Clin. Oral Implant. Res. 2009, 20, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Shimizu, Y.; Asai, S.; Ooya, K. Grafting of deproteinized bone particles inhibits bone resorption after maxillary sinus floor elevation. Clin. Oral Implant. Res. 2004, 15, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Asai, S.; Shimizu, Y.; Ooya, K. Maxillary sinus augmentation model in rabbits: Effect of occluded nasal ostium on new bone formation. Clin. Oral Implant. Res. 2002, 13, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, S.; Shin, S.Y.; Chung, J.H.; Herr, Y.; Lim, H.C. Effectiveness of hydraulic pressure-assisted sinus augmentation in a rabbit sinus model: A preclinical study. Clin. Oral Investig. 2022, 26, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Chen, C.; Shen, X.; Xu, A.; Sharaf, M.A.; Lu, H.; He, F. Bone volume and height changes for lateral window sinus floor elevation using two types of deproteinized bovine bone mineral: A retrospective cohort study of 1–4 years. Clin. Oral Implant. Res. 2024, 35, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Si, M.; Shi, J.; Yang, G.; Shi, Y. Evaluation of three-dimensional contraction of the volume of grafts after staged augmentation of the sinus floor, and an analysis of influential factors. Br. J. Oral Maxillofac. Surg. 2019, 57, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Lewin, S.; Riben, C.; Thor, A.; Öhman-Mägi, C. Bone Volume Assessment Around Dental Implants After Open Maxillary Sinus Elevation Surgery: A Quantitative Approach to CBCT Images. Int. J. Oral Maxillofac. Implant. 2019, 34, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Temmerman, A.; Van Dessel, J.; Cortellini, S.; Jacobs, R.; Teughels, W.; Quirynen, M. Volumetric changes of grafted volumes and the Schneiderian membrane after transcrestal and lateral sinus floor elevation procedures: A clinical, pilot study. J. Clin. Periodontol. 2017, 44, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, T.S. The Structure of Scientific Revolutions; University of Chicago Press: Chicago, IL, USA, 1962. [Google Scholar]
- Copeland, S. On serendipity in science: Discovery at the intersection of chance and wisdom. Synthese 2019, 196, 2385–2406. [Google Scholar] [CrossRef]
- Kant, I. Gesammelte Schriften; Hrsg. Preußische Akademie der Wissenschaften Bd. 1–22, Deutsche Akademie der Wissenschaften zu Berlin (Bd. 23), Akademie der Wissenschaften zu Göttingen (ab Bd. 24); de Gruyter: Berlin, Germany, 1911. [Google Scholar]
- Peirce, C.S. Collected Papers of Charles Sanders Peirce; Harvard University Press: Cambridge, MA, USA, 1931–1935; Volume 1–6. [Google Scholar]
- Lakoff, G.; Johnson, M. Metaphors We Live By; University of Chicago Press: Chicago, IL, USA, 1980. [Google Scholar]


| Authors | Graft | Periods Analyzed | T1 | T2 | ||
|---|---|---|---|---|---|---|
| % Bone | % Graft | % Bone | % Graft | |||
| Lambert et al. [28] | Autogenous | 1 week–5 weeks | 0.3 | 22.7 | 32.7 | 0.0 |
| Bio-Oss | 1 week–6 months | 0.1 | 42.7 | 16 | 34.9 | |
| Yamada et al. [29] | Bio-Oss | 2 weeks–12 weeks | 7.0 | 47.8 | 34.4 | 33.1 |
| Bio-Oss Collagen | 2 weeks–12 weeks | 3.4 | 41.8 | 39.4 | 22.2 | |
| Costa et al. [30] | HA-βTCP granules | 2 weeks–10 weeks | 2.65 | 52.05 | 34.2 | 37.38 |
| HA-βTCP paste | 2 weeks–10 weeks | 0.08 | 79.72 | 23.28 | 48.63 | |
| Iida et al. [31] | Gen-Os | 2 weeks–8 weeks | 6.17 | 35.2 | 26.72 | 9.62 |
| Authors | Graft | Periods Analyzed | Days | BIC% | Dimension Loss Between Periods |
|---|---|---|---|---|---|
| Lambert et al. [28] | Autogenous | 1 week–5 weeks | 18.4 | 13.5 | 42.4% (68.6% after 6 months) |
| Bio-Oss | 1 week–6 months | NA | NA | 9.4% | |
| Yamada et al. [29] | Bio-Oss Collagen | 2 weeks–12 weeks | 62.3 | 28.3 | 16.8% |
| Bio-Oss | 2 weeks–12 weeks | 81.8 | 33.6 | 8.9% | |
| Costa et al. [30] | HA-βTCP granules | 2 weeks–10 weeks | 73.85 a | 36.37 a | 29.3% |
| HA-βTCP paste | 2 weeks–10 weeks | 96.1 a | 34.1 a | 34.0% | |
| Iida et al. [31] | Gen-Os | 2 weeks–8 weeks | 40.4 | 19.1 | 47.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botticelli, D.; Apaza Alccayhuaman, K.A.; Xavier, S.P.; Silva, E.R.; Nakajima, Y.; Baba, S. From Break-Even Point to Dynamic Regenerative Balance: A Conceptual and Quantitative Framework Based on Preclinical Rabbit Sinus Lift Data. Dent. J. 2025, 13, 469. https://doi.org/10.3390/dj13100469
Botticelli D, Apaza Alccayhuaman KA, Xavier SP, Silva ER, Nakajima Y, Baba S. From Break-Even Point to Dynamic Regenerative Balance: A Conceptual and Quantitative Framework Based on Preclinical Rabbit Sinus Lift Data. Dentistry Journal. 2025; 13(10):469. https://doi.org/10.3390/dj13100469
Chicago/Turabian StyleBotticelli, Daniele, Karol Alí Apaza Alccayhuaman, Samuel Porfirio Xavier, Erick Ricardo Silva, Yasushi Nakajima, and Shunsuke Baba. 2025. "From Break-Even Point to Dynamic Regenerative Balance: A Conceptual and Quantitative Framework Based on Preclinical Rabbit Sinus Lift Data" Dentistry Journal 13, no. 10: 469. https://doi.org/10.3390/dj13100469
APA StyleBotticelli, D., Apaza Alccayhuaman, K. A., Xavier, S. P., Silva, E. R., Nakajima, Y., & Baba, S. (2025). From Break-Even Point to Dynamic Regenerative Balance: A Conceptual and Quantitative Framework Based on Preclinical Rabbit Sinus Lift Data. Dentistry Journal, 13(10), 469. https://doi.org/10.3390/dj13100469









