Mechanical and Antimicrobial Evaluation of Chitosan-Coated Elastomeric Orthodontic Modules
Abstract
1. Introduction
2. Materials and Methods
2.1. Chitosan-Coating of Elastomeric Modules
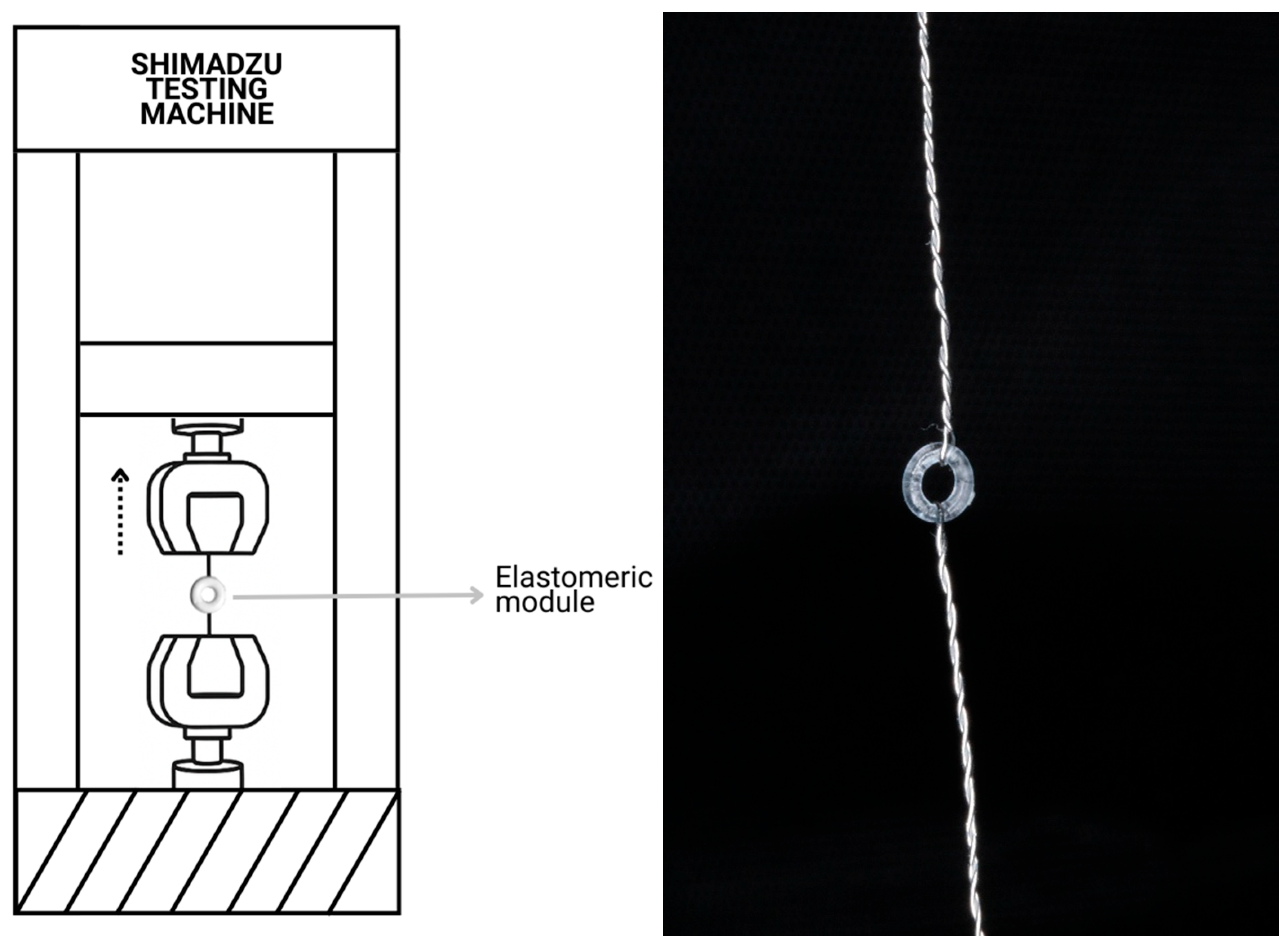
2.2. Physicochemical and Mechanical Characterization
2.3. Antibacterial Activity Assay
2.4. Cell Viability
2.5. Statistical Analysis
3. Results
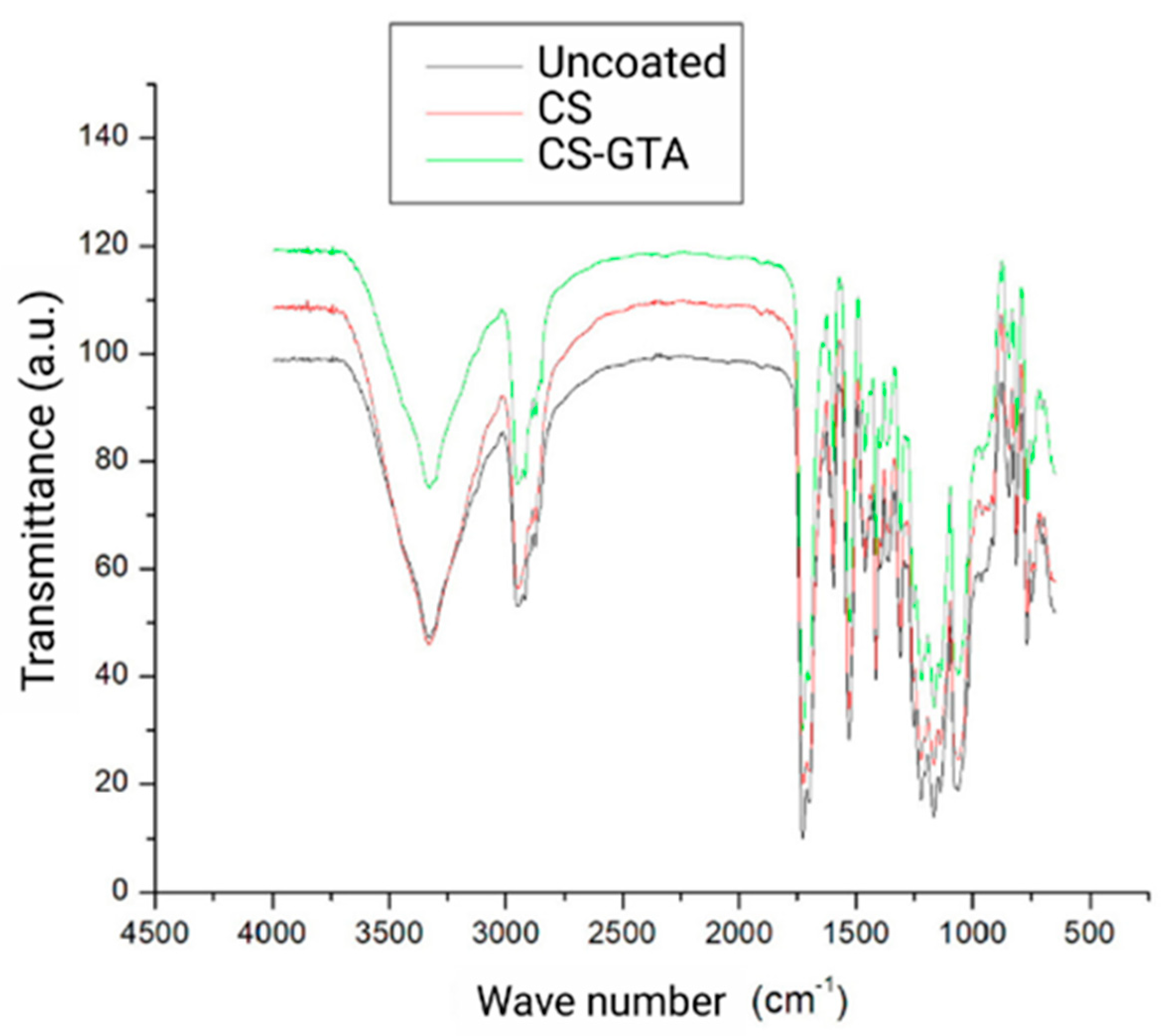
3.1. Physicochemical and Mechanical Characterization
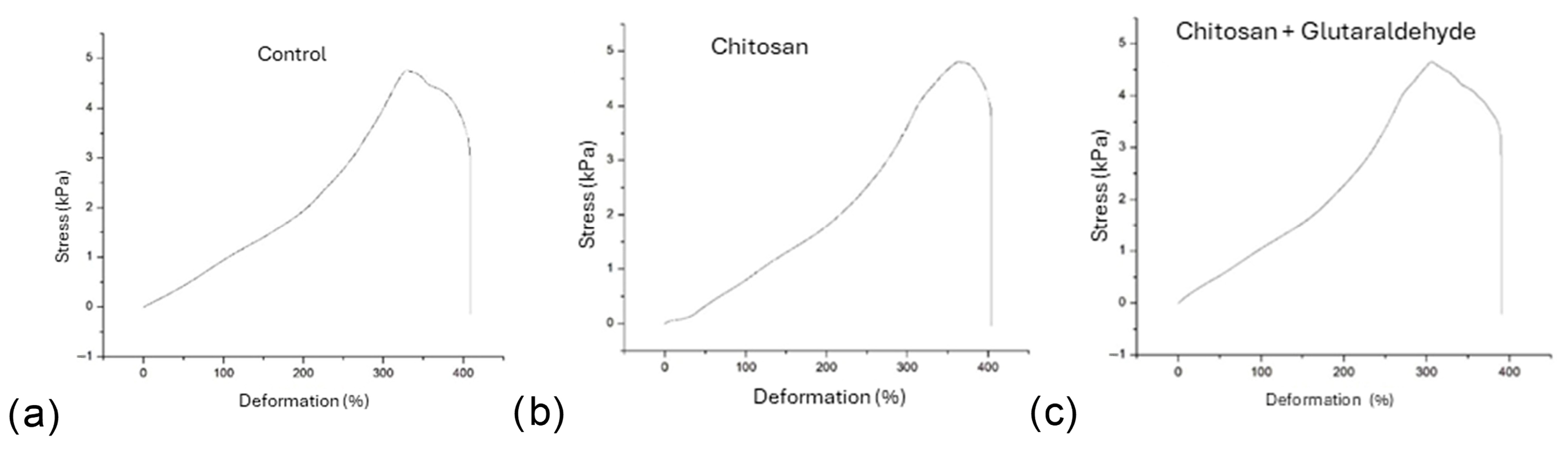
3.2. Mechanical Characterization
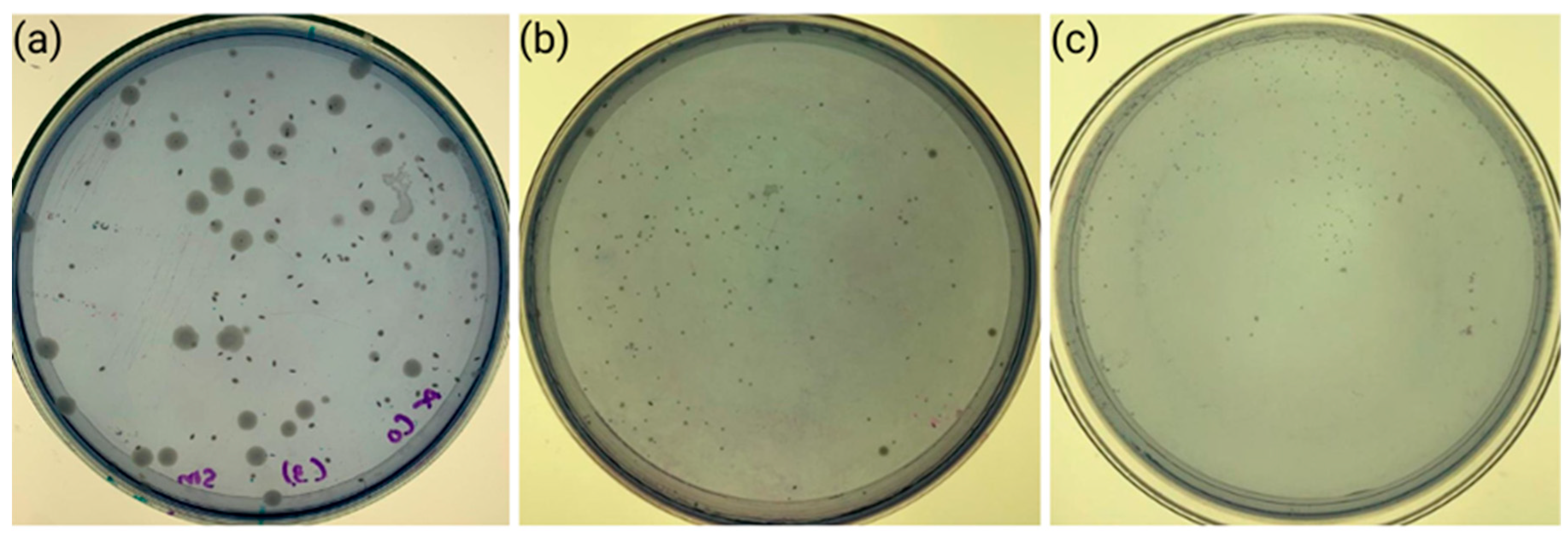
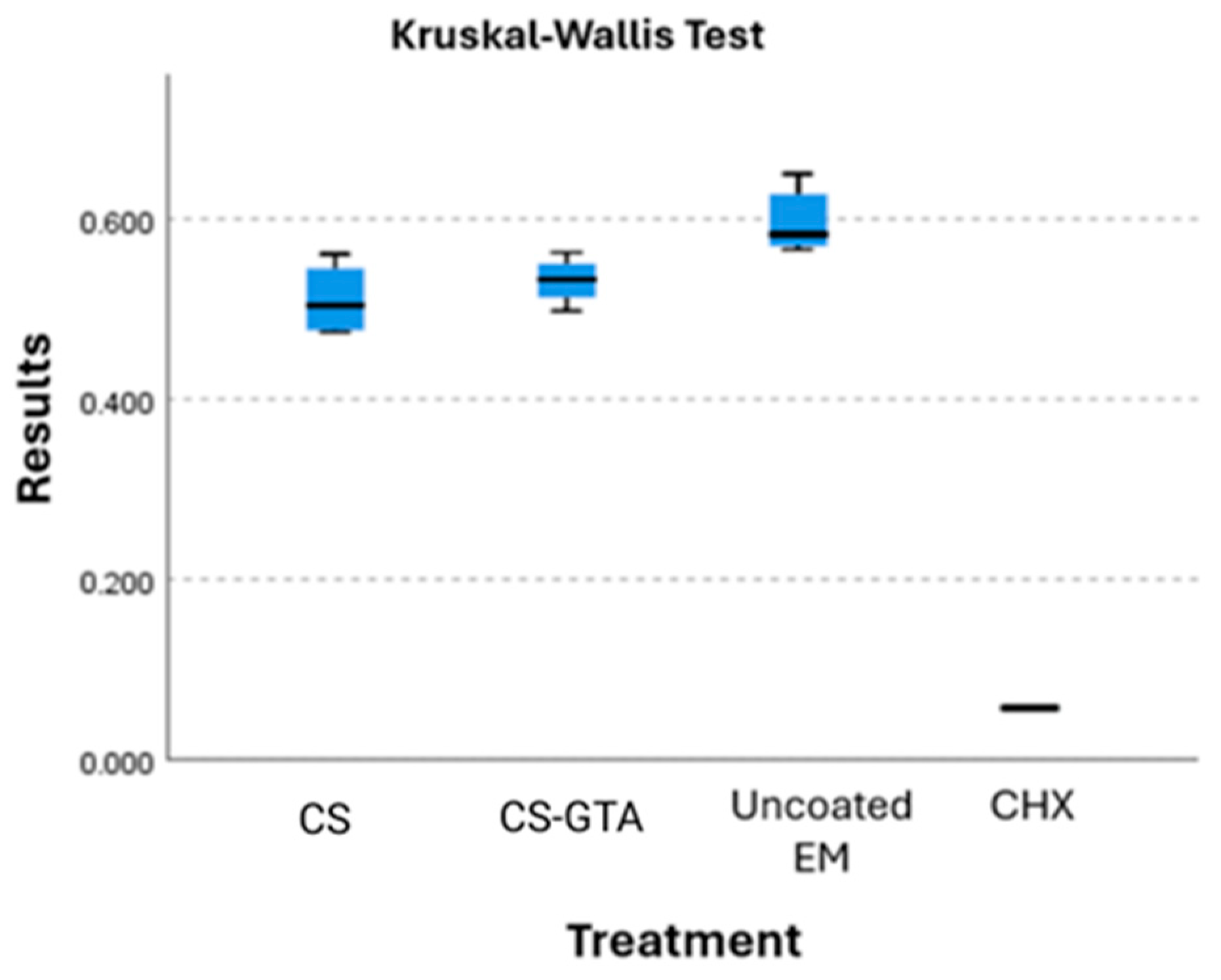
3.3. Antibacterial Activity Assay
3.4. Cell Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamont, R.J.; Hajishengallis, G.N.; Koo, H.; Jenkinson, H.F. Oral Microbiology and Immunology; ASM Books; Wiley: Hoboken, NJ, USA, 2019; ISBN 978-1-68367-290-6. [Google Scholar]
- Perkowski, K.; Baltaza, W.; Conn, D.B.; Marczyńska-Stolarek, M.; Chomicz, L. Examination of Oral Biofilm Microbiota in Patients Using Fixed Orthodontic Appliances in Order to Prevent Risk Factors for Health Complications. Ann. Agric. Environ. Med. 2019, 26, 231–235. [Google Scholar] [CrossRef]
- Mummolo, S.; Nota, A.; Albani, F.; Marchetti, E.; Gatto, R.; Marzo, G.; Quinzi, V.; Tecco, S. Salivary Levels of Streptococcus Mutans and Lactobacilli and Other Salivary Indices in Patients Wearing Clear Aligners versus Fixed Orthodontic Appliances: An Observational Study. PLoS ONE 2020, 15, e0228798. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.-S.; Jung, E.-H.; Kang, S.-M.; Lee, E.-S.; Lee, J.-W.; Kim, B.-I. Improving the Efficacy of Chlorhexidine-Releasing Elastomerics Using a Layer-by-Layer Coating Technique. Dent. Mater. J. 2017, 36, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Migliorati, M.; Isaia, L.; Cassaro, A.; Rivetti, A.; Silvestrini-Biavati, F.; Gastaldo, L.; Piccardo, I.; Dalessandri, D.; Silvestrini-Biavati, A. Efficacy of Professional Hygiene and Prophylaxis on Preventing Plaque Increase in Orthodontic Patients with Multibracket Appliances: A Systematic Review. Eur. J. Orthod. 2015, 37, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Sundararaj, D.; Venkatachalapathy, S.; Tandon, A.; Pereira, A. Critical Evaluation of Incidence and Prevalence of White Spot Lesions during Fixed Orthodontic Appliance Treatment: A Meta-Analysis. J. Int. Soc. Prev. Community Dent. 2015, 5, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Proffit, W.R. Contemporary Orthodontics; Mosby Elsevier: Amsterdam, The Netherlands, 2007; ISBN 978-0-323-04613-8. [Google Scholar]
- Sharma, R.; Sharma, K.; Sawhney, R. Evidence of Variable Bacterial Colonization on Coloured Elastomeric Ligatures during Orthodontic Treatment: An Intermodular Comparative Study. J. Clin. Exp. Dent. 2018, 10, e271–e278. [Google Scholar] [CrossRef]
- Patano, A.; Malcangi, G.; Inchingolo, A.D.; Garofoli, G.; De Leonardis, N.; Azzollini, D.; Latini, G.; Mancini, A.; Carpentiere, V.; Laudadio, C.; et al. Mandibular Crowding: Diagnosis and Management-A Scoping Review. J. Pers. Med. 2023, 13, 774. [Google Scholar] [CrossRef]
- Evangelista, M.B.; Berzins, D.W.; Monaghan, P. Effect of Disinfecting Solutions on the Mechanical Properties of Orthodontic Elastomeric Ligatures. Angle Orthod. 2007, 77, 681–687. [Google Scholar] [CrossRef]
- Nakhaei, S.; Agahi, R.H.; Aminian, A.; Rezaeizadeh, M. Discoloration and Force Degradation of Orthodontic Elastomeric Ligatures. Dent. Press. J. Orthod. 2017, 22, 45–54. [Google Scholar] [CrossRef]
- Forsberg, C.M.; Brattström, V.; Malmberg, E.; Nord, C.E. Ligature Wires and Elastomeric Rings: Two Methods of Ligation, and Their Association with Microbial Colonization of Streptococcus mutans and Lactobacilli. Eur. J. Orthod. 1991, 13, 416–420. [Google Scholar] [CrossRef]
- Brêtas, S.M.; Macari, S.; Elias, A.M.; Ito, I.Y.; Matsumoto, M.A.N. Effect of 0.4% Stannous Fluoride Gel on Streptococci mutans in Relation to Elastomeric Rings and Steel Ligatures in Orthodontic Patients. Am. J. Orthod. Dentofac. Orthop. 2005, 127, 428–433. [Google Scholar] [CrossRef]
- Jeon, H.-S.; Choi, C.-H.; Kang, S.-M.; Kwon, H.-K.; Kim, B.-I. Chlorhexidine-Releasing Orthodontic Elastomerics. Dent. Mater. J. 2015, 34, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Gómora, A.E.; Lara-Carrillo, E.; Robles-Navarro, J.B.; Scougall-Vilchis, R.J.; Hernández-López, S.; Medina-Solís, C.E.; Morales-Luckie, R.A. Biosynthesis of Silver Nanoparticles on Orthodontic Elastomeric Modules: Evaluation of Mechanical and Antibacterial Properties. Molecules 2017, 22, 1407. [Google Scholar] [CrossRef]
- Husain, S.; Al-Samadani, K.H.; Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Qasim, S.B. Chitosan Biomaterials for Current and Potential Dental Applications. Materials 2017, 10, 602. [Google Scholar] [CrossRef]
- Ayala Valencia, G. Efecto Antimicrobiano del Quitosano: Una Revisión de la Literatura. Sci. Agroaliment. 2015, 2, 32–38. [Google Scholar]
- Chuc Gamboa, M.G. Efecto de la Modificación Química y Térmica en las Propiedades de Andamios de Quitosano para Regeneración ósea. Tesis de Doctorado, Centro de Investigación Científica de Yucatán (CICY), Merida, Mexico, 2020. [Google Scholar]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent Advances on Chitosan-Based Micro- and Nanoparticles in Drug Delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Li, B.; Shan, C.-L.; Zhou, Q.; Fang, Y.; Wang, Y.-L.; Xu, F.; Han, L.-R.; Ibrahim, M.; Guo, L.-B.; Xie, G.-L.; et al. Synthesis, Characterization, and Antibacterial Activity of Cross-Linked Chitosan-Glutaraldehyde. Mar. Drugs 2013, 11, 1534–1552. [Google Scholar] [CrossRef] [PubMed]
- Cusihuamán Noa, S.; Talavera Núñez, M.E.; Arenas Chávez, C.; Pacheco Salazar, D.G.; Vera Gonzales, C. Caracterización por Técnicas Espectroscópicas del O-Carboximetilquitosano Obtenido por Derivatización del Quitosano. Rev. Soc. Química Perú 2018, 84, 204–216. [Google Scholar] [CrossRef]
- Cánepa Ivazeta, J.L. Obtención de Quitosanas Con Alto Grado de Desacetilación; Pontificia Universidad Católica del Perú: Lima, Peru, 2018. [Google Scholar]
- Curbelo Hernández, C.; Palacio Dubois, Y.; Fanego Hernández, S. Desacetilación de Quitina Obtenida por vía Química de Exoesqueletos de Camarón Litopenaeus vannamei. Cent. Azúcar 2021, 48, 53–61. [Google Scholar]
- Yu, Q.; Song, Y.; Shi, X.; Xu, C.; Bin, Y. Preparation and Properties of Chitosan Derivative/Poly (Vinyl Alcohol) Blend Film Crosslinked with Glutaraldehyde. Carbohydr. Polym. 2011, 84, 465–470. [Google Scholar] [CrossRef]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for Chitosan Based Antimicrobial Films in Food Applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- D’Almeida, M.; Attik, N.; Amalric, J.; Brunon, C.; Renaud, F.; Abouelleil, H.; Toury, B.; Grosgogeat, B. Chitosan Coating as an Antibacterial Surface for Biomedical Applications. PLoS ONE 2017, 12, e0189537. [Google Scholar] [CrossRef]
- Uysal, T.; Akkurt, M.D.; Amasyali, M.; Ozcan, S.; Yagci, A.; Basak, F.; Sagdic, D. Does a Chitosan-Containing Dentifrice Prevent Demineralization around Orthodontic Brackets? Angle Orthod. 2011, 81, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Sehmi, S.K.; Allan, E.; MacRobert, A.J.; Parkin, I. The Bactericidal Activity of Glutaraldehyde-Impregnated Polyurethane. MicrobiologyOpen 2016, 5, 891–897. [Google Scholar] [CrossRef]
- Jayakrishnan, A.; Jameela, S.R. Glutaraldehyde as a Fixative in Bioprostheses and Drug Delivery Matrices. Biomaterials 1996, 17, 471–484. [Google Scholar] [CrossRef]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium Dyes as Tools in Cell Biology: New Insights into Their Cellular Reduction. In Biotechnology Annual Review; Elsevier: Amsterdam, The Netherlands, 2005; Volume 11, pp. 127–152. ISBN 1387-2656. [Google Scholar]
- American Type Culture Collection (ATCC). ATCC® Strain Resources.
- Gamboa Solana, C.d.C. Actividad Antimicrobiana de Películas de Quitosano Modificado. Maestría Thesis, Universidad Autonoma de Yucatán, Merid, Mexico, 2020. [Google Scholar]
- Valizadeh, S.; Naseri, M.; Babaei, S.; Hosseini, S.M.H.; Imani, A. Development of Bioactive Composite Films from Chitosan and Carboxymethyl Cellulose Using Glutaraldehyde, Cinnamon Essential Oil and Oleic Acid. Int. J. Biol. Macromol. 2019, 134, 604–612. [Google Scholar] [CrossRef]
- Monteiro, O.A.; Airoldi, C. Some Studies of Crosslinking Chitosan-Glutaraldehyde Interaction in a Homogeneous System. Int. J. Biol. Macromol. 1999, 26, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Beppu, M.M.; Vieira, R.S.; Aimoli, C.G.; Santana, C.C. Crosslinking of Chitosan Membranes Using Glutaraldehyde: Effect on Ion Permeability and Water Absorption. J. Membr. Sci. 2007, 301, 126–130. [Google Scholar] [CrossRef]
- Jeong, K.-J.; Song, Y.; Shin, H.-R.; Kim, J.E.; Kim, J.; Sun, F.; Hwang, D.-Y.; Lee, J. In Vivo Study on the Biocompatibility of Chitosan-Hydroxyapatite Film Depending on Degree of Deacetylation. J. Biomed. Mater. Res. A 2017, 105, 1637–1645. [Google Scholar] [CrossRef]
- Escobar-Sierra, D.M.; Perea-Mesa, Y.P. Manufacturing and Evaluation of Chitosan, PVA and Aloe Vera Hydrogels for Skin Applications. Dyna 2017, 84, 134–142. [Google Scholar] [CrossRef]
- Bujňáková, Z.; Dutková, E.; Zorkovská, A.; Baláž, M.; Kováč, J.; Kello, M.; Mojžiš, J.; Briančin, J.; Baláž, P. Mechanochemical Synthesis and in Vitro Studies of Chitosan-Coated InAs/ZnS Mixed Nanocrystals. J. Mater. Sci. 2017, 52, 721–735. [Google Scholar] [CrossRef]
- Ren, X.D.; Liu, Q.S.; Feng, H.; Yin, X.Y. The Characterization of Chitosan Nanoparticles by Raman Spectroscopy. Appl. Mech. Mater. 2014, 665, 367–370. [Google Scholar] [CrossRef]
- Mai, T.T.T.; Ha, P.T.; Pham, H.N.; Le, T.T.H.; Pham, H.L.; Phan, T.B.H.; Tran, D.L.; Nguyen, X.P. Chitosan and O-Carboxymethyl Chitosan Modified Fe3O4 for Hyperthermic Treatment. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 015006. [Google Scholar] [CrossRef]
- Berni Osorio, L.; Makito Osawa Gutierrez, L.; Martinelli de Lima, E.; Gonçalves Mota, E.; Macedo de Menezes, L. Disinfection of Orthodontic Elastomers and Its Effects on Tensile Strength. Turk. J. Orthod. 2022, 35, 22–26. [Google Scholar] [CrossRef]
- Eliades, T.; Eliades, G.; Silikas, N.; Watts, D.C. In Vitro Degradation of Polyurethane Orthodontic Elastomeric Modules. J. Oral Rehabil. 2005, 32, 72–77. [Google Scholar] [CrossRef]
- Pithon, M.M.; Ferraz, C.S.; Rosa, F.C.S.; Rosa, L.P. Sterilizing Elastomeric Chains without Losing Mechanical Properties. Is It Possible? Dent. Press. J. Orthod. 2015, 20, 96–100. [Google Scholar] [CrossRef]
- Stevenson, J.S.; Kusy, R.P. Structural Degradation of Polyurethane-Based Elastomeric Modules. J. Mater. Sci. Mater. Med. 1995, 6, 377–384. [Google Scholar] [CrossRef]
- Losito, K.A.B.; Lucato, A.S.; Tubel, C.A.M.; Correa, C.A.; Santos, J.C.B. dos Force decay in orthodontic elastomeric chains after immersion in disinfection solutions. Braz. J. Oral. Sci. 2014, 13, 266–269. [Google Scholar] [CrossRef]
- Terheyden, H.; Lee, U.; Ludwig, K.; Kreusch, T.; Hedderich, J. Sterilization of Elastic Ligatures for Intraoperative Mandibulomaxillary Immobilization. Br. J. Oral. Maxillofac. Surg. 2000, 38, 299–304. [Google Scholar] [CrossRef]
- McKamey, R.P.; Whitley, J.Q.; Kusy, R.P. Physical and Mechanical Characteristics of a Chlorine-Substituted Poly (Para-Xylylene) Coating on Orthodontic Chain Modules. J. Mater. Sci. Mater. Med. 2000, 11, 407–419. [Google Scholar] [CrossRef]
- Lam, T.V.; Freer, T.J.; Brockhurst, P.J.; Podlich, H.M. Strength Decay of Orthodontic Elastomeric Ligatures. J. Orthod. 2002, 29, 37–43. [Google Scholar] [CrossRef]
- Antony, P.J.; Paulose, J. An In-Vitro Study to Compare the Force Degradation of Pigmented and Non-Pigmented Elastomeric Chains. Indian. J. Dent. Res. 2014, 25, 208–213. [Google Scholar]
- Halimi, A.; Azeroual, M.-F.; Doukkali, A.; El Mabrouk, K.; Zaoui, F. Elastomeric Chain Force Decay in Artificial Saliva: An in Vitro Study. Int. Orthod. 2013, 11, 60–70. [Google Scholar] [CrossRef]
- Dowling, P.A.; Jones, W.B.; Lagerstrom, L.; Sandham, J.A. An Investigation into the Behavioural Characteristics of Orthodontic Elastomeric Modules. Br. J. Orthod. 1998, 25, 197–202. [Google Scholar] [CrossRef]
- Datana, S.; Sengupta, J.; Sharma, V. Structural and Mechanical Characterization of Newer Elastomeric Modules. J. Ind. Orthod. Soc. 2006, 39, 23–29. [Google Scholar]
- Kamarudin, Y.; Skeats, M.K.; Ireland, A.J.; Barbour, M.E. Chlorhexidine Hexametaphosphate as a Coating for Elastomeric Ligatures with Sustained Antimicrobial Properties: A Laboratory Study. Am. J. Orthod. Dentofac. Orthop. 2020, 158, e73–e82. [Google Scholar] [CrossRef]
- Benson, P.E.; Douglas, C.W.I.; Martin, M.V. Fluoridated Elastomers: Effect on the Microbiology of Plaque. Am. J. Orthod. Dentofac. Orthop. 2004, 126, 325–330. [Google Scholar] [CrossRef]
- Doherty, U.B.; Benson, P.E.; Higham, S.M. Fluoride-Releasing Elastomeric Ligatures Assessed with the in Situ Car Ies Model. Eur. J. Orthod. 2002, 24, 371–378. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Lee, D.-Y.; Lee, J.-Y.; Lim, Y.-K. The Effect of Silver Ion-Releasing Elastomers on Mutans Streptococci in Dental Plaque. Korean J. Orthod. 2012, 42, 87–93. [Google Scholar] [CrossRef]
- Shi, P.; Zuo, Y.; Zou, Q.; Shen, J.; Zhang, L.; Li, Y.; Morsi, Y.S. Improved Properties of Incorporated Chitosan Film with Ethyl Cellulose Microspheres for Controlled Release. Int. J. Pharm. 2009, 375, 67–74. [Google Scholar] [CrossRef]
- Garner, S.J.; Nobbs, A.H.; McNally, L.M.; Barbour, M.E. An Antifungal Coating for Dental Silicones Composed of Chlorhexidine Nanoparticles. J. Dent. 2015, 43, 362–372. [Google Scholar] [CrossRef]
- Padois, K.; Bertholle, V.; Pirot, F.; Hyunh, T.T.N.; Rossi, A.; Colombo, P.; Falson, F.; Sonvico, F. Chlorhexidine Salt-Loaded Polyurethane Orthodontic Chains: In Vitro Release and Antibacterial Activity Studies. AAPS PharmSciTech 2012, 13, 1446–1450. [Google Scholar] [CrossRef] [PubMed]
- Sarasam, A.R.; Brown, P.; Khajotia, S.S.; Dmytryk, J.J.; Madihally, S.V. Antibacterial Activity of Chitosan-Based Matrices on Oral Pathogens. J. Mater. Sci. Mater. Med. 2008, 19, 1083–1090. [Google Scholar] [CrossRef]
- Frigaard, J.; Jensen, J.L.; Galtung, H.K.; Hiorth, M. The Potential of Chitosan in Nanomedicine: An Overview of the Cytotoxicity of Chitosan Based Nanoparticles. Front. Pharmacol. 2022, 13, 880377. [Google Scholar] [CrossRef] [PubMed]
- Raviña, M.; Cubillo, E.; Olmeda, D.; Novoa-Carballal, R.; Fernandez-Megia, E.; Riguera, R.; Sánchez, A.; Cano, A.; Alonso, M.J. Hyaluronic Acid/Chitosan-g-Poly (Ethylene Glycol) Nanoparticles for Gene Therapy: An Application for pDNA and siRNA Delivery. Pharm. Res. 2010, 27, 2544–2555. [Google Scholar] [CrossRef]
- Zoe, L.H.; David, S.R.; Rajabalaya, R. Chitosan Nanoparticle Toxicity: A Comprehensive Literature Review of in Vivo and in Vitro Assessments for Medical Applications. Toxicol. Rep. 2023, 11, 83–106. [Google Scholar] [CrossRef]
- Zaboon, M.H.; Saleh, A.A.; Al-Lami, H.S. Synthesis, Characterization and Cytotoxicity Investigation of Chitosan-Amino Acid Derivatives Nanoparticles in Human Breast Cancer Cell Lines. J. Mex. Chem. Soc. 2021, 65, 178–188. [Google Scholar] [CrossRef]
- Anitha, A.; Rani, V.D.; Krishna, R.; Sreeja, V.; Selvamurugan, N.; Nair, S.; Tamura, H.; Jayakumar, R. Synthesis, Characterization, Cytotoxicity and Antibacterial Studies of Chitosan, O-Carboxymethyl and N, O-Carboxymethyl Chitosan Nanoparticles. Carbohydr. Polym. 2009, 78, 672–677. [Google Scholar] [CrossRef]
- Ivanova, N.; Ermenlieva, N.; Simeonova, L.; Kolev, I.; Slavov, I.; Karashanova, D.; Andonova, V. Chlorhexidine-Silver Nanoparticle Conjugation Leading to Antimicrobial Synergism but Enhanced Cytotoxicity. Pharmaceutics 2023, 15, 2298. [Google Scholar] [CrossRef]
- Liu, J.X.; Werner, J.; Kirsch, T.; Zuckerman, J.D.; Virk, M.S. Cytotoxicity Evaluation of Chlorhexidine Gluconate on Human Fibroblasts, Myoblasts, and Osteoblasts. J. Bone Jt. Infect. 2018, 3, 165–172. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.

| Material | YS * (%) | σY (MPa) | MD (%) | σmax (MPa) | |
|---|---|---|---|---|---|
| Uncoated | 336 ± 14.4 | a | 5.06 ± 0.31 | 409 ± 11.9 | 5.12 ± 0.28 |
| CS | 324 ± 18 | ab | 4.93 ± 0.26 | 398 ± 14.1 | 4.94 ± 0.26 |
| CS-GTA | 314 ± 11.3 | b | 4.74 ± 0.22 | 393 ± 18.5 | 4.82 ± 0.24 |
| Experimental Coatings | S. mutans | S. sobrinus | S. sobrinus + S. mutans |
|---|---|---|---|
| Ch | |||
| Mean | 208 | 906.666667 | 1544 |
| SD | 60.39867548 | 154.108187 | 421.729771 |
| CS-GTA | |||
| Mean | 645.3333333 | 528 | 316 |
| SD | 184.2751566 | 276.781502 | 150.359569 |
| Control | |||
| Mean | 733.3333333 | 1116 | 1368.333333 |
| SD | 110.01 | 86.0697392 | 165.9889555 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrán-Novelo, L.G.; Aguilar-Pérez, F.J.; De La Garza-Ramos, M.A.; Cienfuegos-Sarmiento, A.A.; Herrera-Atoche, J.R.; Chuc-Gamboa, M.G.; Rodríguez-Chávez, J.A.; Cauich-Rodríguez, J.V. Mechanical and Antimicrobial Evaluation of Chitosan-Coated Elastomeric Orthodontic Modules. Dent. J. 2025, 13, 447. https://doi.org/10.3390/dj13100447
Beltrán-Novelo LG, Aguilar-Pérez FJ, De La Garza-Ramos MA, Cienfuegos-Sarmiento AA, Herrera-Atoche JR, Chuc-Gamboa MG, Rodríguez-Chávez JA, Cauich-Rodríguez JV. Mechanical and Antimicrobial Evaluation of Chitosan-Coated Elastomeric Orthodontic Modules. Dentistry Journal. 2025; 13(10):447. https://doi.org/10.3390/dj13100447
Chicago/Turabian StyleBeltrán-Novelo, Lucía Gabriela, Fernando Javier Aguilar-Pérez, Myriam Angélica De La Garza-Ramos, Arturo Abraham Cienfuegos-Sarmiento, José Rubén Herrera-Atoche, Martha Gabriela Chuc-Gamboa, Jacqueline Adelina Rodríguez-Chávez, and Juan Valerio Cauich-Rodríguez. 2025. "Mechanical and Antimicrobial Evaluation of Chitosan-Coated Elastomeric Orthodontic Modules" Dentistry Journal 13, no. 10: 447. https://doi.org/10.3390/dj13100447
APA StyleBeltrán-Novelo, L. G., Aguilar-Pérez, F. J., De La Garza-Ramos, M. A., Cienfuegos-Sarmiento, A. A., Herrera-Atoche, J. R., Chuc-Gamboa, M. G., Rodríguez-Chávez, J. A., & Cauich-Rodríguez, J. V. (2025). Mechanical and Antimicrobial Evaluation of Chitosan-Coated Elastomeric Orthodontic Modules. Dentistry Journal, 13(10), 447. https://doi.org/10.3390/dj13100447









