Proof-of-Concept Digital-Physical Workflow for Clear Aligner Manufacturing
Abstract
1. Introduction
2. Materials and Methods
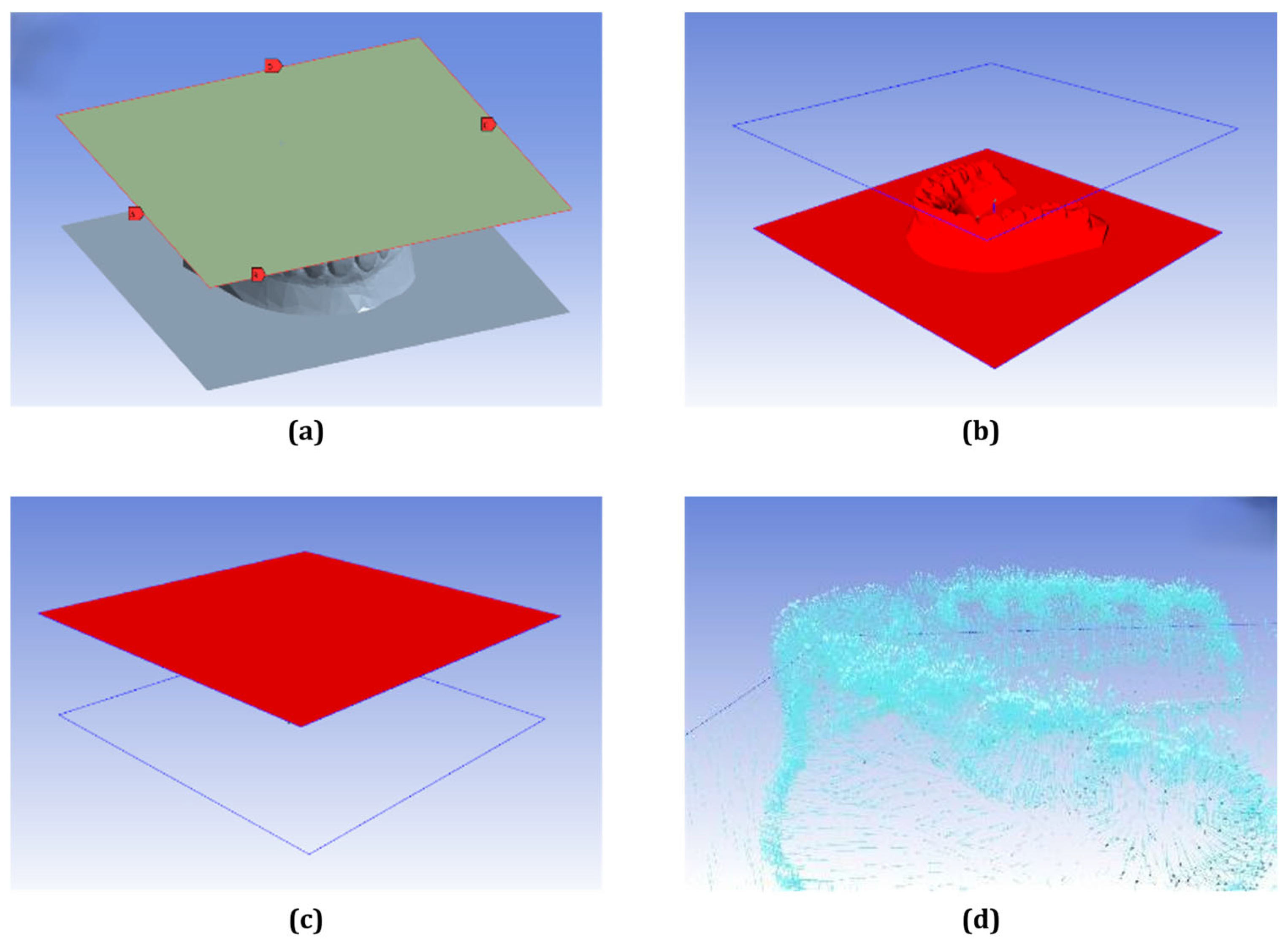
2.1. Thermoforming Error Analysis
2.2. Materials, Thermoforming Parameters, and Measurement Methodology
2.3. Numerical Modeling of the Thermoforming Process
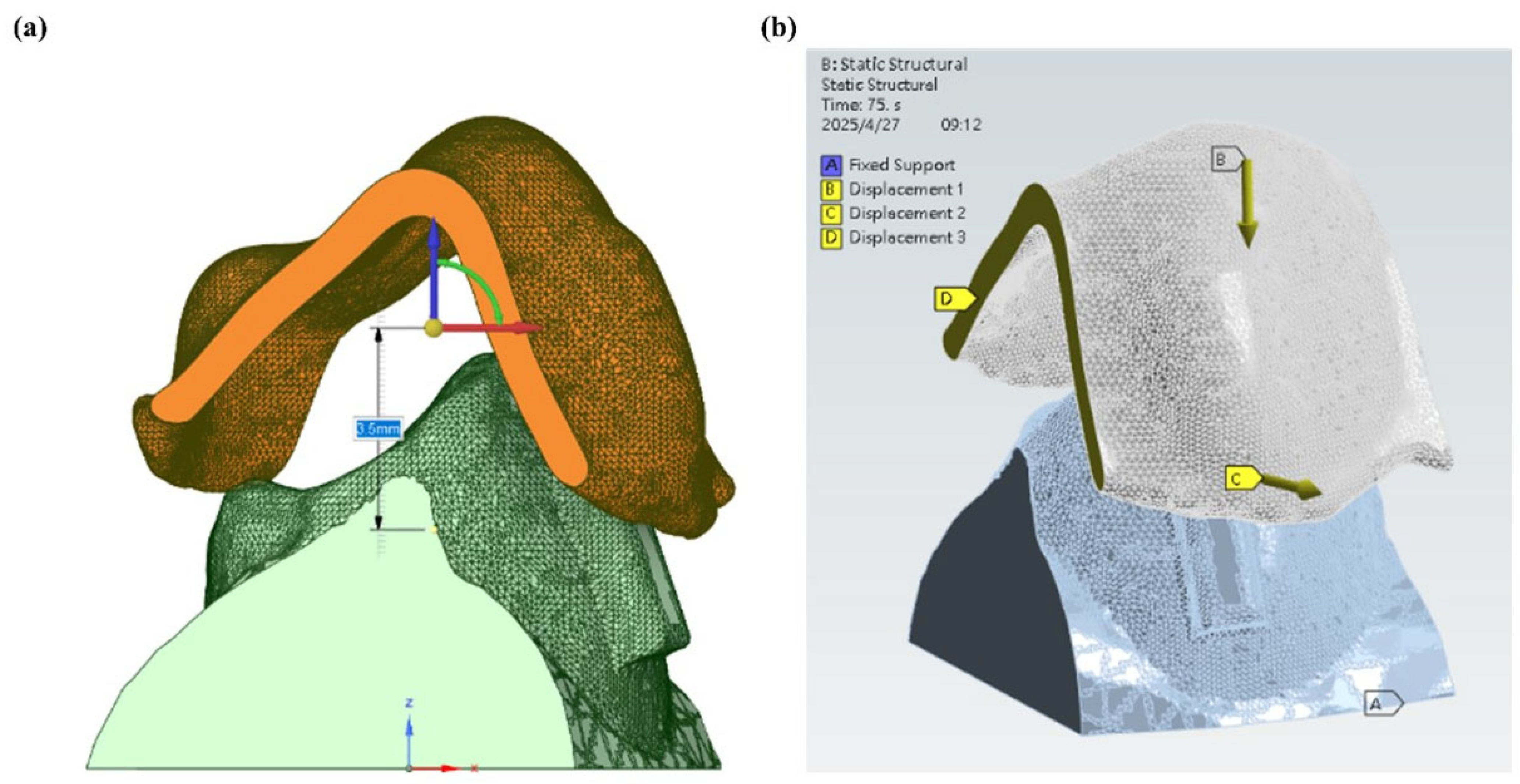
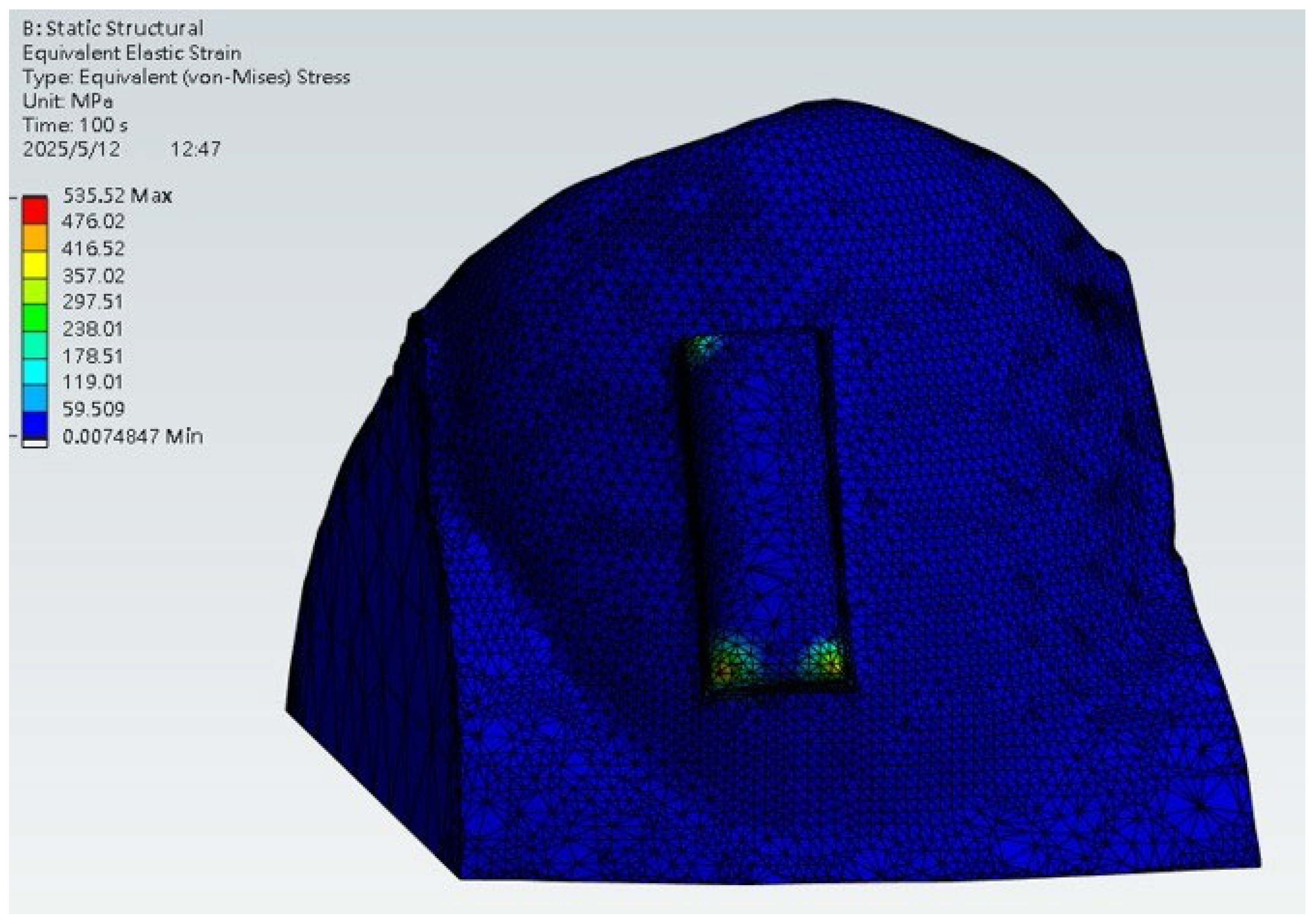
2.4. Setup for Stress Analysis During Wear
3. Results and Discussion
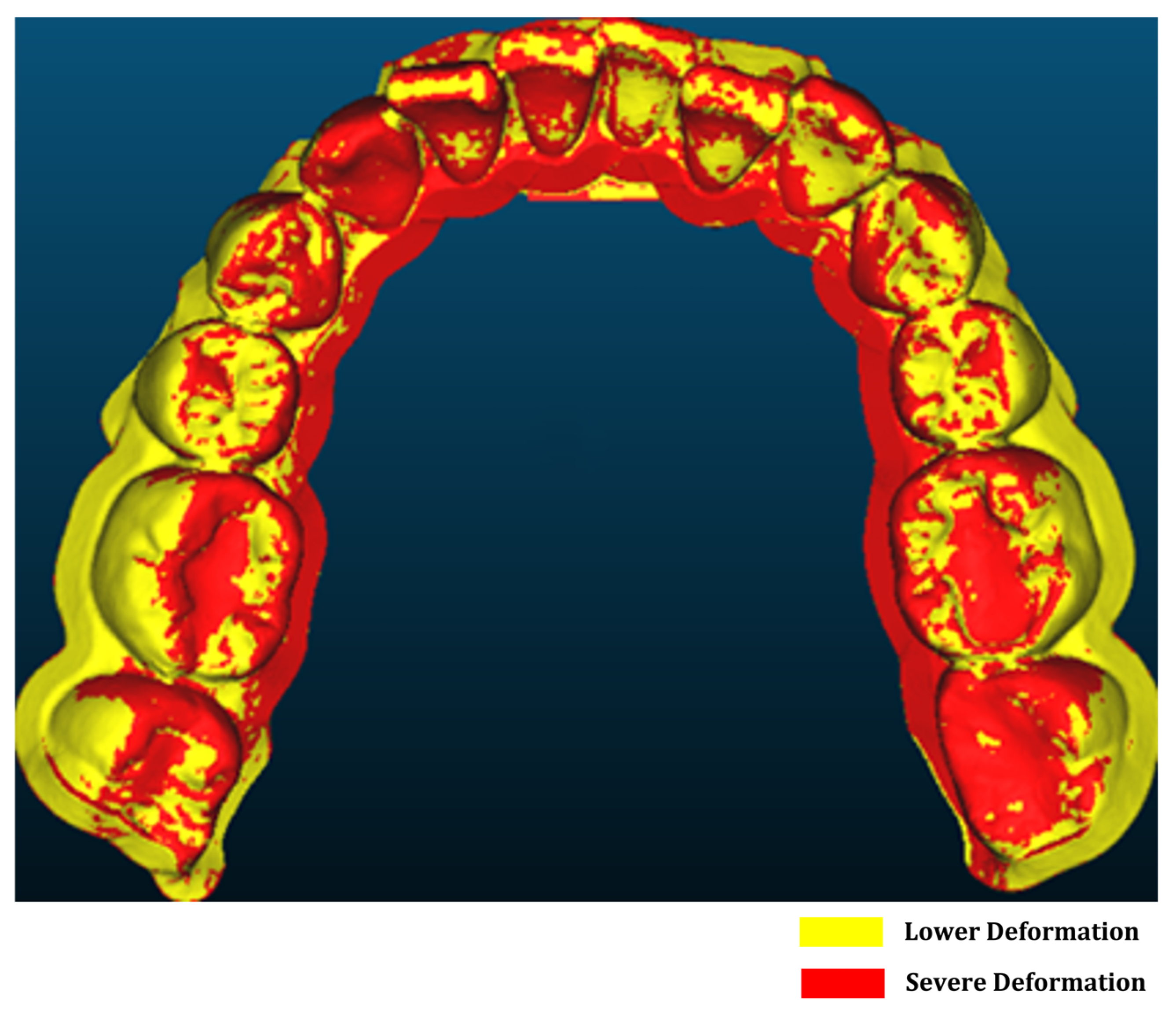
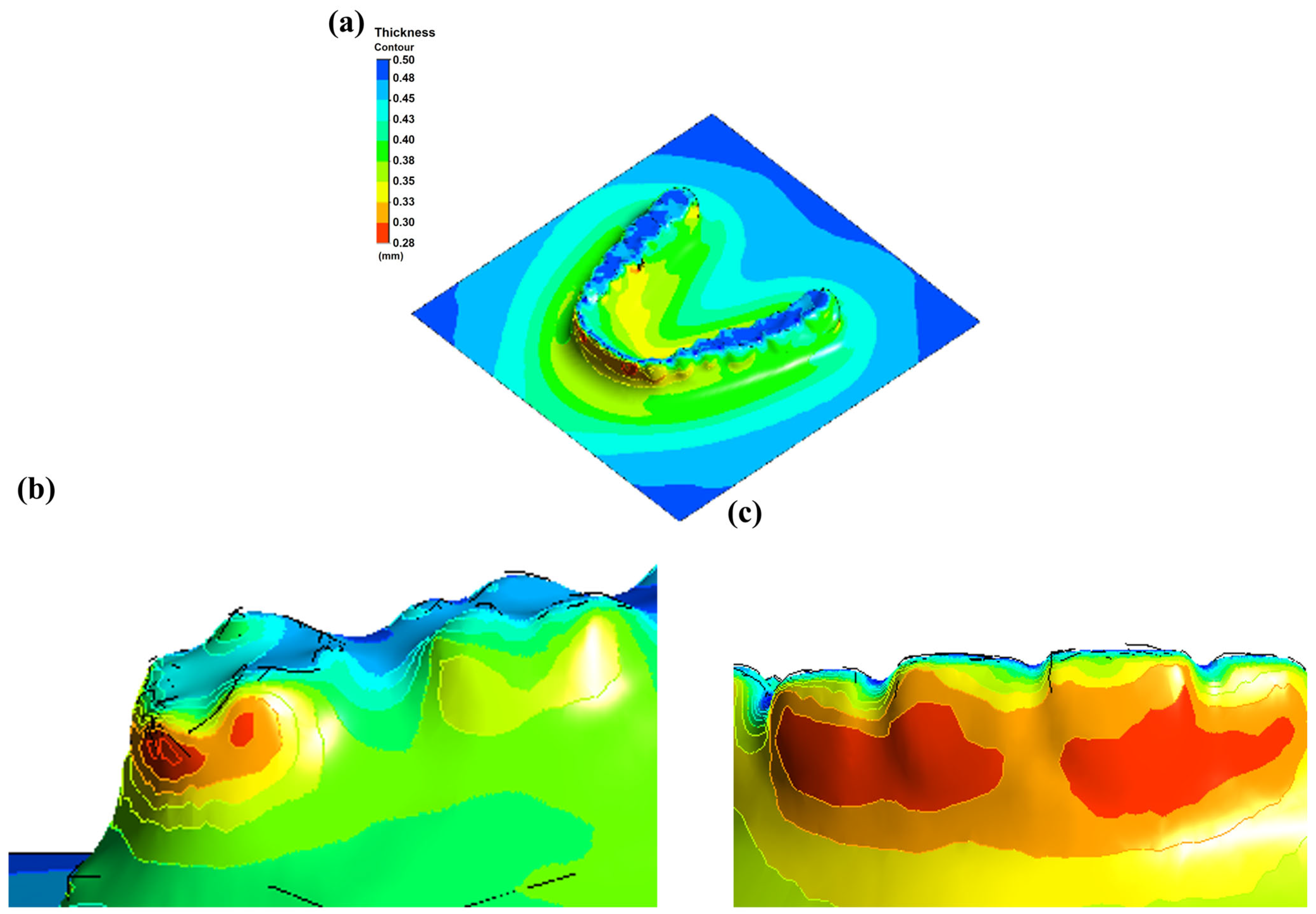
3.1. Forming Error Analysis Results
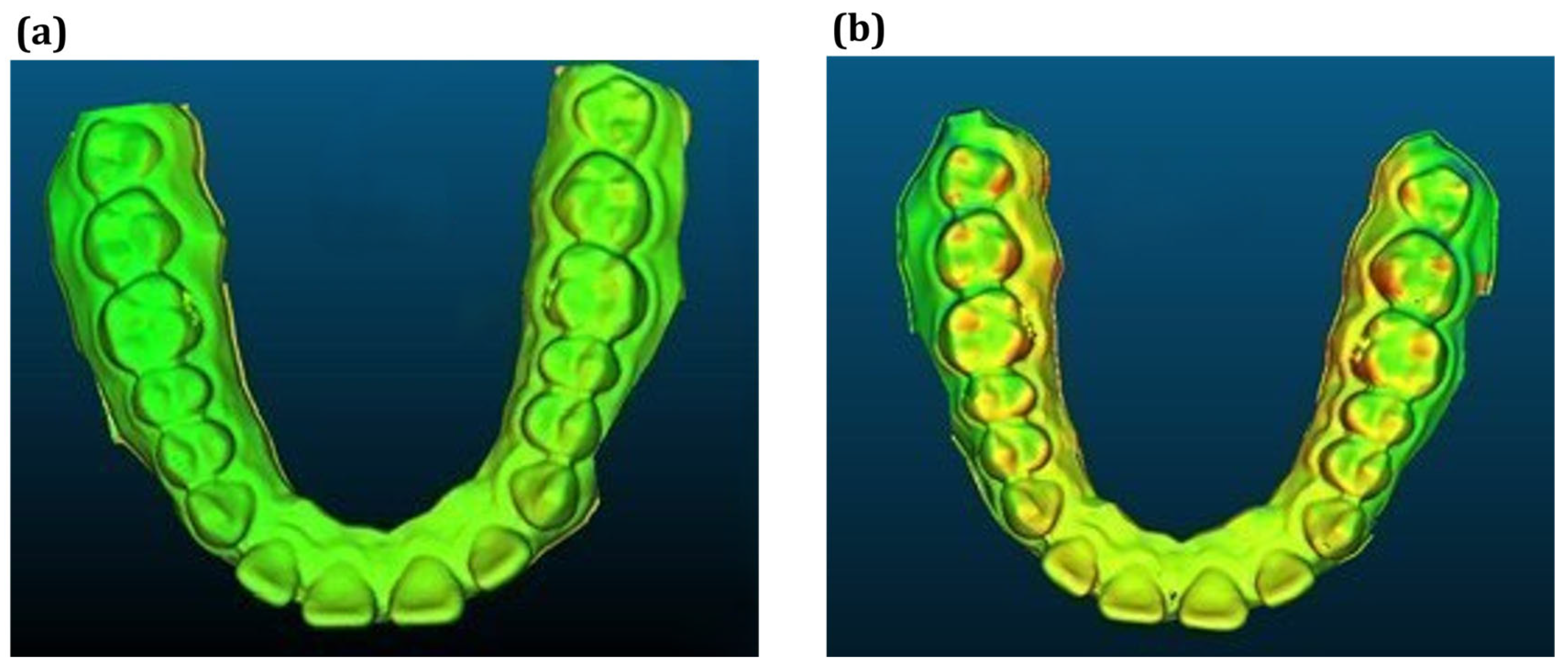
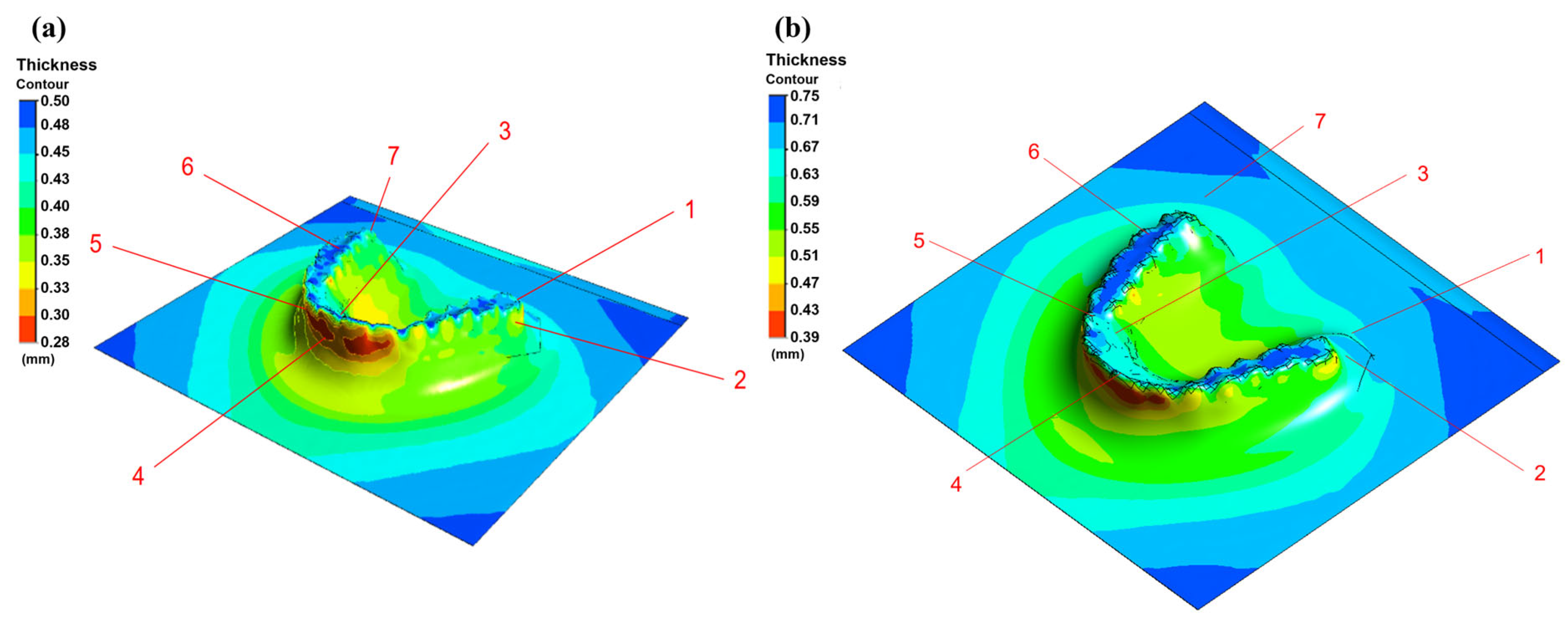
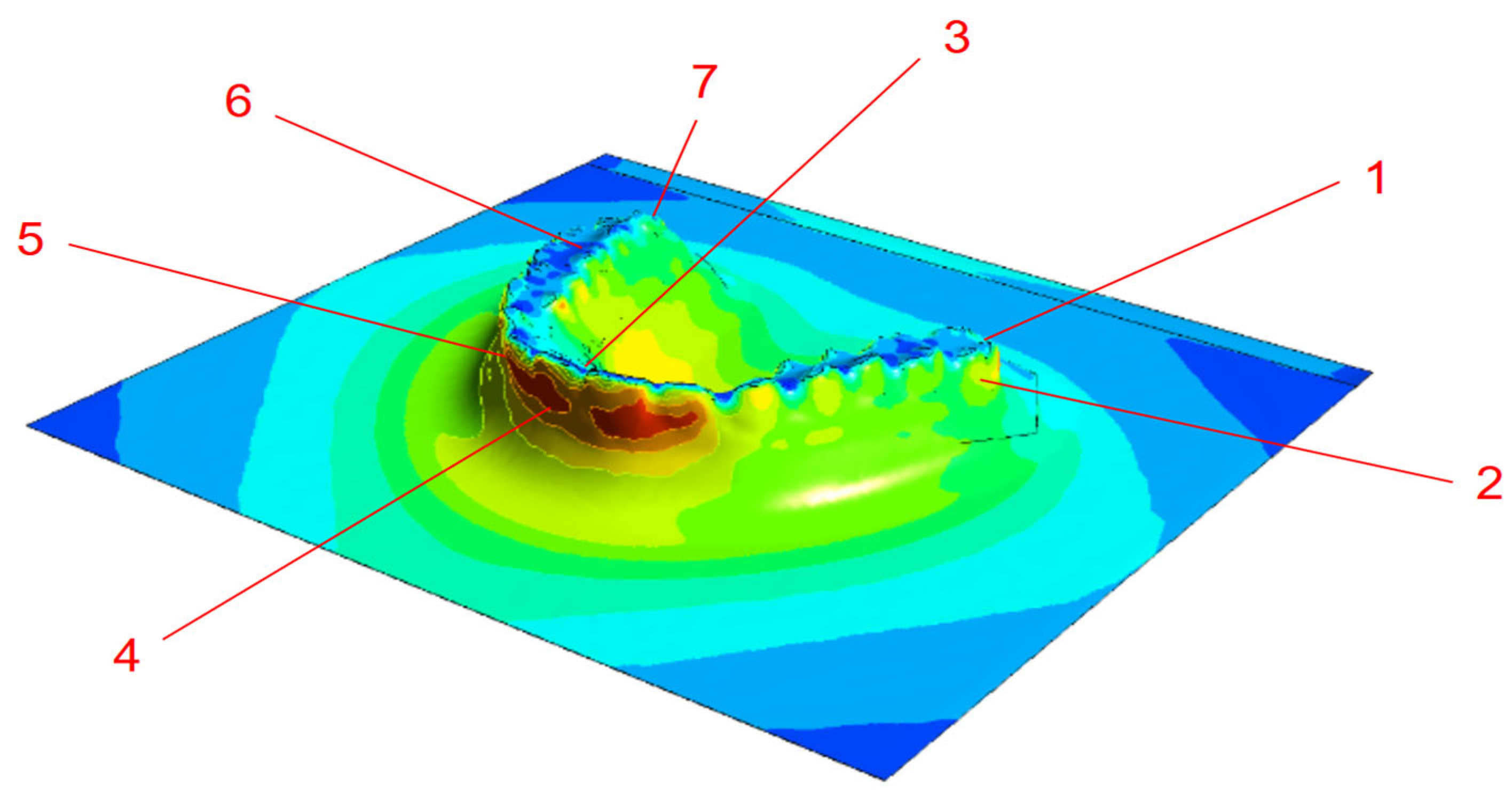
3.2. Thermoforming Simulation Results
3.3. Stress Analysis During Wear
3.4. Comparison Between Traditional and Proposed Workflow
4. Conclusions
- Positive-thermoforming achieved superior fidelity, reducing geometric deviations to 0.3–0.4 mm compared with 0.48–1.06 mm under negative pressure.
- PETG sheet thickness simulations correlated well with experimental measurements with deviations within 10% and supported by 95% confidence intervals, confirming predictive potential.
- Stress analysis showed concentrated load transfer at aligner–attachment interfaces, aligning with clinically expected force delivery patterns, though the simplified model (single tooth, displacement driven) should be interpreted as illustrative.
- Overall, the workflow highlights how numerical simulation and controlled processing can improve structural consistency and predictability in aligner manufacturing.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dipalma, G.; Inchingolo, A.D.; Fiore, A.; Balestriere, L.; Nardelli, P.; Casamassima, L.; Di Venere, D.; Palermo, A.; Inchingolo, F.; Inchingolo, A.M. The Differential Impact of Clear Aligners and Fixed Orthodontic Appliances on Periodontal Health: A Systematic Review. Children 2025, 12, 138. [Google Scholar] [CrossRef]
- Ardila, C.M. Addressing Mucosal Ulcers during Orthodontic Treatment: An Urgent Call for Preventive Strategies. World J. Clin. Cases 2024, 12, 6420–6424. [Google Scholar] [CrossRef]
- AlDahash, F.; AlShamali, D.; AlBander, W.; Bakhsh, R.; AlMadhi, W.; AlSenani, S. Oral Mucosal Ulceration during Orthodontic Treatment: The Perception of Patients and Knowledge and Attitude of the Orthodontic Practitioners. J. Fam. Med. Prim. Care 2020, 9, 5537. [Google Scholar] [CrossRef]
- Hartogsohn, C.R.; Sonnesen, L. Clear Aligner Treatment: Indications, Advantages, and Adverse Effects—A Systematic Review. Dent. J. 2025, 13, 40. [Google Scholar] [CrossRef]
- Hosny, M.A.A.; Alasmari, F.S.; Alsaidi, N.M.; Alsharif, H.M.; Alshareef, S.A.; Aldwyyan, N.F.; Alahmadi, R.Y.; Almutairi, R.A.; Almutairi, B.M.; Alhemaidi, G.S.; et al. Indications, Advantages, Disadvantages and Effectiveness of Invisalign Aligners. Int. J. Community Med. Public. Health 2021, 8, 5064. [Google Scholar] [CrossRef]
- Rossini, G.; Parrini, S.; Castroflorio, T.; Deregibus, A.; Debernardi, C.L. Efficacy of Clear Aligners in Controlling Orthodontic Tooth Movement: A Systematic Review. Angle Orthod. 2015, 85, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Wajekar, N.; Pathak, S.; Mani, S. Rise & Review of Invisalign Clear Aligner System. IP Indian. J. Orthod. Dentofac. Res. 2022, 8, 7–11. [Google Scholar] [CrossRef]
- Tamer, I.; Öztas, E.; Marsan, G. Orthodontic Treatment with Clear Aligners and the Scientific Reality behind Their Marketing: A Literature Review. Turkish J. Orthod. 2019, 32, 241–246. [Google Scholar] [CrossRef]
- Albarki, Z.; Weir, T.; Meade, M.J. Efficacy of Planned Root Torque Changes of Instanding Maxillary Lateral Incisors with the Invisalign Appliance. Am. J. Orthod. Dentofac. Orthop. 2025, 168, 367–378. [Google Scholar] [CrossRef]
- Grassia, V.; Ronsivalle, V.; Isola, G.; Nucci, L.; Leonardi, R.; Lo Giudice, A. Accuracy (Trueness and Precision) of 3D Printed Orthodontic Models Finalized to Clear Aligners Production, Testing Crowded and Spaced Dentition. BMC Oral Health 2023, 23, 352. [Google Scholar] [CrossRef]
- Mangano, F.; Gandolfi, A.; Luongo, G.; Logozzo, S. Intraoral Scanners in Dentistry: A Review of the Current Literature. BMC Oral Health 2017, 17, 149. [Google Scholar] [CrossRef]
- Pillai, S.; Upadhyay, A.; Khayambashi, P.; Farooq, I.; Sabri, H.; Tarar, M.; Lee, K.T.; Harb, I.; Zhou, S.; Wang, Y.; et al. Dental 3d-Printing: Transferring Art from the Laboratories to the Clinics. Polymers 2021, 13, 157. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Kamble, R.; Kanani, H. Revolutionizing Smiles: Advancing Orthodontics Through Digital Innovation. Cureus 2024, 16, e64086. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, M.J.; Kumar, A.; Lin, S.; Jeng, J. Reducing Mechanical Anisotropy in Material Extrusion Process Using Bioinspired Architectured Lattice Structures. Addit. Manuf. 2023, 66, 103480. [Google Scholar] [CrossRef]
- Prajapati, M.J.; Kumar, A.; Lin, S.C.; Jeng, J.Y. Closed-Cell Metamaterial Composites 3D Printed with Hybrid FFF Process for Tunable Mechanical and Functional Properties. Thin-Walled Struct. 2023, 192, 111168. [Google Scholar] [CrossRef]
- Al Rashid, A.; Ahmed, W.; Khalid, M.Y.; Koç, M. Vat Photopolymerization of Polymers and Polymer Composites: Processes and Applications. Addit. Manuf. 2021, 47, 102279. [Google Scholar] [CrossRef]
- Elshazly, T.M.; Keilig, L.; Alkabani, Y.; Ghoneima, A.; Abuzayda, M.; Talaat, S.; Bourauel, C.P. Primary Evaluation of Shape Recovery of Orthodontic Aligners Fabricated from Shape Memory Polymer (A Typodont Study). Dent. J. 2021, 9, 31. [Google Scholar] [CrossRef]
- Yu, X.; Li, G.; Zheng, Y.; Gao, J.; Fu, Y.; Wang, Q.; Huang, L.; Pan, X.; Ding, J. “Invisible” Orthodontics by Polymeric “Clear” Aligners Molded on 3D-Printed Personalized Dental Models. Regen. Biomater. 2022, 9, rbac007. [Google Scholar] [CrossRef]
- Alawdi, G.M.; Al Fahad, M.F.; Al Muzher, S.B.; Alfaifi, A.H.; Hazeem, A.M.; Dakheel, R.S.; Jan, R.H.; Al-Qutub, L.M.; Alharbi, L.H.; Khalil, A. Does Invisalign Outperform Fixed Appliance in Treating Vertical Discrepancies? Cureus 2024, 16, e65973. [Google Scholar] [CrossRef]
- Eliades, T.; Panayi, N.; Papageorgiou, S.N. From Biomimetics to Smart Materials and 3D Technology: Applications in Orthodontic Bonding, Debonding, and Appliance Design or Fabrication. Jpn. Dent. Sci. Rev. 2023, 59, 403–411. [Google Scholar] [CrossRef]
- Elsaharty, M.; Hafez, A.; Abdelwarith, A. Accuracy of 3D Printing Versus Milling in Fabrication of Clear Aligners Dental Models. Al-Azhar J. Dent. Sci. 2023, 26, 263–276. [Google Scholar] [CrossRef]
- Rodrigues, R.B.; de Carvalho, A.J.D.; Felipe E Silva, B.V.; Simamoto-Júnior, P.C.; Novais, V.R. Impact of Radiotherapy in Chemical Composition and Mechanical Properties of Human Cervical Dentin: An in Vitro Study. J. Appl. Oral Sci. 2025, 33, e20240279. [Google Scholar] [CrossRef] [PubMed]
- Petousis, M.; Michailidis, N.; Saltas, V.; Papadakis, V.; Spiridaki, M.; Mountakis, N.; Argyros, A.; Valsamos, J.; Nasikas, N.K.; Vidakis, N. Mechanical and Electrical Properties of Polyethylene Terephthalate Glycol/Antimony Tin Oxide Nanocomposites in Material Extrusion 3D Printing. Nanomaterials 2024, 14, 761. [Google Scholar] [CrossRef] [PubMed]
- Matias, M.; Listik, E.; Heidorn, V.; de Souza, E.C.; da Silva Rios, C.A.; Arcanjo, J.M.; Pierard, L.M.G.; Fontes, T.S.; Nahás, A.C.R. Comparison of GAP Width of Thermoformed Clear Aligners Produced by Different 3D Printers. Brazilian Dent. Sci. 2025, 28, e4696. [Google Scholar] [CrossRef]
- Grzebieluch, W.; Grajzer, M.; Mikulewicz, M. Comparative Analysis of Fused Deposition Modeling and Digital Light Processing Techniques for Dimensional Accuracy in Clear Aligner Manufacturing. Med. Sci. Monit. 2023, 29, e940922. [Google Scholar] [CrossRef]
- Zhao, M.; Xu, L. ETC-PCR: Coarse-to-Fine Edge-Triangle Compatibility for Robust and Accurate Point Cloud Registration. IEEE Access 2025, 13, 102659–102674. [Google Scholar] [CrossRef]
- Liao, X.; Huang, F.; Chen, W.; Cai, X.; Lu, Y.; Zhou, Z. The Thickness and Gap Width of Aligners Made of Three Types of Thermoforming Materials: An in-Vitro Study. Semin. Orthod. 2025. [Google Scholar] [CrossRef]
- Staderini, E.; Chiusolo, G.; Guglielmi, F.; Papi, M.; Perini, G.; Tepedino, M.; Gallenzi, P. Effects of Thermoforming on the Mechanical, Optical, Chemical, and Morphological Properties of PET-G: In Vitro Study. Polymers 2024, 16, 203. [Google Scholar] [CrossRef]
- Online Materials Information Resource—MatWeb. Available online: http://www.matweb.com/ (accessed on 22 July 2025).
- Laohachaiaroon, P.; Samruajbenjakun, B.; Chaichanasiri, E. Initial Displacement and Stress Distribution of Upper Central Incisor Extrusion with Clear Aligners and Various Shapes of Composite Attachments Using the Finite Element Method. Dent. J. 2022, 10, 114. [Google Scholar] [CrossRef]
- Cao, H.; Hua, X.; Yang, L.; Aoki, K.; Shang, S.; Lin, D. A Systematic Review of Biomechanics of Clear Aligners by Finite Element Analysis. BMC Oral Health 2025, 25, 1026. [Google Scholar] [CrossRef]
- Chopra, S.; Bansal, P.; Bansal, P. Essix Appliance- An Innovation Modification for use as Temporary Bridge- A Case Report. J. Adv. Med. Dent. Sci. Res. 2020, 8, 184–186. [Google Scholar] [CrossRef]
- Hamed, N.; Hassan, I.; Elghoul, D.; Elabbassy, E.; Zaky, A. Invitro Evaluation of Forces Delivered by Aligners Fabricated Using Positive Air Pressure versus Negative Air Pressure. Egypt. Dent. J. 2024, 70, 53–58. [Google Scholar] [CrossRef]
- Wiechens, B.; Brockmeyer, P.; Erfurth-Jach, T.; Hahn, W. Force Delivery Modification of Removable Thermoplastic Appliances Using Hilliard Precision Thermopliers for Tipping an Upper Central Incisor. Clin. Oral Investig. 2022, 26, 6105–6118. [Google Scholar] [CrossRef]
- Ye, N.; Brown, B.E.; Mantell, S.C.; Larson, B.E.; Gruenheid, T.; Fok, A.S. Validation of Finite-Element-Simulated Orthodontic Forces Produced by Thermoplastic Aligners: Effect of Aligner Geometry and Creep. J. Mech. Behav. Biomed. Mater. 2024, 160, 106755. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; He, L.; Xuan, Q.; Liao, Y.; Dai, J.; Lei, D. Phase-Change VO2-Based Thermochromic Smart Windows. Light. Sci. Appl. 2024, 13, 255. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, A.; Nakano, H.; Kataoka, Y.; Maki, K. Evaluation of Mechanical Tests Conducted before and after Thermoforming of Aligners. Showa Univ. J. Med. Sci. 2023, 35, 121–130. [Google Scholar] [CrossRef]
- Rajasekaran, A.; Chaudhari, P.K. Integrated Manufacturing of Direct 3D-Printed Clear Aligners. Front. Dent. Med. 2023, 3, 1089627. [Google Scholar] [CrossRef]
- Cremonini, F.; Pancari, C.; Brucculeri, L.; Karami Shabankare, A.; Lombardo, L. Force Expressed by 3D-Printed Aligners with Different Thickness and Design Compared to Thermoformed Aligners: An in Vitro Study. Appl. Sci. 2025, 15, 2911. [Google Scholar] [CrossRef]
- Pascadopoli, M.; Zampetti, P.; Nardi, M.G.; Pellegrini, M.; Scribante, A. Smartphone Applications in Dentistry: A Scoping Review. Dent. J. 2023, 11, 243. [Google Scholar] [CrossRef]
- Kim, S.; Shin, J.; Lee, E.; Park, S.; Jeong, T.; Hwang, J.; Seo, H. Comparative Analysis of Deep-Learning-Based Bone Age Estimation between Whole Lateral Cephalometric and the Cervical Vertebral Region in Children. J. Clin. Pediatr. Dent. 2024, 48, 191–199. [Google Scholar] [CrossRef]


| Property | PETG Sheet | Tooth Tissue |
|---|---|---|
| Density | 1.28 g/cm3 | 2.1 g/cm3 |
| Young’s Modulus | 2 GPa | 18 GPa |
| Poisson’s Ratio | 0.33 | 0.3 |
| Ultimate Tensile Strength | – | 105 MPa |
| Ultimate Compressive Strength | – | 300 MPa |
| Comparison Stage | Deviation Range (mm) |
|---|---|
| Original vs. Post-cured model | −0.12~0.14 |
| Post-cured vs. Positive-pressure model | −0.19~0.24 |
| Post-cured vs. Negative-pressure model | −0.33~0.56 |
| Original vs. Positive-pressure model | −0.30~0.40 |
| Original vs. Negative-pressure model | −0.48~1.06 |
| 0.05 mm | 0.75 mm | |||||||
|---|---|---|---|---|---|---|---|---|
| Point | Measured (mm) | 95% CI (mm) | Simulated (mm) | Error (%) | Measured (mm) | 95% CI (mm) | Simulated (mm) | Error (%) |
| 1 | 0.42 ± 0.02 | 0.37–0.47 | 0.43 | −2% | 0.71 ± 0.05 | 0.59–0.83 | 0.73 | 3% |
| 2 | 0.35 ± 0.04 | 0.25–0.45 | 0.35 | 0% | 0.58 ± 0.02 | 0.53–0.63 | 0.54 | 7% |
| 3 | 0.43 ± 0.07 | 0.26–0.6 | 0.41 | 4% | 0.71 ± 0.02 | 0.66–0.81 | 0.70 | 4% |
| 4 | 0.32 ± 0.05 | 0.2–0.44 | 0.31 | 3% | 0.48 ± 0.04 | 0.38–0.58 | 0.47 | 2% |
| 5 | 0.32 ± 0.02 | 0.27–0.37 | 0.31 | 3% | 0.42± 0.07 | 0.24–0.58 | 0.47 | 8% |
| 6 | 0.33 ± 0.07 | 0.16–0.5 | 0.31 | 6% | 0.7± 0.03 | 0.55–0.85 | 0.73 | 4% |
| 7 | 0.47 ± 0.03 | 0.4–0.55 | 0.49 | −4% | 0.64± 0.02 | 0.59–0.69 | 0.66 | 3% |
| Parameters | Traditional Methodology | Digital Design Workflow | Reference |
|---|---|---|---|
| Accuracy | Prone to manual error and material inaccuracies, such as thickness variations and thinning. | Direct printing enables uniform thickness distribution and improved accuracy in gap widths. | [28,36] |
| Repeatability | Manual trimming and heating cycles can lead to variability. | Digital CAD/CAM files ensure reproducibility and allow automated pre-fabrication quality checks. | [6,37] |
| Fabrication time | Involves multiple steps (printing, thermoforming, trimming) | Eliminates physical modeling, printing, and post-processing, reducing overall time. | [38,39] |
| Customization | Manual process limits customization | Full digital customization via CAD modeling. | [28,39] |
| Clinical Predictability | Poor fitting and variability are force delivery is predominant. | Allows more personalized biomechanics and potentially more predictable outcomes once validated. | [38,39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-H.; Chou, I.-C.; Prajapati, M.J.; Wang, Y.-H.; Le, P.-K.; Jiang, C.-P. Proof-of-Concept Digital-Physical Workflow for Clear Aligner Manufacturing. Dent. J. 2025, 13, 454. https://doi.org/10.3390/dj13100454
Huang S-H, Chou I-C, Prajapati MJ, Wang Y-H, Le P-K, Jiang C-P. Proof-of-Concept Digital-Physical Workflow for Clear Aligner Manufacturing. Dentistry Journal. 2025; 13(10):454. https://doi.org/10.3390/dj13100454
Chicago/Turabian StyleHuang, Shih-Hao, I-Chiang Chou, Mayur Jiyalal Prajapati, Yu-Hsiang Wang, Po-Kai Le, and Cho-Pei Jiang. 2025. "Proof-of-Concept Digital-Physical Workflow for Clear Aligner Manufacturing" Dentistry Journal 13, no. 10: 454. https://doi.org/10.3390/dj13100454
APA StyleHuang, S.-H., Chou, I.-C., Prajapati, M. J., Wang, Y.-H., Le, P.-K., & Jiang, C.-P. (2025). Proof-of-Concept Digital-Physical Workflow for Clear Aligner Manufacturing. Dentistry Journal, 13(10), 454. https://doi.org/10.3390/dj13100454









