Clinical Performance of Extra-Short (≤5.5 mm) Compared to Longer Implants Splinted under the Same Prosthesis: A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Ethical Considerations
2.3. Participants
2.3.1. Inclusion Criteria
- Age ≥ 18 years.
- Clinical indication for placing a FPD supported by a maximum of 4 implants in the posterior regions.
- Sufficient bone height to place implants of at least 6.5 mm in length.
- Availability for follow-up observation.
- Signed informed consent.
2.3.2. Exclusion Criteria
- Need for bone-augmentation surgery prior to implant placement.
- Smoking > 10 cigarettes/day.
- Patients with poorly controlled diabetes.
- Patients on chronic treatment with non-steroidal anti-inflammatory drugs (NSAIDs).
- Patients on oral or intravenous bisphosphonates.
- Patients undergoing chemotherapy or radiotherapy.
- Patients on systemic corticosteroids.
2.4. Randomization
2.4.1. Sequence Generation
2.4.2. Allocation Concealment Mechanisms
2.4.3. Implementation
2.5. Interventions
The Following Visit Schedule Was Employed for All the Participants
2.6. Outcomes
2.7. Sample Size Calculation
2.8. Blinding
2.9. Statistical Methods
2.10. Report/Publication Guidelines
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kondo, T.; Kanayama, K.; Egusa, H.; Nishimura, I. Current perspectives of residual ridge resorption: Pathological activation of oral barrier osteoclasts. J. Prosthodont. Res. 2023, 67, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, A.K.; Wada, M.; Ikebe, K.; Maeda, Y. To what extent residual alveolar ridge can be preserved by implant? A systematic review. Int. J. Implant Dent. 2016, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Fanghänel, J.; Proff, P.; Dietze, S.; Bayerlein, T.; Mack, F.; Gedrange, T. The morphological and clinical relevance of mandibular and maxillary bone structures for implantation. Folia Morphol. 2006, 65, 49–53. [Google Scholar]
- Terheyden, H.; Meijer, G.J.; Raghoebar, G.M. Vertical bone augmentation and regular implants versus short implants in the vertically deficient posterior mandible: A systematic review and meta-analysis of randomized studies. Int. J. Oral Maxillofac. Surg. 2021, 50, 1249–1258. [Google Scholar] [CrossRef]
- Sghaireen, M.G.; Shrivastava, D.; Alnusayri, M.O.; Alahmari, A.D.; Aldajani, A.M.; Srivastava, K.C.; Alam, M.K. Bone Grafts in Dental Implant Management: A Narrative Review. Curr. Pediatr. Rev. 2022, 19, 15–20. [Google Scholar] [CrossRef]
- Ravidà, A.; Serroni, M.; Borgnakke, W.S.; Romandini, M.; Wang, I.I.; Arena, C.; Annunziata, M.; Cecoro, G.; Saleh, M.H.A. Short (≤6 mm) compared with ≥10-mm dental implants in different clinical scenarios: A systematic review of randomized clinical trials with meta-analysis, trial sequential analysis and quality of evidence grading. J. Clin. Periodontol. 2024, 51, 936–965. [Google Scholar] [CrossRef]
- Starch-Jensen, T.; Nielsen, H.B. Sandwich osteotomy of the atrophic posterior mandible with interpositional autogenous bone block graft compared with bone substitute material: A systematic review and meta-analysis. Br. J. Oral Maxillofac. Surg. 2020, 58, e237–e247. [Google Scholar] [CrossRef]
- García-Ochoa, A.P.; Pérez-González, F.; Moreno, A.N.; Sánchez-Labrador, L.; Brinkmann, J.C.-B.; Martínez-González, J.M.; Martínez, J.L.-Q. Complications associated with inferior alveolar nerve reposition technique for simultaneous implant-based rehabilitation of atrophic mandibles. A systematic literature review. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 390–396. [Google Scholar] [CrossRef]
- Schwartz, S.R. Short Implants: An Answer to a Challenging Dilemma? Dent. Clin. N. Am. 2020, 64, 279–290. [Google Scholar] [CrossRef]
- Hsu, Y.T.; Rosen, P.S.; Choksi, K.; Shih, M.C.; Ninneman, S.; Lee, C.T. Complications of sinus floor elevation procedure and management strategies: A systematic review. Clin. Implant Dent. Relat. Res. 2022, 24, 740–765. [Google Scholar] [CrossRef]
- Thoma, D.S.; Zeltner, M.; Hüsler, J.; Hämmerle, C.H.; Jung, R.E. EAO Supplement Working Group 4—EAO CC 2015 Short implants versus sinus lifting with longer implants to restore the posterior maxilla: A systematic review. Clin. Oral Implant Res. 2015, 26, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Kermanshah, H.; Keshtkar, A.; Hassani, A.; Bitaraf, T. Comparing short implants to standard dental implants: A systematic review and meta-analysis of randomized controlled trials with extended follow-up. Evid. Based Dent. 2023, 24, 192–193. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Wu, X.; Yan, Q.; Shi, B. Are short implants (≤8.5 mm) reliable in the rehabilitation of completely edentulous patients: A systematic review and meta-analysis. J. Prosthet. Dent. 2024, 131, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Carosi, P.; Lorenzi, C.; Lio, F.; Laureti, M.; Ferrigno, N.; Arcuri, C. Short implants (≤6 mm) as an alternative treatment option to maxillary sinus lift. Int. J. Oral Maxillofac. Surg. 2021, 50, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Haas, R.; Sporniak-Tutak, K.; Garcia, A.; Taylor, T.D.; Tutak, M.; Pohl, V.; Hämmerle, C.H.F. Randomized controlled multi-centre study comparing shorter dental implants (6 mm) to longer dental implants (11-15 mm) in combination with sinus floor elevation procedures: 10-year data. J Clin Periodontol. 2024, 51, 499–509. [Google Scholar] [CrossRef]
- Lai, H.C.; Si, M.S.; Zhuang, L.F.; Shen, H.; Liu, Y.L.; Wismeijer, D. Long-term outcomes of short dental implants supporting single crowns in posterior region: A clinical retrospective study of 5–10 years. Clin. Oral Implant Res. 2013, 24, 230–237. [Google Scholar] [CrossRef]
- Naenni, N.; Sahrmann, P.; Schmidlin, P.R.; Attin, T.; Wiedemeier, D.B.; Sapata, V.; Hämmerle, C.H.F.; Jung, R.E. Five-Year Survival of Short Single-Tooth Implants (6 mm): A Randomized Controlled Clinical Trial. J. Dent. Res. 2018, 97, 887–892. [Google Scholar] [CrossRef]
- Telleman, G.; Raghoebar, G.M.; Vissink, A.; den Hartog, L.; Slater, J.J.H.; Meijer, H.J. A systematic review of the prognosis of short (<10 mm) dental implants placed in the partially edentulous patient. J. Clin. Periodontol. 2011, 38, 667–676. [Google Scholar]
- Wang, Y.; Jiang, J.; Guan, Y.; Si, M.; He, F. Retrospective Study of Short Versus Standard Posterior Implants and Analysis of Implant Failure Risk Factors. Int. J. Oral Maxillofac. Implant. 2021, 36, 1129–1136. [Google Scholar] [CrossRef]
- Al-Johany, S.S.; Al Amri, M.D.; Alsaeed, S.; Alalola, B. Dental Implant Length and Diameter: A Proposed Classification Scheme. J. Prosthodont. 2017, 26, 252–260. [Google Scholar] [CrossRef]
- Hashemi, S.; Tabatabaei, S.; Baghaei, K.; Fathi, A.; Atash, R. Long-Term Clinical Outcomes of Single Crowns or Short Fixed Partial Dentures Supported by Short (≤6 mm) Dental Implants: A Systematic Review. Eur. J. Dent. 2024, 18, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Mendes, P.A.; Silva, V.E.A.; da Costa, D.V.; de Pinho, M.M.; Chambrone, L.; Zenóbio, E.G. Effectiveness of Extra-Short (<6 mm) Implants Compared to Standard-Length Implants Associated with Bone Graft: Systematic Review. Int. J. Oral Maxillofac. Implant. 2023, 38, 29–36. [Google Scholar] [CrossRef]
- Badaró, M.M.; Mendoza Marin, D.O.; Pauletto, P.; Simek Vega Gonçalves, T.M.; Porporatti, A.L.; De Luca Canto, G. Failures in Single Extra-Short Implants (≤6 mm): A Systematic Review and Meta-analysis. Int. J. Oral Maxillofac. Implant. 2021, 36, 669–689. [Google Scholar] [CrossRef] [PubMed]
- Grunau, O.; Terheyden, H. Lateral augmentation of the sinus floor followed by regular implants versus short implants in the vertically deficient posterior maxilla: A systematic review and timewise meta-analysis of randomized studies. Int. J. Oral Maxillofac. Surg. 2023, 52, 813–824. [Google Scholar] [CrossRef]
- Guljé, F.L.; Raghoebar, G.M.; Gareb, B.; Vissink, A.; Meijer, H.J.A. Single crown restorations supported by 6-mm implants in the resorbed posterior mandible: A 10-year prospective case series. Clin Implant Dent Relat Res. 2024, 26, 642–650. [Google Scholar] [CrossRef]
- Torgerson, D.J.; Roberts, C. Understanding controlled trials. Randomisation methods: Concealment. BMJ 1999, 319, 375–376. [Google Scholar] [CrossRef]
- Anitua, E.; Carda, C.; Andia, I. A novel drilling procedure and subsequent bone autograft preparation: A technical note. Int. J. Oral Maxillofac. Implant. 2007, 22, 138–145. [Google Scholar]
- Bolle, C.; Felice, P.; Barausse, C.; Pistilli, V.; Trullenque-Eriksson, A.; Esposito, M. 4 mm long vs longer implants in augmented bone in posterior atrophic jaws: 1-year post-loading results from a multicentre randomised controlled trial. Eur. J. Oral Implant. 2018, 11, 31–47. [Google Scholar]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. PLoS Med. 2010, 7, e1000251. [Google Scholar] [CrossRef]
- Emfietzoglou, R.; Dereka, X. Survival Rates of Short Dental Implants (≤6 mm) Used as an Alternative to Longer (>6 mm) Implants for the Rehabilitation of Posterior Partial Edentulism: A Systematic Review of RCTs. Dent. J. 2024, 12, 185. [Google Scholar] [CrossRef]
- Mangano, F.G.; Shibli, J.A.; Sammons, R.L.; Iaculli, F.; Piattelli, A.; Mangano, C. Short (8-mm) locking-taper implants supporting single crowns in posterior region: A prospective clinical study with 1-to 10-years of follow-up. Clin. Oral Implant Res. 2014, 25, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Alkhraisat, M.H.; Eguia, A. Single-crown restorations in premolar-molar regions: Short (≤6.5 mm) vs. longer implants: Retrospective cohort study. Int. J. Implant Dent. 2022, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Alkhraisat, M.H.; Piñas, L.; Orive, G. Efficacy of biologically guided implant site preparation to obtain adequate primary implant stability. Ann. Anat. Anat. Anz. 2015, 199, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Alkhraisat, M.H. Fifteen-Year Follow-up of Short Dental Implants in the Completely Edentulous Jaw: Submerged Versus Nonsubmerged Healing. Implant Dent. 2019, 28, 551–555. [Google Scholar] [CrossRef]
- Quispe-López, N.; Guadilla, Y.; Gómez-Polo, C.; López-Valverde, N.; Flores-Fraile, J.; Montero, J. The influence of implant depth, abutment height and mucosal phenotype on peri-implant bone levels: A 2-year clinical trial. J. Dent. 2024, 148, 105264. [Google Scholar] [CrossRef]
- Del Amo, F.S.; Romero-Bustillos, M.; Catena, A.; Galindo-Moreno, P.; Sánchez-Suárez, J.M.; Sánchez, R.; Garaicoa-Pazmino, C. Effect of Abutment Height on Marginal Bone Loss Around Dental Implants: A Systematic Review. Int. J. Prosthodont. 2024, 37, 95–102. [Google Scholar] [CrossRef]

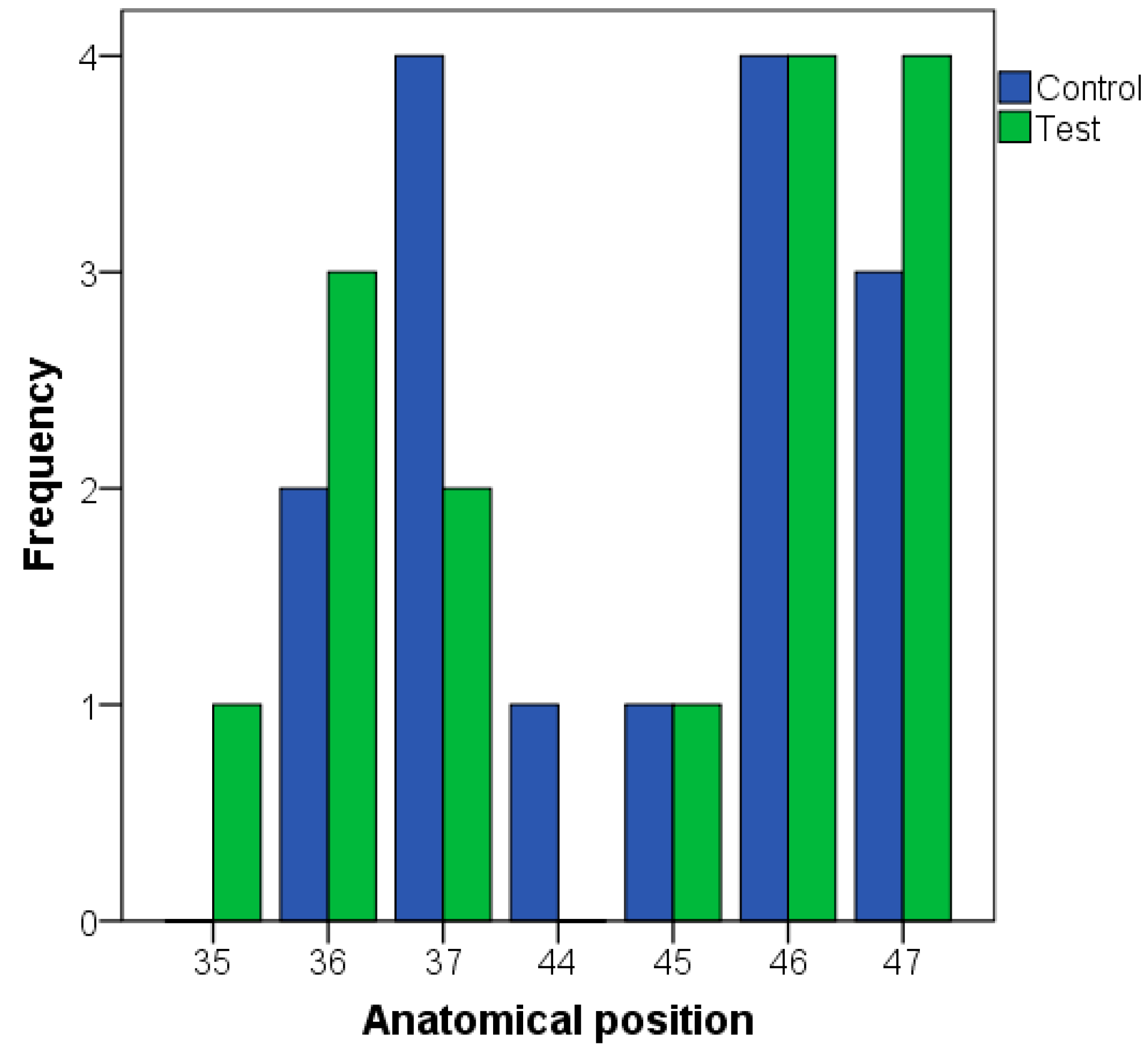
| Variable | N |
|---|---|
| Female/male | 7/8 |
| Mean age (years; SD) | 67 ± 9 |
| Medical History | |
| Arterial hypertension | 5 |
| Hypercholesteremia | 4 |
| Allergy to drugs | 3 |
| Smoking habit (≤10 cigarettes/day) | 2 |
| Extraoral cancer | 2 |
| Obstructive sleep apnea | 1 |
| Hypothyroidism | 1 |
| Diabetes mellites type II | 1 |
| Dementia | 1 |
| Autoimmune hepatitis | 1 |
| Dental History | |
| Parafunction habits | 7 |
| Temporomandibular join disorders | 4 |
| Gingivitis | 1 |
| Variable | CONTROL | TEST | p-Value | |
|---|---|---|---|---|
| Diameter (mm) | 3.00 | 0 | 1 | 0.407 a |
| 3.30 | 3 | 3 | ||
| 3.50 | 5 | 2 | ||
| 3.75 | 2 | 5 | ||
| 4.00 | 5 | 3 | ||
| 4.25 | 0 | 1 | ||
| Anatomical position | Lower premolars | 2 | 2 | 0.808 a |
| Lower molars | 13 | 13 | ||
| Bone type | II | 10 | 10 | 0.574 a |
| III | 4 | 5 | ||
| IV | 1 | 0 | ||
| Bone density (units) (mean; SD) | 636;184 | 580;110 | 0.183 b | |
| Insertion torque (Ncm) (median; range) | 60; 10 to 70 | 60; 10 to 65 | 0.888 c | |
| Loading protocol | Immediate | 13 | 13 | 0.701 d |
| Delayed | 2 | 2 | ||
| Variable | CONTROL | TEST | p-Value | |
|---|---|---|---|---|
| Mesial MBL—Loading (mm) | Mean; SD | 0.60; 0.62 | 0.48; 0.39 | 0.608 a |
| Mesial MBL—12 months (mm) | Mean; SD | 0.40; 0.56 | 0.26; 0.49 | 0.430 a |
| Distal MBL—Loading (mm) | Mean; SD | 0.35; 0.48 | 0.42; 0.48 | 0.649 a |
| Distal MBL—12 months (mm) | Mean; SD | 0.38; 0.44 | 0.23; 0.58 | 0.444 a |
| Change in mesial MBL (mm) | Median; range | −0.03; −1.75 to 0.45 | −0.1; −1.19 to 0.24 | 0.955 b |
| Change in distal MBL (mm) | Median; range | 0.08; −1.45 to 0.72 | −0.05; −1.41 to 0.27 | 0.118 b |
| Implant survival | 15 | 15 | - | |
| Bleeding on probing—12 months | 2 | 2 | - | |
| Pocket proping depth (mm) | Median; range | 1.5; 1.0 to 3.0 | 1.7; 1.3 to 3.2 | 0.844 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anitua, E.; Montalvillo, A.; Eguia, A.; Alkhraisat, M.H. Clinical Performance of Extra-Short (≤5.5 mm) Compared to Longer Implants Splinted under the Same Prosthesis: A Randomized Clinical Trial. Dent. J. 2024, 12, 292. https://doi.org/10.3390/dj12090292
Anitua E, Montalvillo A, Eguia A, Alkhraisat MH. Clinical Performance of Extra-Short (≤5.5 mm) Compared to Longer Implants Splinted under the Same Prosthesis: A Randomized Clinical Trial. Dentistry Journal. 2024; 12(9):292. https://doi.org/10.3390/dj12090292
Chicago/Turabian StyleAnitua, Eduardo, Adriana Montalvillo, Asier Eguia, and Mohammad Hamdan Alkhraisat. 2024. "Clinical Performance of Extra-Short (≤5.5 mm) Compared to Longer Implants Splinted under the Same Prosthesis: A Randomized Clinical Trial" Dentistry Journal 12, no. 9: 292. https://doi.org/10.3390/dj12090292
APA StyleAnitua, E., Montalvillo, A., Eguia, A., & Alkhraisat, M. H. (2024). Clinical Performance of Extra-Short (≤5.5 mm) Compared to Longer Implants Splinted under the Same Prosthesis: A Randomized Clinical Trial. Dentistry Journal, 12(9), 292. https://doi.org/10.3390/dj12090292







