Evaluation of Cytotoxicity of the Dental Materials TheraCal LC, TheraCal PT, ApaCal ART and Biodentine Used in Vital Pulp Therapy: In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Extraction of Test Materials
2.3. Evaluation of Cytotoxicity of Test Materials Using the MTT Assay
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Innes, N.P.T.; Frencken, J.E.; Bjørndal, L.; Maltz, M.; Manton, D.J.; Ricketts, D.; Van Landuyt, K.; Banerjee, A.; Campus, G.; Doméjean, S.; et al. Managing Carious Lesions: Consensus Recommendations on Terminology. Adv. Dent. Res. 2016, 28, 49–57. [Google Scholar] [CrossRef]
- Duncan, H.F.; Galler, K.M.; Tomson, P.L.; Simon, S.; El-Karim, I.; Kundzina, R.; Krastl, G.; Dammaschke, T.; Fransson, H.; Markvart, M.; et al. European Society of Endodontology Position Statement: Management of Deep Caries and the Exposed Pulp. Int. Endod. J. 2019, 52, 923–934. [Google Scholar] [CrossRef]
- Wells, C.; Dulong, C.; McCormack, S. Vital Pulp Therapy for Endodontic Treatment of Mature Teeth: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines. 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK546327/ (accessed on 18 April 2024).
- Hanna, S.N.; Alfayate, R.P.; Prichard, J. Vital Pulp Therapy an Insight over the Available Literature and Future Expectations. Eur. Endod. J. 2020, 5, 46–53. [Google Scholar] [PubMed]
- Novotna, B.; Harvan, L.; Somolova, L.; Morozova, Y.; Voborna, I. Osetreni kazu blizkeho zubni dreni a metoda odlozene exkavace. Czech Dent. J. 2021, 121, 83–89. [Google Scholar]
- Duncan, H.; El-Karim, I.A. Vital Pulp Treatment; Wiley-Blackwell: Hoboken, NJ, USA, 2024; ISBN 1119930405. [Google Scholar]
- Alleman, D.S.; Magne, P. A Systematic Approach to Deep Caries Removal End Points: The Peripheral Seal Concept in Adhesive Dentistry. Quintessence Int. 2012, 43, 197–208. [Google Scholar] [PubMed]
- Didilescu, A.C.; Cristache, C.M.; Andrei, M.; Voicu, G.; Perlea, P. The Effect of Dental Pulp-Capping Materials on Hard-Tissue Barrier Formation: A Systematic Review and Meta-Analysis. J. Am. Dent. Assoc. 2018, 149, 903–917.e4. [Google Scholar] [CrossRef] [PubMed]
- Davaie, S.; Hooshmand, T.; Ansarifard, S. Different Types of Bioceramics as Dental Pulp Capping Materials: A Systematic Review. Ceram. Int. 2021, 47, 20781–20792. [Google Scholar] [CrossRef]
- Krifka, S.; Seidenader, C.; Hiller, K.A.; Schmalz, G.; Schweikl, H. Oxidative Stress and Cytotoxicity Generated by Dental Composites in Human Pulp Cells. Clin. Oral Investig. 2012, 16, 215–224. [Google Scholar] [CrossRef]
- Iaculli, F.; Rodríguez-Lozano, F.J.; Briseño-Marroquín, B.; Wolf, T.G.; Spagnuolo, G.; Rengo, S. Vital Pulp Therapy of Permanent Teeth with Reversible or Irreversible Pulpitis: An Overview of the Literature. J. Clin. Med. 2022, 11, 4016. [Google Scholar] [CrossRef]
- Ha, W.; Kahler, B.; Walsh, L.J. Classification and Nomenclature of Commercial Hygroscopic Dental Cements. Eur. Endod. J. 2017, 2, 27. [Google Scholar] [CrossRef]
- Lee, S.J.; Monsef, M.; Torabinejad, M. Sealing Ability of a Mineral Trioxide Aggregate for Repair of Lateral Root Perforations. J. Endod. 1993, 19, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Cervino, G.; Laino, L.; D’Amico, C.; Russo, D.; Nucci, L.; Amoroso, G.; Gorassini, F.; Tepedino, M.; Terranova, A.; Gambino, D.; et al. Mineral Trioxide Aggregate Applications in Endodontics: A Review. Eur. J. Dent. 2020, 14, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Prati, C.; Gandolfi, M.G. Calcium Silicate Bioactive Cements: Biological Perspectives and Clinical Applications. Dent. Mater. 2015, 31, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Dawood, A.E.; Parashos, P.; Wong, R.H.K.; Reynolds, E.C.; Manton, D.J. Calcium Silicate-Based Cements: Composition, Properties, and Clinical Applications. J. Investig. Clin. Dent. 2017, 8, e12195. [Google Scholar] [CrossRef] [PubMed]
- Kadali, N.; Krishna Alla, R.; Guduri, V.; Av, R.; Sajjan Mc, S.; Venkateswara Raju 5 1 Lecturer, R. Mineral Trioxide Aggregate: An Overview of Composition, Properties and Clinical Applications. Int. J. Dent. Mater. 2020, 2, 11–18. [Google Scholar] [CrossRef]
- Gasperi, T.L.; da Silveira, J.d.A.C.; Schmidt, T.F.; Teixeira, C.d.S.; Garcia, L.d.F.R.; Bortoluzzi, E.A. Physical-Mechanical Properties of a Resin-Modified Calcium Silicate Material for Pulp Capping. Braz. Dent. J. 2020, 31, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Suh, B.I. Cytotoxicity and Biocompatibility of Resin-Free and Resin-Modified Direct Pulp Capping Materials: A State-of-the-Art Review. Dent. Mater. J. 2017, 36, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Žižka, R.; Šedý, J.; Škrdlant, J.; Kučera, P.; Čtvrtlík, R.; Tomáštík, J. Kalciumsilikátové Cementy. 1. Část: Vlastnosti a Rozdělení. LKS Časopis České Stomatol. Komory 2018, 28, 37–43. [Google Scholar]
- Domingos Pires, M.; Cordeiro, J.; Vasconcelos, I.; Alves, M.; Quaresma, S.A.; Ginjeira, A.; Camilleri, J. Effect of Different Manipulations on the Physical, Chemical and Microstructural Characteristics of Biodentine. Dent. Mater. 2021, 37, e399–e406. [Google Scholar] [CrossRef]
- Docimo, R.; Carrante, V.F.; Costacurta, M. The Physical-Mechanical Properties and Biocompatibility of Biodentine: A Review. J. Osseointegration 2021, 13, 47–50. [Google Scholar] [CrossRef]
- Kadali, N.S.; Alla, R.K.; AV, R.; MC, S.S.; Raju Mantena, S.; Raju, R.V. An Overview of Composition, Properties, and Applications of Biodentine. Int. J. Dent. Mater. 2021, 3, 120–126. [Google Scholar] [CrossRef]
- Awawdeh, L.; Al-Qudah, A.; Hamouri, H.; Chakra, R.J. Outcomes of Vital Pulp Therapy Using Mineral Trioxide Aggregate or Biodentine: A Prospective Randomized Clinical Trial. J. Endod. 2018, 44, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Poggio, C.; Arciola, C.R.; Beltrami, R.; Monaco, A.; Dagna, A.; Lombardini, M.; Visai, L. Cytocompatibility and Antibacterial Properties of Capping Materials. Sci. World J. 2014, 2014, 181945. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, D.; Song, D.; Kim, H.M.; Kim, S.Y. Biocompatibility and Bioactivity of Set Direct Pulp Capping Materials on Human Dental Pulp Stem Cells. Materials 2020, 13, 3925. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.L.; Soler-Doria, A.; López-García, S.; García-Bernal, D.; Rodríguez-Lozano, F.J.; Lozano, A.; Llena, C.; Forner, L.; Guerrero-Gironés, J.; Melo, M. Comparative Biological Properties and Mineralization Potential of 3 Endodontic Materials for Vital Pulp Therapy: Theracal PT, Theracal LC, and Biodentine on Human Dental Pulp Stem Cells. J. Endod. 2021, 47, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Ye, J.R.; Asiri, N.M.; Chae, Y.K.; Choi, S.C.; Nam, O.H. Biocompatibility and Bioactivity of a Dual-Cured Resin-Based Calcium Silicate Cement: In Vitro and in Vivo Evaluation. J. Endod. 2024, 50, 235–242. [Google Scholar] [CrossRef]
- Anthrayose, P.; Aggarwal, A.; Yadav, S.; Nawal, R.R.; Talwar, S. Microscopic and Elemental Characterization of Hydrated Dental Pulp Capping Agents. J. Conserv. Dent. Endod. 2021, 24, 496–501. [Google Scholar]
- Bakir, Ş.; Bakir, E.P.; Akbiyik, S.Y. Evaluation of the Bond Strength of Resin-Modified Glass Ionomer Enhanced with Bioactive Glass to Composite Resin with Different Dental Adhesive Systems. Anal. Quant. Cytopathol. Histopathol. 2021, 43, 235–241. [Google Scholar]
- Akbiyik, S.Y.; Bakir, E.P.; Bakir, S. Evaluation of the Bond Strength of Different Pulp Capping Materials to Dental Adhesive Systems: An In Vitro Study. J. Adv. Oral Res. 2021, 12, 286–295. [Google Scholar] [CrossRef]
- Karadas, M.; Köse, T.E.; Atıcı, M.G. Comparison of Radiopacity of Dentin Replacement Materials. J. Dent. Mater. Tech. 2020, 9, 195–202. [Google Scholar]
- Alazrag, M.A.; Abu-Seida, A.M.; El-Batouty, K.M.; El Ashry, S.H. Marginal Adaptation, Solubility and Biocompatibility of TheraCal LC Compared with MTA-Angelus and Biodentine as a Furcation Perforation Repair Material. BMC Oral Health 2020, 20, 298. [Google Scholar] [CrossRef] [PubMed]
- Javier Rodríguez-Lozano, F.; López-García, S.; García-Bernal, D.; Sanz, J.L.; Lozano, A.; Pecci-Lloret, M.P.; Melo, M.; López-Ginés, C.; Forner, L.; Rodríguez-Lozano, F.J. Cytocompatibility and Bioactive Properties of the New Dual-Curing Resin-Modified Calcium Silicate-Based Material for Vital Pulp Therapy. Clin. Oral Investig. 2021, 25, 5009–5024. [Google Scholar] [CrossRef] [PubMed]
- The Dentine In A Capsule Or More Article | Septodont Learning. Available online: https://www.septodontlearning.co.uk/cpd-training/biodentine/the-dentine-in-a-capsule-or-more-article (accessed on 30 May 2024).
- ČSN EN ISO 10993-5 (855220). Available online: https://www.technicke-normy-csn.cz/csn-en-iso-10993-5-855220-232664.html (accessed on 30 May 2024).
- ISO 10993-5:2009-Biological evaluation of medical devices—Part 5: Tests for in vitro cytotoxicity. Available online: https://www.iso.org/standard/36406.html (accessed on 30 May 2024).
- Lozano-Guillén, A.; López-García, S.; Rodríguez-Lozano, J.F.; Luis Sanz, J.; Lozano, A.; Llena, C.; Forner, L. Comparative Cytocompatibility of the New Calcium Silicate-Based Cement NeoPutty versus NeoMTA Plus and MTA on Human Dental Pulp Cells: An in Vitro Study. Clin. Oral Investig. 2022, 26, 7219–7228. [Google Scholar] [CrossRef] [PubMed]
- Corral, C.; Negrete, P.; Estay, J.; Osorio, S.; Covarrubias, C.; de Junior, O.B.; Barud, H. Radiopacity and Chemical Assessment of New Commercial Calcium Silicate-Based Cements. Int. J. Odontostomatol. 2018, 12, 262–268. [Google Scholar] [CrossRef][Green Version]
- Esen, M.; Guven, Y.; Seyhan, M.F.; Ersev, H.; Tuna-Ince, E.B. Evaluation of the Genotoxicity, Cytotoxicity, and Bioactivity of Calcium Silicate-Based Cements. BMC Oral Health 2024, 24, 119. [Google Scholar] [CrossRef] [PubMed]
- Birant, S.; Gokalp, M.; Duran, Y.; Koruyucu, M.; Akkoc, T.; Seymen, F. Cytotoxicity of NeoMTA Plus, ProRoot MTA and Biodentine on Human Dental Pulp Stem Cells. J. Dent. Sci. 2021, 16, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Lang, O.; Kohidai, L.; Kohidai, Z.; Dobo-Nagy, C.; Csomo, K.B.; Lajko, M.; Mozes, M.; Keki, S.; Deak, G.; Tian, K.V.; et al. Cell Physiological Effects of Glass Ionomer Cements on Fibroblast Cells. Toxicol. Vitr. 2019, 61, 104627. [Google Scholar] [CrossRef]
- Quiñonez-Ruvalcaba, F.; Bermúdez-Jiménez, C.; Aguilera-Galavíz, L.A.; Villanueva-Sánchez, F.G.; García-Cruz, S.; Gaitán-Fonseca, C. Histopathological Biocompatibility Evaluation of TheraCal PT, NeoMTA, and MTA Angelus in a Murine Model. J. Funct. Biomater. 2023, 14, 202. [Google Scholar] [CrossRef]
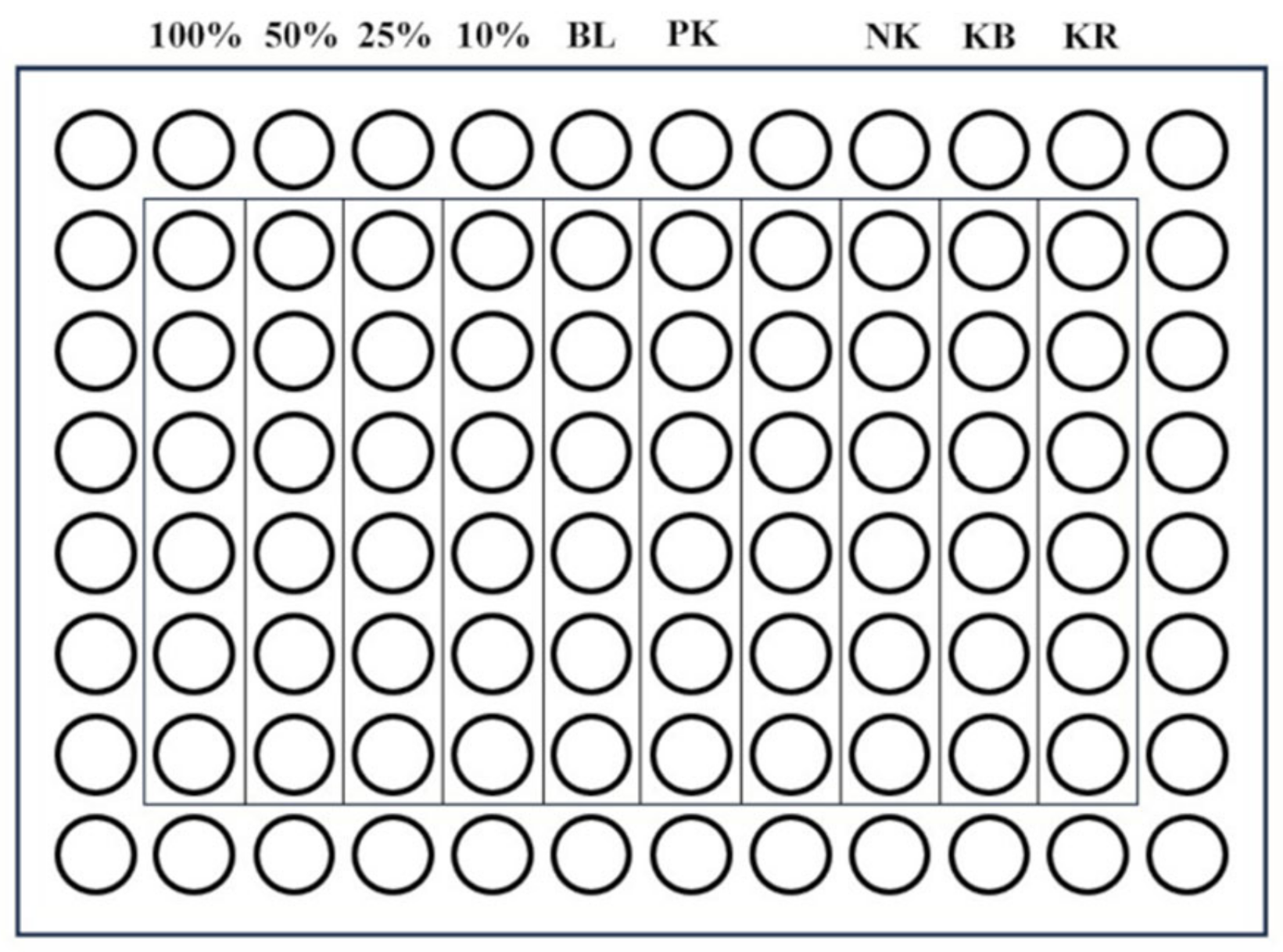

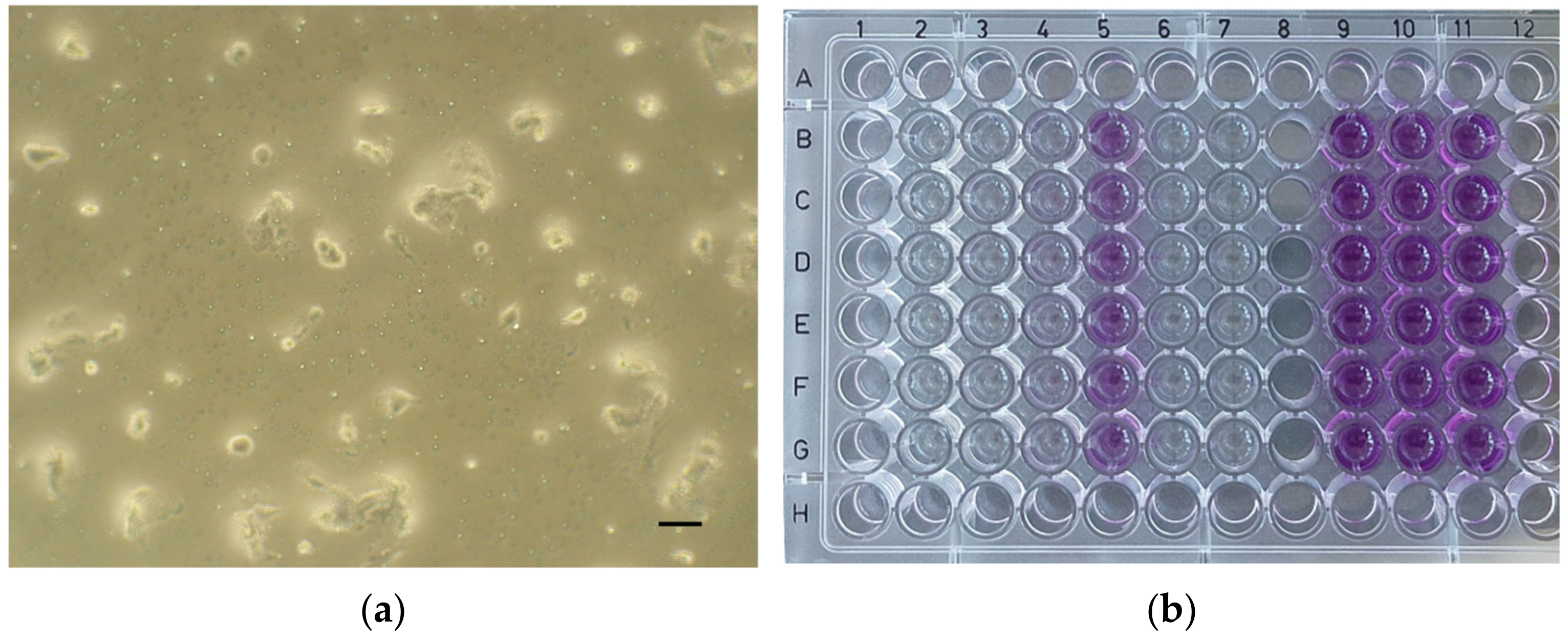
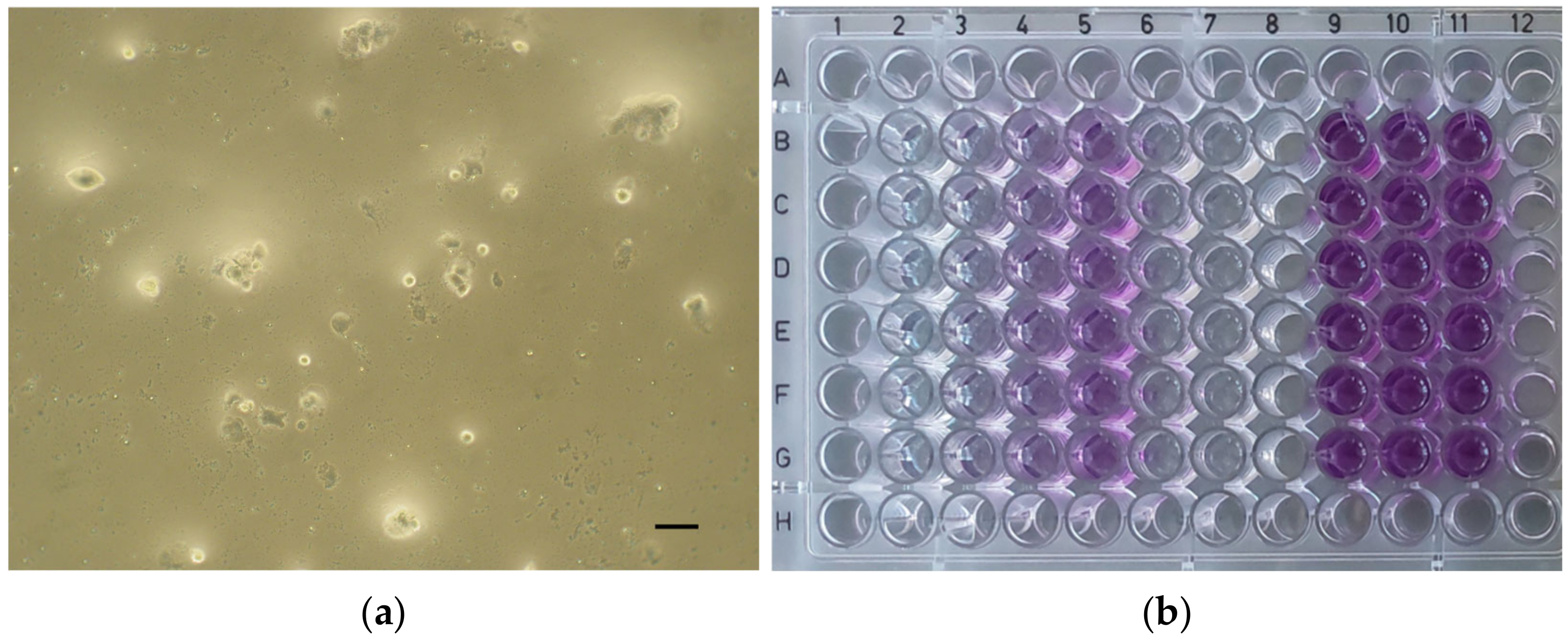
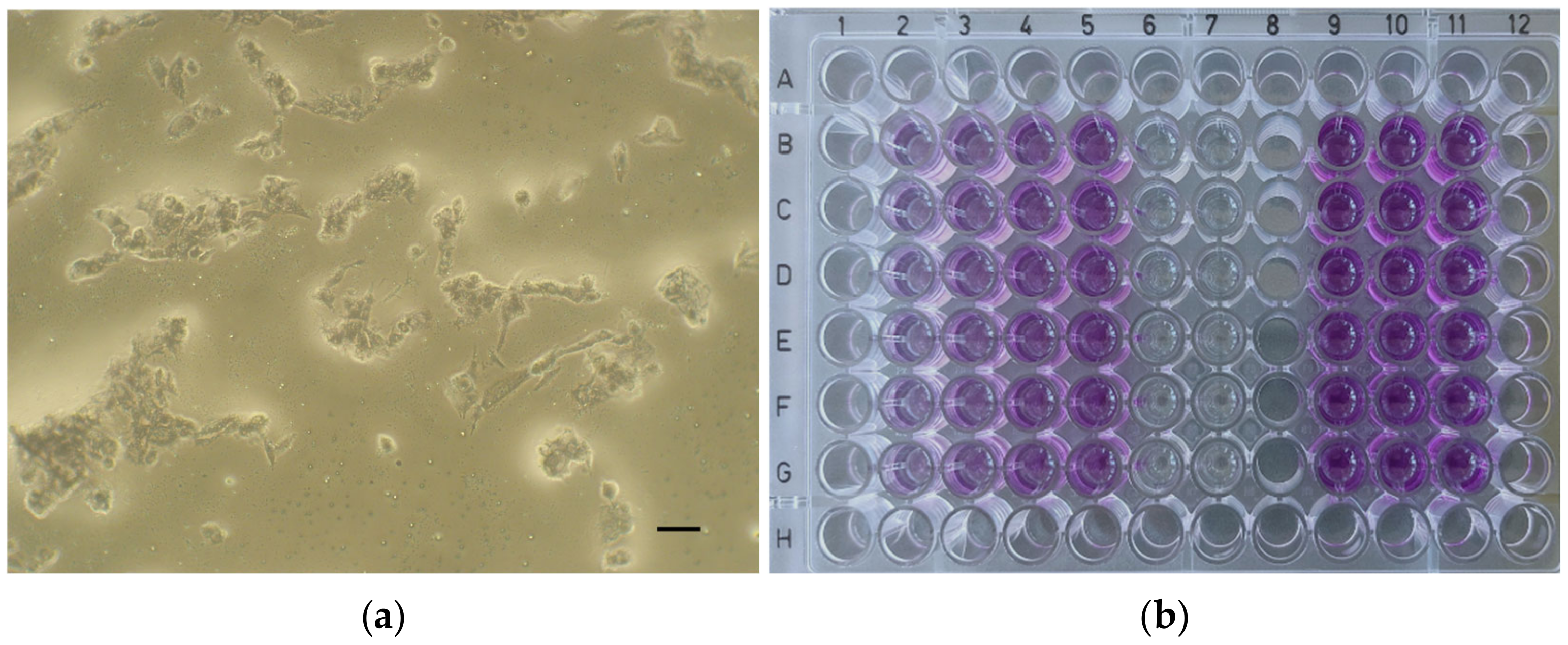


| Material | Form | Manufacturer’s Brochure | Composition According to Literature |
|---|---|---|---|
| ApaCal ART | Premixed syringe | Urethane Dimethacrylate, Triethylene Glycol Dimethacrylate, Calcium Hydroxide, Tricalcium Phosphate, Hydroxyapatite, Photoinitiators, Stabilisers, Radiopacifiers | Urethane Dimethacrylate, Triethylene Glycol Dimethacrylate, Calcium Hydroxide, Tricalcium Phosphate, Hydroxyapatite, Photoinitiators, Stabilisers, Barium Zirconate [32] |
| TheraCal LC | Premixed syringe | Resin-modified Calcium Silicate | Calcium Silicate (Portland cement type III) (30–50%), Bis-GMA (Bisphenol A Diglycidyl Meth-acrylate) (5–10%), PEGDMA (Polyethylene Glycol Dimethacrylate), Barium Zirconate (1–5%), [32,33] Strontium Glass, Fumed Silica) [34] |
| TheraCal PT | Dual syringe with automix tip | Resin-modified Calcium Silicate | SG-Mix cement (50–75%), Bis-GMA (5–10%), Barium Zirconate (1–5%), Ytterbium Fluoride (1–5%), Initiator (<1%) [34] |
| Biodentine | Powder capsule and liquid ampule (1 powder capsule: 1 single-dose container of liquid) | Tricalcium Silicate powder; Aqueous Calcium Chloride solution Excipients | Powder: Tricalcium Silicate (80.1%), Dicalcium Silicate, Calcium Carbonate (14.9%), Zirconium Oxide (5–10%); Liquid: Water, Calcium-Chloride-Modified Polycarboxylate (10–25%) [23,33,35] |
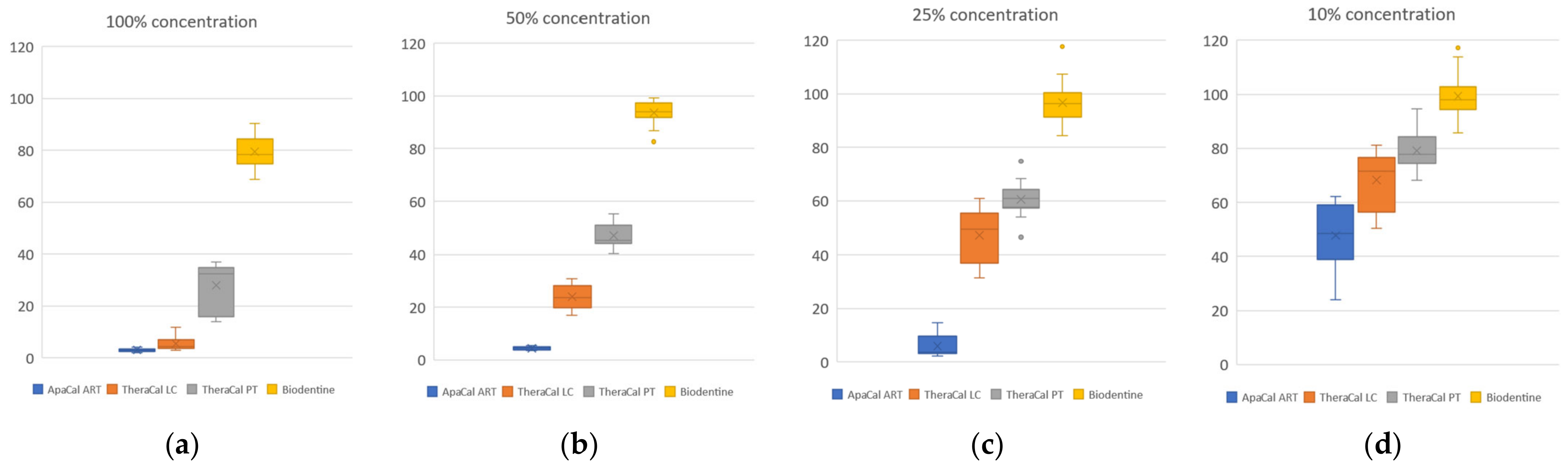
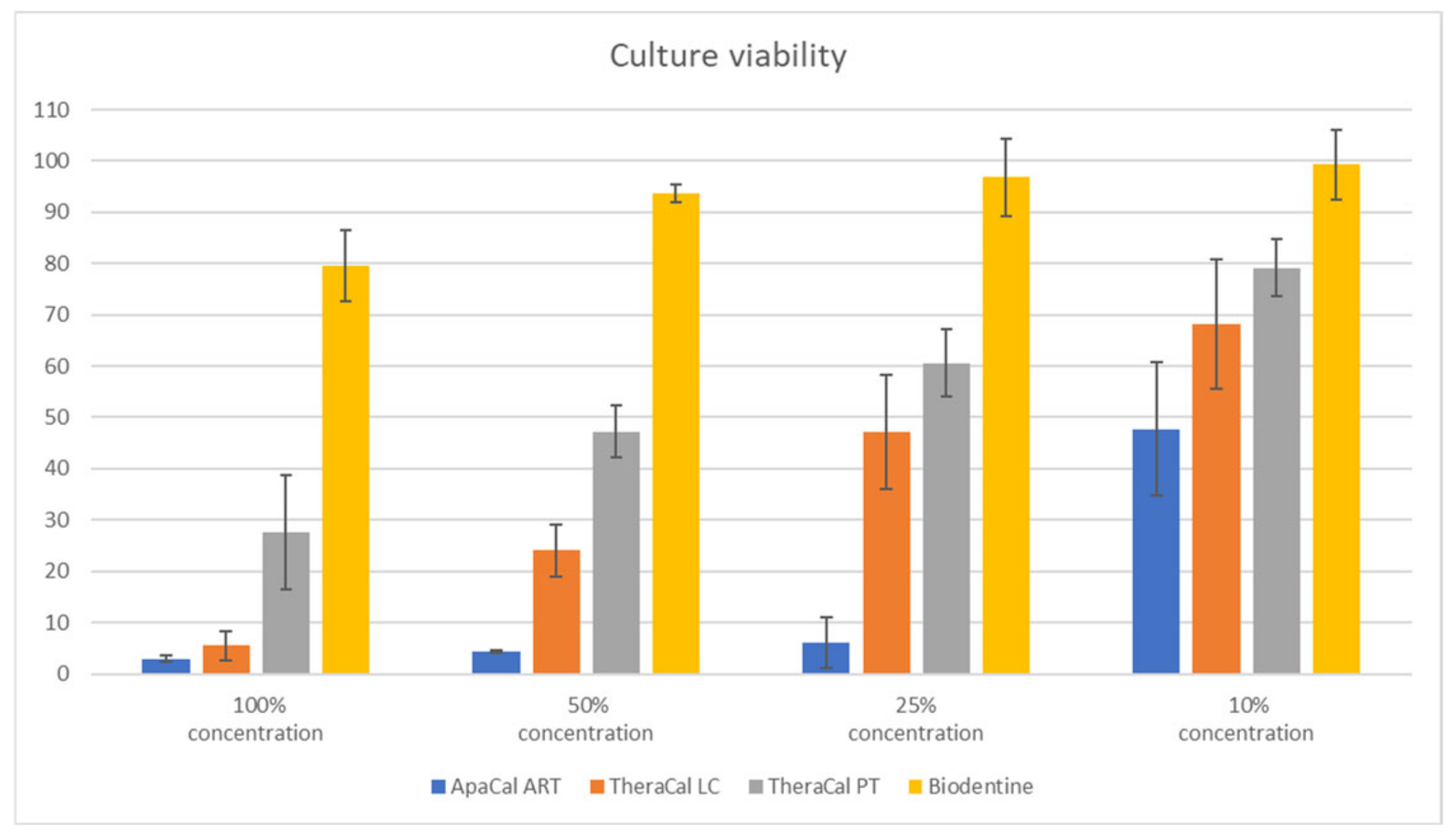
| Material Extract | 100% Concentration | 50% Concentration | 25% Concentration | 10% Concentration |
|---|---|---|---|---|
| ApaCal ART | 2.98 | 4.34 | 6.12 | 47.71 |
| TheraCal LC | 5.54 | 24.05 | 47.22 | 68.18 |
| TheraCal PT | 27.63 | 47.24 | 60.63 | 79.18 |
| Biodentine | 79.49 | 93.67 | 96.79 | 99.29 |
| Extract Concentration | Material | N | Culture Viability | p (ANOVA) | |
|---|---|---|---|---|---|
| Mean | Std. Deviation | ||||
| 100% | ApaCal ART | 3 | 2.98 | 0.60 | <0.0001 |
| TheraCal LC | 3 | 5.54 | 2.83 | ||
| TheraCal PT | 3 | 27.63 | 11.04 | ||
| Biodentine | 3 | 79.49 | 6.88 | ||
| 50% | ApaCal ART | 3 | 4.34 | 0.22 | <0.0001 |
| TheraCal LC | 3 | 24.05 | 5.15 | ||
| TheraCal PT | 3 | 47.24 | 5.06 | ||
| Biodentine | 3 | 93.67 | 1.75 | ||
| 25% | ApaCal ART | 3 | 6.12 | 4.96 | <0.0001 |
| TheraCal LC | 3 | 47.22 | 11.11 | ||
| TheraCal PT | 3 | 60.63 | 6.67 | ||
| Biodentine | 3 | 96.79 | 7.58 | ||
| 10% | ApaCal ART | 3 | 47.71 | 13.03 | 0.002 |
| TheraCal LC | 3 | 68.18 | 12.51 | ||
| TheraCal PT | 3 | 79.18 | 5.60 | ||
| Biodentine | 3 | 99.29 | 6.85 | ||
| Extract Concentration | Material | Reference Material | Mean Difference | p |
|---|---|---|---|---|
| 100% | ApaCal ART | Biodentine | −76.51667 | <0.0001 |
| TheraCal LC | Biodentine | −73.95333 | <0.0001 | |
| TheraCal PT | Biodentine | −51.86000 | <0.0001 | |
| 50% | ApaCal ART | Biodentine | −89.32667 | <0.0001 |
| TheraCal LC | Biodentine | −69.62333 | <0.0001 | |
| TheraCal PT | Biodentine | −46.43000 | <0.0001 | |
| 25% | ApaCal ART | Biodentine | −90.66667 | <0.0001 |
| TheraCal LC | Biodentine | −49.56667 | 0.0002 | |
| TheraCal PT | Biodentine | −36.15333 | 0.001 | |
| 10% | ApaCal ART | Biodentine | −51.58000 | 0.001 |
| TheraCal LC | Biodentine | −31.11000 | 0.013 | |
| TheraCal PT | Biodentine | −20.11333 | 0.095 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novotná, B.; Holík, P.; Morozova, Y.; Rosa, M.; Galandáková, A.; Langová, K. Evaluation of Cytotoxicity of the Dental Materials TheraCal LC, TheraCal PT, ApaCal ART and Biodentine Used in Vital Pulp Therapy: In Vitro Study. Dent. J. 2024, 12, 249. https://doi.org/10.3390/dj12080249
Novotná B, Holík P, Morozova Y, Rosa M, Galandáková A, Langová K. Evaluation of Cytotoxicity of the Dental Materials TheraCal LC, TheraCal PT, ApaCal ART and Biodentine Used in Vital Pulp Therapy: In Vitro Study. Dentistry Journal. 2024; 12(8):249. https://doi.org/10.3390/dj12080249
Chicago/Turabian StyleNovotná, Barbora, Pavel Holík, Yuliya Morozova, Matej Rosa, Adéla Galandáková, and Kateřina Langová. 2024. "Evaluation of Cytotoxicity of the Dental Materials TheraCal LC, TheraCal PT, ApaCal ART and Biodentine Used in Vital Pulp Therapy: In Vitro Study" Dentistry Journal 12, no. 8: 249. https://doi.org/10.3390/dj12080249
APA StyleNovotná, B., Holík, P., Morozova, Y., Rosa, M., Galandáková, A., & Langová, K. (2024). Evaluation of Cytotoxicity of the Dental Materials TheraCal LC, TheraCal PT, ApaCal ART and Biodentine Used in Vital Pulp Therapy: In Vitro Study. Dentistry Journal, 12(8), 249. https://doi.org/10.3390/dj12080249






