Microbial Leakage through Three Different Implant–Abutment Interfaces on Morse Taper Implants In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Implant and Abutment Selection and Constitution of Study Groups
2.3. Collecting Negative Control and Abutment Fixation
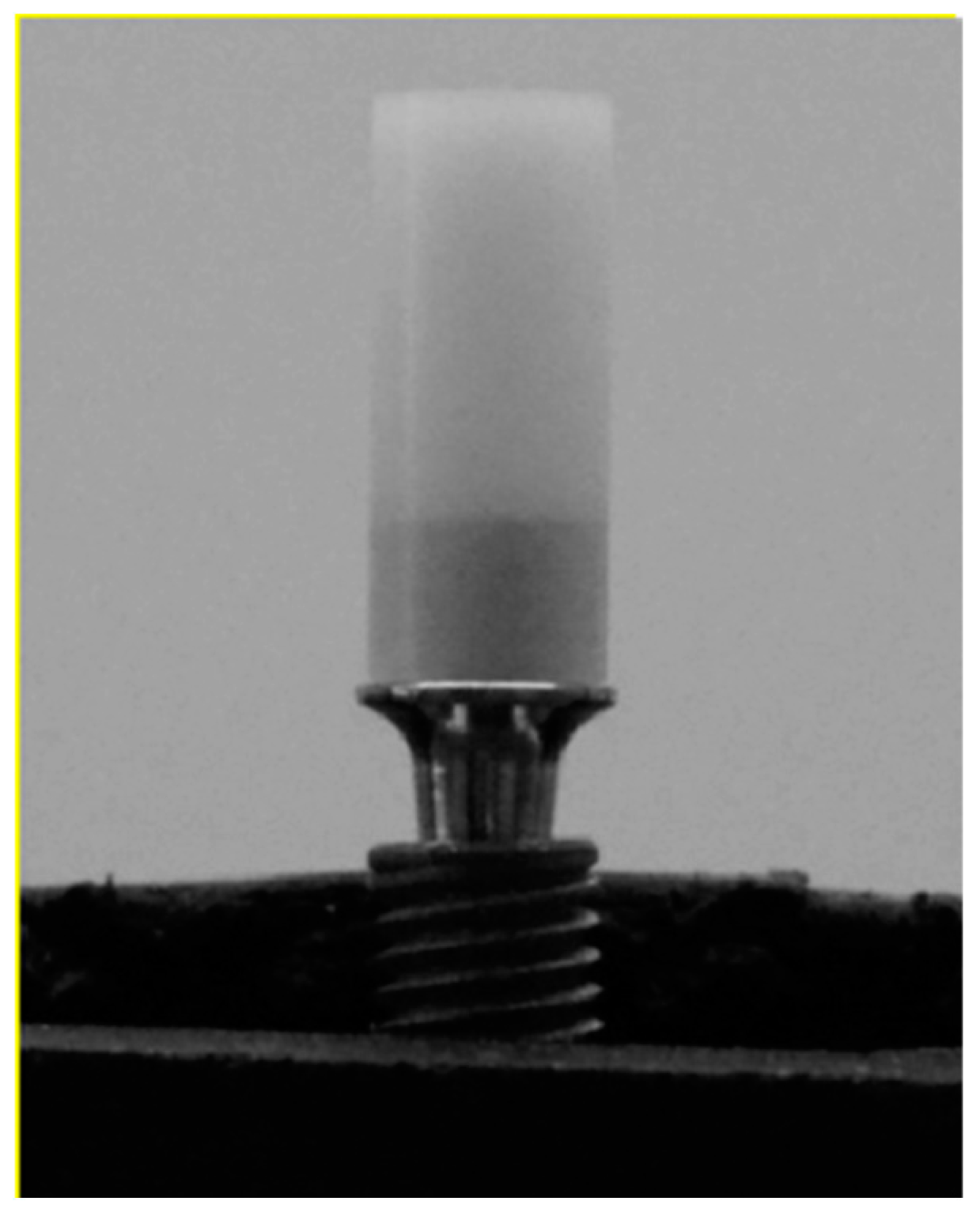
2.4. Polyurethane Bases and Sample Positioning
2.5. The 3D Crowns
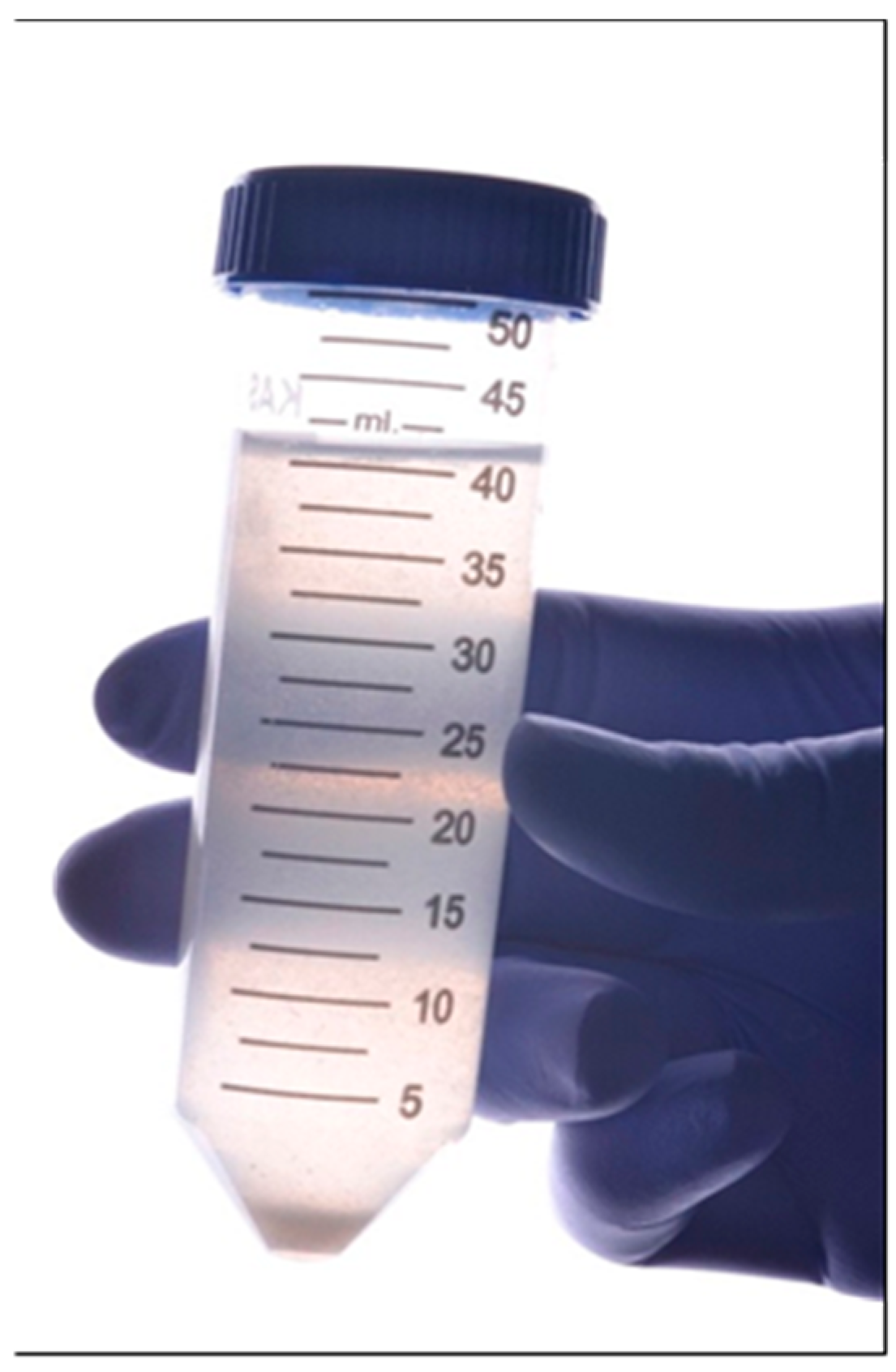
2.6. Saliva Collecting
2.7. Final Preparation of Specimens and Contamination Test
2.8. Thermomechanical Cycling
2.9. Thermocycling Test
2.10. Assessment of Microbial Leakage Using Checkerboard DNA–DNA Hybridization Method
2.11. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howe, M.S.; Keys, W.; Richards, D. Long-term (10-year) dental implant survival: A systematic review and sensitivity meta-analysis. J. Dent. 2019, 84, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Chowdhary, R.; Kumari, S. Microleakage at the different implant abutment interface: A systematic review. J. Clin. Diagn. Res. 2017, 11, ZE10–ZE15. [Google Scholar] [CrossRef] [PubMed]
- Dibart, S.; Warbington, M.; Su, M.F.; Skobe, Z. In vitro evaluation of the implant-abutment bacterial seal: The locking taper system. Int. J. Oral Maxillofac. Implants 2005, 20, 732–737. [Google Scholar] [PubMed]
- Callan, D.P.; O’Mahony, A.; Cobb, C.M. Loss of crestal bone around dental implants: A retrospective study. Implant. Dent. 1998, 7, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Bella, A.P.G.S.N.; Tuzita, A.S.; Suffredini, I.B.; Kojima, A.N.; Giovani, E.M.; Mesquita, A.M.M. Bacterial infiltration and detorque at the implant abutment Morse taper interface after masticatory simulation. Sci. Rep. 2022, 12, 17103. [Google Scholar] [CrossRef] [PubMed]
- Rismanchian, M.; Hatami, M.; Badrian, H.; Khalighinejad, N.; Goroohi, H. Evaluation of microgap size and microbial leakage in the connection area of 4 abutments with Straumann (ITI) implant. J. Oral Implantol. 2012, 38, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, K.B.; Bergamo, E.T.P.; Cruz, V.D.M.; Ramalho, I.S.; Lino, L.F.D.O.; Bonfante, E.A. Three-dimensional misfit between Ti-base abutments and implants evaluated by replica technique. J. Appl. Oral Sci. 2020, 28, e20200343. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Smith, C.; Martin, L.; Haffajee, J.A.; Uzel, N.G.; Goodson, J.M. Use of checkerboard DNA–DNA hybridization to study complex microbial ecosystems. Oral Microbiol. Immunol. 2004, 19, 352–362. [Google Scholar] [CrossRef]
- do Nascimento, C.; de Albuquerque, R.F.; Monesi, N.; Candido-Silva, J.A. Alternative method for direct DNA probe labeling and detection using the checkerboard hybridization format. J. Clin. Microbiol. 2010, 48, 3039–3040. [Google Scholar] [CrossRef]
- Jörnéus, L.; Jemt, T.; Carlsson, L. Loads and designs of screw joints for single crowns supported by osseointegrated implants. Int. J. Oral Maxillofac. Implants 1992, 7, 353–359. [Google Scholar]
- Bello Taborda, M.B.; Yaguinuma Gonçalves, G.S.; Alves de Sousa, C.; Gonçalves Assunção, W. Analysis of torque maintenance and fracture resistance after fatigue in retention screws made of different metals for screw-retained implant-borne prosthesis joints. Int. J. Dent. 2021, 2021, 9693239. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Smith, C.; Martin, L.; Paster, B.J.; Dewhirst, F.E.; Levin, A.E. “Checkerboard” DNA-DNA hybridization. Biotechniques 1994, 17, 788–792. [Google Scholar] [PubMed]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, W.; Ribeiro, R.F.; Sato, S.; Pedrazzi, V. Microleakage into and from two-stage implants: An in vitro comparative study. Int. J. Oral Maxillofac. Implants 2011, 26, 56–62. [Google Scholar] [PubMed]
- Passos, S.P.; Gressler May, L.; Faria, R.; Özcan, M.; Bottino, M.A. Implant-abutment gap versus microbial colonization: Clinical significance based on a literature review. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, C.; Ikeda, L.N.; Pita, M.S.; Pedroso e Silva, R.C.; Pedrazzi, V.; de Albuquerque Junior, R.F.; Ribeiro, R.F. Marginal fit and microbial leakage along the implant-abutment interface of fixed partial prostheses: An in vitro analysis using checkerboard DNA-DNA hybridization. J. Prosthet. Dent. 2015, 114, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Love, R.M.; Jenkinson, H.F. Invasion of dentinal tubules by oral bacteria. Crit. Rev. Oral Biol. Med. 2002, 13, 171–183. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, C.A.; Taborda, M.B.B.; Momesso, G.A.C.; Rocha, E.P.; Dos Santos, P.H.; Santiago-Júnior, J.F.; Assunção, W.G. Materials sealing preventing biofilm formation in implant/abutment joints: Which is the most effective? a systematic review and meta-analysis. J. Oral Implantol. 2020, 46, 163–171. [Google Scholar] [CrossRef]
- Vélez, J.; Peláez, J.; López-Suárez, C.; Agustín-Panadero, R.; Tobar, C.; Suárez, M.J. Influence of implant connection, abutment design and screw insertion torque on implant-abutment misfit. J. Clin. Med. 2020, 9, 2365. [Google Scholar] [CrossRef]
- Quek, C.E.; Tan, K.B.; Nicholls, J.I. Load fatigue performance of a single-tooth implant abutment system: Effect of diameter. Int. J. Oral Maxillofac. Implants 2006, 21, 929–936. [Google Scholar]
- Aloise, J.P.; Curcio, R.; Laporta, M.Z.; Rossi, L.; da Silva, A.M.A.; Rapoport, A. Microbial leakage through the implant-abutment interface of Morse taper implants in vitro. Clin. Oral Implants Res. 2010, 21, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.E.M.; Soares, G.G.; Teixeira, H.C.; Takenaka, I.K.T.M.; Diniz, S.O.F.; de Andrade, M.A.P.; Cardoso, V.N.; de Araújo, I.D. Efficacy of (99m)Tc-labeled ceftizoxime in the diagnosis of subclinical infections associated with titanium implants in rats. Surg. Infect. 2015, 16, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Asmarz, H.Y.; Magrin, G.L.; Prado, A.M.; Passoni, B.B.; Magalhães Benfatti, C.A. Evaluation of removal torque and internal surface alterations in frictional Morse taper connections after mechanical loading associated or not with oral biofilm. Int. J. Oral Maxillofac. Implants 2021, 36, 492–501. [Google Scholar] [CrossRef]
- Shah, K.K.; Sivaswamy, V. A literature review on implant abutment types, materials, and fabrication processes. J. Long. Term. Eff. Med. Implants 2022, 33, 57–66. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.W.; Kim, J.H.; Truong, V.M.; Park, Y.S. The impact of Morse taper implant design on microleakage at implant-healing abutment interface. Dent. Mater. J. 2022, 41, 767–773. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, C.; Miani, P.K.; Pedrazzi, V.; Gonçalves, R.B.; Ribeiro, R.F.; Faria, A.C.L.; Macedo, A.P.; Albuquerque, R.F., Jr. Leakage of saliva through the implant-abutment interface: In vitro evaluation of three different implant connections under unloaded and loaded conditions. Int. J. Oral Maxillofac. Implants 2012, 27, 551–560. [Google Scholar]
- Molinero-Mourelle, P.; Roccuzzo, A.; Yilmaz, B.; Lam, W.Y.H.; Pow, E.H.N.; Highsmith, J.D.R.; Gómez-Polo, M. Microleakage assessment of CAD-CAM Cobalt-Chrome and Zirconia abutments on a conical connection dental implant: A comparative in vitro study. Clin. Oral Implants Res. 2022, 33, 945–952. [Google Scholar] [CrossRef]
- Teixeira, W. In Vitro Evaluation of Bacterial Leakage at the Implant/Abutment Interface after Application of Gel Containing Metronidazole in Cone-Morse Connections Type. Ph.D. Thesis, School of Dentistry of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil, 2019; 66p. [Google Scholar]
- Jaworski, M.E.; Melo, A.C.M.; Picheth, C.M.T.; Sartori, I.A.d.M. Analysis of the bacterial seal at the implant-abutment interface in external-hexagon and Morse taper-connection implants: An in vitro study using a new methodology. Int. J. Oral Maxillofac. Implants 2012, 27, 1091–1095. [Google Scholar] [PubMed]
- Ardakani, M.R.T.; Meimandi, M.; Amid, R.; Pourahmadie, A.D.; Shidfar, S. In vitro comparison of microbial leakage of the implant-healing abutment interface in four connection systems. J. Oral Implantol. 2019, 45, 350–355. [Google Scholar] [CrossRef]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef]
- Garaicoa, J.L.; Bates, A.M.; Avila-Ortiz, G.; Brogden, K.A. Antimicrobial prosthetic surfaces in the oral cavity—A perspective on creative approaches. Microorganisms 2020, 8, 1247. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Huang, Y.W.; Zhu, L.; Weltman, R. Prevalences of peri-implantitis and peri-implant mucositis: Systematic review and meta-analysis. J. Dent. 2017, 62, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Jepsen, S.; Stadlinger, B.; Terheyden, H. Peri-implantitis and its prevention. Clin. Oral Implants Res. 2019, 30, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Bollen, C.M. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J. Clin. Periodontol. 1995, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Weart, R.B.; Lee, A.H.; Chien, A.C.; Haeusser, D.P.; Hill, N.S.; Levin, P.A. A metabolic sensor governing cell size in bacteria. Cell 2007, 130, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Cosyn, J.; Eghbali, A.; De Bruyn, H.; Collys, K.; Cleymaet, R.; De Rouck, T. Immediate single-tooth implants in the anterior maxilla: 3-year results of a case series on hard and soft tissue response and aesthetics. J. Clin. Periodontol. 2011, 38, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.M.; Nogueira-Filho, G.; Tenenbaum, H.C.; Lai, J.Y.; Brito, C.; Doering, H.; Nonhoff, J. Performance of conical abutment (Morse taper) connection implants: A systematic review. J. Biomed. Mater. Res. A 2014, 102, 552–574. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Huang, X.; Zhou, X.; Li, M.; Ren, B.; Peng, X.; Cheng, L. Influence of dental prosthesis and restorative materials interface on oral biofilms. Int. J. Mol. Sci. 2018, 19, 3157. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.-S.; Kranz, H.T.; Schneider, M.; Tietze, J.P.; Piwowarcyk, A.; Kuzius, T.; Arnold, W.; Naumova, E.A. Biofilm formation on different dental restorative materials in the oral cavity. BMC Oral Health 2020, 20, 162. [Google Scholar] [CrossRef]
- do Nascimento, C.; Miani, P.K.; Watanabe, E.; Pedrazzi, V.; de Albuqerque, R.F. In vitro evaluation of bacterial leakage along the implant-abutment interface of an external-hex implant after saliva incubation. Int. J. Oral Maxillofac. Implants 2011, 26, 782–787. [Google Scholar]
- do Nascimento, C.; Barbosa, R.E.S.; Issa, J.P.M.; Watanabe, E.; Ito, I.Y.; Albuquerque, R.F. Bacterial leakage along the implant-abutment interface of premachined or cast components. Int. J. Oral Maxillofac. Surg. 2008, 37, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Delgado-Ruiz, R.A.; Prados Frutos, J.C.; Prados-Privado, M.; Dedavid, B.A.; Granero-Marín, J.M.; Guirado, J.L.C. Misfit of three different implant-abutment connections before and after cyclic load application: An in vitro study. Int. J. Oral Maxillofac. Implants 2017, 32, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Radovanović, S.; Delibasic, B.; Blaya, J.A.; Penarrocha, D.; Rakic, M. The predictive value of microbiological findings on teeth, internal and external implant portions in clinical decision making. Clin. Oral Implant Res. 2017, 28, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D.; Zaura, E. Dental biofilm: Ecological interactions in health and disease. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S12–S22. [Google Scholar] [CrossRef] [PubMed]
- Baggi, L.; Di Girolamo, M.; Mirisola, C.; Calcaterra, R. Microbiological evaluation of bacterial and mycotic seal in implant systems with different implant-abutment interfaces and closing torque values. Implant Dent. 2013, 22, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Karkowska-Kuleta, J.; Satala, D.; Smolarz, M.; Zawrotniak, M.; Rapala-Kozik, M. Fungi—A component of the oral microbiome involved in periodontal diseases. Adv. Exp. Med. Biol. 2022, 1373, 113–138. [Google Scholar] [PubMed]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Periodontol. 2018, 89 (Suppl. S1), S267–S290. [Google Scholar] [CrossRef] [PubMed]
- Zandim-Barcelos, D.L.; Carvalho, G.G.d.; Sapata, V.M.; Villar, C.C.; Hämmerle, C.; Romito, G.A. Implant-based factor as possible risk for peri-implantitis. Braz. Oral Res. 2019, 33 (Suppl. S1), e067. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.H.; Wang, H.L. Breaking the wave of peri-implantitis. Periodontology 2000 2020, 84, 145–160. [Google Scholar] [CrossRef]
- Shibli, J.A.; Melo, L.; Ferrari, D.S.; Figueiredo, L.C.; Faveri, M.; Feres, M. Composition of supra- and subgingival biofilm of subjects with healthy and diseased implants. Clin. Oral Implant. Res. 2008, 19, 975–982. [Google Scholar] [CrossRef]
- Máximo, M.B.; de Mendonça, A.C.; Renata Santos, V.; Figueiredo, L.C.; Feres, M.; Duarte, P.M. Short-term clinical and microbiological evaluations of peri-implant diseases before and after mechanical anti-infective therapies. Clin. Oral Implant Res. 2009, 20, 99–108. [Google Scholar] [CrossRef]
- Lafaurie, G.I.; Sabogal, M.A.; Castillo, D.M.; Rincón, M.V.; Gómez, L.A.; Lesmes, Y.A.; Chambrone, L. Microbiome and microbial biofilm profiles of peri-implantitis: A systematic review. J. Periodontol. 2017, 88, 1066–1089. [Google Scholar] [CrossRef] [PubMed]
- Gujar, A.N.; Al-Hazmi, A.; Raj, A.T.; Patil, S. Microbial profile in different orthodontic appliances by checkerboard DNA-DNA hybridization: An in-vivo study. Am. J. Orthod. Dentofac. Orthop. 2020, 157, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Sahin, C.; Ayyildiz, S. Correlation between microleakage and screw loosening at implant-abutment connection. J. Adv. Prosthodont. 2014, 6, 35–38. [Google Scholar] [CrossRef]
- D’Ercole, S.; Tripodi, D.; Marzo, G.; Bernardi, S.; Continenza, M.A.; Piattelli, A.; Iaculli, F.; Mummolo, S. Microleakage of bacteria in different implant-abutment assemblies: An in vitro study. J. Appl. Biomater. Funct. Mater. 2015, 13, e174–e180. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, D.; D’Ercole, S.; Iaculli, F.; Piattelli, A.; Perrotti, V.; Iezzi, G. Degree of bacterial microleakage at the implant-abutment junction in cone Morse tapered implants under loaded and unloaded conditions. J. Appl. Biomater. Funct. Mater. 2015, 13, e367–e371. [Google Scholar] [CrossRef] [PubMed]
- Guerra, E.; Pereira, C.; Faria, R.; Jorge, A.O.; Bottino, M.A.; de Melo, R.M. The impact of conical and nonconical abutments on bacterial infiltration at the implant-abutment interface. Int. J. Periodontics Restor. Dent. 2016, 36, 825–831. [Google Scholar] [CrossRef]
- Sinjari, B.; D’Addazio, G.; De Tullio, I.; Traini, T.; Caputi, S. Peri-implant bone resorption during healing abutment placement: The effect of a 0.20 chlorhexidine gel vs. placebo—A randomized double blind controlled human study. BioMed Res. Int. 2018, 2018, 5326340. [Google Scholar] [CrossRef]








| Thermomechanical Loading | Thermocycling | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | 1st Quartile | Median | Mean | 3rd Quartile | Max | Min | 1st Quartile | Median | Mean | 3rd Quartile | Max | |
| Candida tropicalis | 499,465 | 516,712 | 534,038 | 547,554 | 557,708 | 651,489 | 400,259 | 523,034 | 537,468 | 527,930 | 542,365 | 556,524 |
| Candida glabrata | 471,179 | 544,496 | 602,260 | 599,857 | 615,837 | 811,410 | 431,936 | 503,807 | 561,537 | 563,680 | 621,410 | 699,708 |
| Candida dubliniensis | 475,402 | 504,454 | 539,168 | 544,929 | 567,558 | 683,705 | 512,943 | 573,779 | 595,362 | 583,010 | 604,592 | 628,377 |
| Candida albicans | 503,754 | 514,799 | 534,297 | 552,731 | 584,323 | 649,825 | 480,652 | 512,195 | 523,533 | 551,795 | 563,133 | 679,463 |
| S. pneumoniae | 504,896 | 567,937 | 597,443 | 587,463 | 621,210 | 642,731 | 595,870 | 598,772 | 612,444 | 625,121 | 638,792 | 679,728 |
| S. gallolyticus | 507,870 | 546,434 | 579,044 | 587,641 | 615,521 | 697,752 | 589,889 | 638,313 | 655,077 | 644,569 | 661,366 | 678,234 |
| V. parvula | 539,243 | 590,354 | 597,497 | 606,167 | 635,940 | 658,358 | 567,120 | 585,344 | 593,449 | 588,217 | 596,322 | 598,849 |
| T. denticola | 483,362 | 578,609 | 656,805 | 660,446 | 763,000 | 828,167 | 561,773 | 586,472 | 647,304 | 653,646 | 714,479 | 758,203 |
| T. forsythia | 558,789 | 611,798 | 683,665 | 746,543 | 755,839 | 134,4715 | 659,659 | 704,875 | 722,092 | 723,360 | 740,576 | 789,596 |
| S. sobrinus | 661,538 | 699,976 | 726,574 | 750,250 | 806,908 | 895,655 | 691,838 | 786,421 | 844,923 | 820,197 | 878,699 | 899,104 |
| S. sanguinis | 530,248 | 594,529 | 638,470 | 651,256 | 652,013 | 819,170 | 504,070 | 633,840 | 691,386 | 651,957 | 709,502 | 720,986 |
| S. salivarius | 542,248 | 568,458 | 622,717 | 622,594 | 704,591 | 816,027 | 583,503 | 600,370 | 649,205 | 659,604 | 708,438 | 756,500 |
| S. pasteuri | 480,058 | 558,181 | 602,310 | 622,594 | 704,591 | 816,027 | 587,990 | 600,285 | 619,728 | 628,646 | 648,089 | 687,136 |
| S. parasanguinis | 536,446 | 5,707,749 | 629,047 | 630,151 | 667,066 | 757,201 | 550,919 | 605,175 | 636,416 | 640,084 | 671,325 | 736,586 |
| S. oralis | 474,413 | 568,393 | 652,822 | 634,822 | 704,656 | 762,701 | 530,628 | 543,113 | 591,199 | 604,967 | 653,053 | 706,844 |
| S. mutans | 487,655 | 546,186 | 620,196 | 603,589 | 658,292 | 703,153 | 457,503 | 467,810 | 509,795 | 538,970 | 580,955 | 678,787 |
| S. moorei | 567,414 | 624,352 | 644,285 | 655,223 | 660,830 | 787,577 | 566,710 | 577,428 | 638,138 | 642,531 | 703,241 | 727,137 |
| S. mitis | 479,870 | 570,950 | 608,519 | 613,243 | 623,248 | 858,306 | 574,245 | 607,696 | 630,267 | 632,519 | 655,090 | 699,222 |
| S. constellatus | 545,565 | 555,355 | 610,398 | 637,886 | 684,581 | 885,305 | 511,381 | 556,467 | 588,400 | 574,351 | 606,284 | 609,222 |
| S. aureus | 487,191 | 540,175 | 675,949 | 635,201 | 702,206 | 769,954 | 587,478 | 594,220 | 612,905 | 652,605 | 671,291 | 797,133 |
| P. putida | 516,300 | 619,464 | 650,428 | 647,966 | 698,450 | 786,884 | 558,804 | 586,594 | 633,324 | 624,951 | 671,681 | 674,352 |
| P. nigrescens | 501,925 | 574,848 | 610,700 | 608,865 | 637,100 | 707,094 | 587,177 | 632,085 | 655,643 | 640,732 | 664,291 | 664,466 |
| P. micra | 481,842 | 597,128 | 614,948 | 614,355 | 662,351 | 635,758 | 560,538 | 605,321 | 649,838 | 638,953 | 683,470 | 695,596 |
| P. melaninogenica | 581,484 | 642,451 | 656,114 | 658,453 | 679,316 | 748,853 | 525,768 | 573,214 | 651,422 | 659,362 | 737,570 | 808,836 |
| P. intermedia | 515,419 | 567,509 | 622,782 | 613,075 | 654,738 | 692,820 | 422,016 | 459,808 | 558,247 | 563,794 | 66,223 | 716,664 |
| P. gingivalis | 537,493 | 574,899 | 620,351 | 613,752 | 651,885 | 690,923 | 583,254 | 612,617 | 648,828 | 643,132 | 679,342 | 691,620 |
| P. endodontalis | 509,354 | 575,225 | 616,594 | 608,420 | 649,087 | 685,654 | 565,105 | 566,852 | 577,432 | 577,239 | 587,819 | 588,988 |
| P. anaerobius | 537,745 | 568,076 | 600,039 | 607,396 | 624,819 | 715,654 | 598,955 | 599,954 | 610,824 | 613,199 | 624,069 | 632,193 |
| P. aeruginosa | 481,647 | 582,853 | 640,709 | 624,453 | 681,893 | 751,444 | 565,730 | 591,237 | 599,990 | 606,724 | 615,477 | 661,188 |
| M. salivarium | 514,754 | 551,419 | 610,696 | 594,794 | 631,804 | 662,789 | 537,426 | 538,582 | 555,831 | 577,334 | 594,583 | 660,247 |
| K. pneumoniae | 478,531 | 542,816 | 599,017 | 589,771 | 634,740 | 681,970 | 511,323 | 573,528 | 616,948 | 618,669 | 662,088 | 729,458 |
| F. nucleatum | 390,358 | 509,930 | 539,019 | 552,677 | 624,528 | 662,581 | 629,171 | 632,180 | 655,032 | 657,829 | 680,680 | 692,081 |
| E. faecalis | 449,873 | 524,863 | 540,289 | 551,201 | 589,501 | 656,899 | 615,639 | 663,619 | 684,774 | 675,718 | 696,873 | 717,685 |
| E. corrodens | 392,714 | 578,014 | 610,590 | 580,026 | 627,132 | 684,806 | 567,392 | 586,274 | 666,448 | 662,937 | 743,111 | 751,458 |
| C. rectus | 423,191 | 526,447 | 663,110 | 615,102 | 676,448 | 752,398 | 612,597 | 633,473 | 640,603 | 647,565 | 654,695 | 696,458 |
| C. gengivalis | 474,206 | 591,170 | 650,446 | 641,002 | 674,923 | 787,770 | 608,877 | 628,253 | 668,521 | 667,024 | 707,292 | 722,178 |
| B. fragilis | 508,267 | 546,144 | 620,278 | 609,245 | 668,141 | 693,737 | 560,138 | 637,204 | 666,793 | 647,105 | 676,692 | 694,699 |
| Aaa | 476,387 | 569,010 | 612,387 | 615,976 | 632,509 | 521,162 | 521,162 | 571,564 | 633,465 | 622,224 | 685,226 | 698,605 |
| C. coli | 451,384 | 571,859 | 626,636 | 607,283 | 672,237 | 707,912 | 602,417 | 614,768 | 632,057 | 635,732 | 653,021 | 676,396 |
| L. casei | 565,001 | 581,528 | 646,157 | 638,162 | 683,994 | 724,165 | 479,441 | 588,346 | 646,438 | 624,235 | 682,327 | 724,623 |
| Thermomechanical Loading | Thermocycling | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | 1st Quartile | Median | Mean | 3rd Quartile | Max | Min | 1st Quartile | Median | Mean | 3rd Quartile | Max | |
| Candida tropicalis | 496,592 | 515,731 | 526,960 | 525,193 | 53,851º | 548,276 | 489,379 | 506,751 | 512,887 | 516,540 | 522,676 | 551,008 |
| Candida glabrata | 518,446 | 527,305 | 534,480 | 538,439 | 542,217 | 582,655 | 501,586 | 508,209 | 515,254 | 527,091 | 534,136 | 576,270 |
| Candida dubliniensis | 477,982 | 517,257 | 529,519 | 525,692 | 545,424 | 565,209 | 470,631 | 490,024 | 512,224 | 506,412 | 528,612 | 530,569 |
| Candida albicans | 473,474 | 494,370 | 523,611 | 516,886 | 529,699 | 572,128 | 492,392 | 499,021 | 510,583 | 517,413 | 528,993 | 556,093 |
| S. pneumoniae | 482,061 | 508,639 | 522,327 | 518,340 | 537,917 | 544,420 | 487,890 | 500,679 | 533,283 | 531,922 | 564,526 | 573,230 |
| S. gallolyticus | 399,066 | 507,491 | 528,371 | 520,918 | 554,330 | 565,274 | 489,946 | 498,905 | 521,177 | 525,008 | 547,280 | 567,733 |
| V. parvula | 512,181 | 526,347 | 536,916 | 531,959 | 540,894 | 542,268 | 386,188 | 452,881 | 487,084 | 477,951 | 512,154 | 551,449 |
| T. denticola | 543,743 | 558,818 | 563,354 | 568,994 | 572,934 | 613,872 | 542,853 | 560,909 | 569,516 | 565,391 | 573,999 | 579,678 |
| T. forsythia | 532,057 | 543,820 | 560,373 | 561,235 | 580,801 | 585,616 | 507,034 | 530,541 | 550,539 | 552,844 | 572,841 | 603,264 |
| S. sobrinus | 519,783 | 537,888 | 549,026 | 551,081 | 557,541 | 610,955 | 569,753 | 573,052 | 577,485 | 580,099 | 584,533 | 595,673 |
| S. sanguinis | 473,787 | 514,390 | 533,482 | 531,100 | 554,510 | 580,375 | 329,607 | 416,505 | 477,452 | 468,591 | 529,538 | 589,852 |
| S. salivarius | 475,515 | 525,172 | 534,335 | 530,916 | 544,926 | 559,998 | 515,742 | 518,324 | 531,689 | 533,916 | 547,281 | 556,545 |
| S. pasteuri | 459,655 | 494,762 | 521,916 | 516,966 | 540,400 | 560,938 | 481,242 | 485,587 | 491,090 | 489,862 | 495,364 | 496,024 |
| S. parasanguinis | 551,532 | 555,873 | 560,776 | 562,206 | 564,969 | 583,635 | 522,089 | 535,622 | 548,198 | 544,030 | 556,606 | 557,635 |
| S. oralis | 547,741 | 547,741 | 557,186 | 566,023 | 570,717 | 583,620 | 561,110 | 569,979 | 578,776 | 580,457 | 588,353 | 604,966 |
| S. mutans | 521,082 | 545,371 | 571,449 | 563,677 | 582,176 | 597,851 | 548,784 | 551,513 | 561,157 | 565,602 | 575,245 | 591,308 |
| S. moorei | 506,457 | 554,874 | 565,164 | 560,009 | 573,283 | 599,603 | 544,476 | 549,398 | 560,877 | 564,643 | 576,123 | 592,343 |
| S. mitis | 526,259 | 533,981 | 548,766 | 555,798 | 574,910 | 595,837 | 100,000 | 426,065 | 535,121 | 428,344 | 537,400 | 543,136 |
| S. constellatus | 527,892 | 560,203 | 569,269 | 566,945 | 574,598 | 592,919 | 547,831 | 562,346 | 567,613 | 563,918 | 569,184 | 572,616 |
| S. aureus | 548,861 | 556,266 | 563,362 | 564,513 | 574,890 | 578,580 | 526,178 | 569,788 | 584,634 | 571,746 | 586,592 | 591,541 |
| P. putida | 500,590 | 541,112 | 549,882 | 547,336 | 560,674 | 585,879 | 503,974 | 527,182 | 537,623 | 537,638 | 548,080 | 571,334 |
| P. nigrescens | 532,600 | 545,662 | 557,655 | 564,862 | 588,724 | 594,985 | 555,658 | 556,225 | 561,188 | 566,100 | 566,562 | 568,365 |
| P. micra | 402,709 | 550,502 | 561,360 | 543,071 | 568,187 | 622,768 | 307,382 | 493,785 | 557,719 | 501,157 | 565,090 | 581,808 |
| P. melaninogenica | 495,919 | 555,366 | 562,177 | 563,158 | 575,509 | 613,968 | 534,323 | 539,015 | 545,649 | 549,336 | 555,970 | 571,724 |
| P. intermedia | 544,669 | 557,460 | 569,073 | 571,801 | 580,986 | 613,662 | 564,974 | 569,463 | 571,896 | 572,860 | 575,293 | 582,673 |
| P. gingivalis | 489,725 | 504,471 | 535,813 | 527,119 | 546,902 | 554,464 | 507,347 | 518,645 | 536,165 | 538,127 | 555,646 | 572,833 |
| P. endodontalis | 441,086 | 486,754 | 502,020 | 496,015 | 507,733 | 529,601 | 407,109 | 447,178 | 468,629 | 476,382 | 497,832 | 561,159 |
| P. anaerobius | 479,681 | 521,592 | 532,805 | 539,360 | 537,340 | 573,804 | 529,878 | 549,986 | 559,938 | 553,762 | 563,714 | 565,295 |
| P. aeruginosa | 503,678 | 522,435 | 562,092 | 553,281 | 578,722 | 601,961 | 556,387 | 557,108 | 557,552 | 563,401 | 563,845 | 582,113 |
| M. salivarium | 521,811 | 535,519 | 565,304 | 558,432 | 574,149 | 591,321 | 541,602 | 559,289 | 572,156 | 567,911 | 580,778 | 585,729 |
| K. pneumoniae | 542,472 | 568,314 | 577,548 | 581,477 | 595,568 | 617,113 | 559,294 | 566,629 | 570,637 | 578,279 | 582,288 | 612,548 |
| F. nucleatum | 560,132 | 564,347 | 570,906 | 575,682 | 581,220 | 619,010 | 543,114 | 547,686 | 557,899 | 559,287 | 569,501 | 578,237 |
| E.faecalis | 545,878 | 564,308 | 576,484 | 573,064 | 581,512 | 603,714 | 557,411 | 560,442 | 562,146 | 569,102 | 570,806 | 594,704 |
| E. corrodens | 552,590 | 568,619 | 579,075 | 578,317 | 587,834 | 608,508 | 567,342 | 567,979 | 578,663 | 578,561 | 589,245 | 589,577 |
| C. rectus | 532,558 | 561,741 | 575,519 | 577,864 | 593,144 | 615,889 | 544,577 | 565,388 | 572,980 | 569,702 | 577,294 | 588,274 |
| C. gengivalis | 537,625 | 545,711 | 556,653 | 559,280 | 571,189 | 583,272 | 521,654 | 542,848 | 556,176 | 553,238 | 566,565 | 578,945 |
| B. fragilis | 514,676 | 553,184 | 567,165 | 562,438 | 573,858 | 600,250 | 558,006 | 567,206 | 572,172 | 575,094 | 580,060 | 597,996 |
| Aaa | 520,565 | 531,841 | 549,008 | 548,850 | 565,558 | 580,608 | 534,334 | 535,150 | 540,761 | 547,236 | 552,847 | 573,088 |
| C. coli | 528,294 | 553,992 | 561,322 | 558,542 | 572,180 | 575,593 | 553,865 | 565,098 | 575,156 | 571,711 | 581,769 | 582,669 |
| L. casei | 531,608 | 573,140 | 583,355 | 580,824 | 595,654 | 604,658 | 551,711 | 557,724 | 57,660 | 576,619 | 596,555 | 599,446 |
| Thermomechanical Loading | Thermocycling | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | 1st Quartile | Median | Mean | 3rd Quartile | Max | Min | 1st Quartile | Median | Mean | 3rd Quartile | Max | |
| Candida tropicalis | 521,879 | 526,899 | 532,735 | 535,348 | 540,132 | 568,524 | 532,127 | 544,172 | 550,925 | 546,923 | 553,676 | 553,717 |
| Candida glabrata | 509,069 | 519,093 | 521,232 | 524,599 | 531,062 | 547,197 | 523,553 | 523,558 | 527,277 | 528,696 | 532,414 | 536,675 |
| Candida dubliniensis | 502,067 | 511,557 | 515,934 | 516,141 | 522,602 | 525,501 | 523,273 | 524,197 | 524,826 | 525,243 | 525,872 | 528,050 |
| Candida albicans | 505,054 | 517,696 | 520,209 | 519,744 | 524,708 | 529,092 | 518,324 | 519,122 | 521,380 | 521,729 | 523,987 | 525,834 |
| S. pneumoniae | 508,163 | 509,542 | 517,133 | 518,351 | 524,156 | 535,767 | 505,438 | 512,514 | 516,580 | 517,004 | 521,070 | 529,418 |
| S. gallolyticus | 508,370 | 511,941 | 512,435 | 514,700 | 515,767 | 524,863 | 512,751 | 517,757 | 520,574 | 519,627 | 522,444 | 524,608 |
| V. parvula | 498,994 | 505,425 | 510,424 | 509,845 | 514,491 | 519,270 | 509,489 | 511,837 | 512,774 | 515,817 | 516,755 | 528,232 |
| T. denticola | 492,806 | 510,774 | 518,132 | 517,028 | 525,095 | 540,757 | 509,681 | 510,765 | 526,990 | 524,016 | 530,242 | 532,403 |
| T. forsythia | 505,347 | 519,426 | 522,475 | 524,148 | 529,123 | 547,926 | 510,002 | 515,201 | 521,239 | 525,246 | 531,285 | 548,505 |
| S. sobrinus | 595,786 | 510,908 | 515,635 | 520,340 | 535,644 | 541,346 | 516,657 | 519,578 | 522,600 | 526,706 | 529,727 | 544,967 |
| S. sanguinis | 499,460 | 506,609 | 509,465 | 511,159 | 514,431 | 525,458 | 511,951 | 514,355 | 515,938 | 525,292 | 526,876 | 557,342 |
| S. salivarius | 499,330 | 507,500 | 512,509 | 511,075 | 515,363 | 519,720 | 506,010 | 507,446 | 500,883 | 508,136 | 509,200 | 509,516 |
| S. pasteuri | 502,444 | 507,500 | 512,509 | 511,075 | 515,363 | 519,720 | 506,276 | 513,591 | 516,103 | 519,186 | 521,698 | 538,260 |
| S. parasanguinis | 506,311 | 506,901 | 507,235 | 510,155 | 510,431 | 523,784 | 509,446 | 512,415 | 516,258 | 521,968 | 525,811 | 545,913 |
| S. oralis | 502,672 | 503,168 | 507,264 | 513,903 | 516,239 | 556,053 | 509,773 | 512,126 | 519,338 | 519,623 | 526,834 | 530,043 |
| S. mutans | 502,274 | 506,689 | 509,707 | 509,551 | 510,936 | 518,002 | 510,262 | 511,036 | 513,776 | 523,327 | 526,067 | 555,495 |
| S. moorei | 502,459 | 506,118 | 508,818 | 509,641 | 512,814 | 521,343 | 495,690 | 504,196 | 512,701 | 514,469 | 523,858 | 535,015 |
| S. mitis | 496,140 | 508,397 | 512,622 | 510,891 | 516,285 | 521,740 | 509,202 | 512,173 | 515,134 | 515,491 | 518,452 | 522,494 |
| S. constellatus | 499,229 | 504,608 | 513,249 | 510,944 | 515,374 | 520,901 | 502,799 | 507,690 | 510,825 | 517,339 | 520,474 | 544,905 |
| S. aureus | 502,340 | 506,297 | 513,276 | 512,750 | 516,756 | 524,366 | 503,241 | 510,870 | 515,086 | 518,742 | 522,958 | 541,558 |
| P. putida | 502,727 | 509,252 | 512,796 | 516,473 | 520,938 | 540,759 | 513,027 | 515,250 | 519,531 | 525,870 | 530,151 | 551,392 |
| P. nigrescens | 499,713 | 505,889 | 514,311 | 513,822 | 521,081 | 526,972 | 508,985 | 509,206 | 512,414 | 515,173 | 518,382 | 526,878 |
| P. micra | 499,866 | 509,486 | 514,798 | 513,336 | 518,662 | 519,904 | 495,886 | 505,933 | 514,731 | 514,545 | 523,343 | 532,833 |
| P. melaninogenica | 516,074 | 519,036 | 521,372 | 520,954 | 522,910 | 526,552 | 515,292 | 524,258 | 528,527 | 527,933 | 532,202 | 539,287 |
| P. intermedia | 492,805 | 510,600 | 512,890 | 512,164 | 518,208 | 520,554 | 502,698 | 512,800 | 516,485 | 522,552 | 525,937 | 553,340 |
| P. gingivalis | 496,318 | 510,253 | 513,687 | 514,056 | 519,108 | 525,049 | 506,885 | 508,754 | 511,323 | 521,321 | 523,890 | 555,753 |
| P. endodontalis | 500,108 | 507,253 | 510,450 | 510,585 | 513,594 | 522,991 | 510,414 | 512,129 | 514,838 | 518,472 | 521,181 | 533,797 |
| P. anareobius | 499,789 | 508,881 | 513,569 | 512,332 | 518,974 | 521,314 | 510,178 | 512,684 | 513,693 | 521,200 | 522,209 | 547,236 |
| P. aeruginosa | 502,333 | 504,190 | 506,289 | 508,465 | 512,226 | 517,909 | 499,685 | 504,556 | 515,162 | 514,296 | 524,903 | 527,176 |
| M. salivarium | 502,480 | 503,394 | 508,459 | 508,819 | 513,308 | 517,638 | 505,885 | 509,168 | 512,009 | 513,433 | 516,273 | 523,829 |
| K. pneumoniae | 503,118 | 507,107 | 509,697 | 510,426 | 514,686 | 517,074 | 512,896 | 515,614 | 516,578 | 517,487 | 518,450 | 523,895 |
| F. nucleatum | 496,246 | 509,891 | 513,442 | 512,987 | 517,171 | 529,364 | 506,459 | 506,717 | 511,210 | 512,268 | 516,762 | 520,195 |
| E.faecalis | 502,648 | 509,671 | 516,600 | 513,598 | 517,098 | 523,633 | 503,046 | 508,314 | 511,641 | 510,392 | 513,718 | 515,240 |
| E. corrodens | 499,102 | 507,130 | 511,786 | 510,290 | 514,304 | 517,051 | 502,761 | 507,881 | 511,606 | 513,627 | 517,352 | 528,535 |
| C. rectus | 503,240 | 504,524 | 508,209 | 509,771 | 513,324 | 521,964 | 503,162 | 506,076 | 508,482 | 511,234 | 513,640 | 524,809 |
| C. gengivalis | 496,277 | 509,774 | 508,597 | 511,429 | 518,305 | 525,247 | 500,159 | 502,528 | 510,465 | 512,300 | 520,237 | 528,113 |
| B. fragilis | 500,472 | 506,942 | 508,975 | 508,685 | 511,356 | 515,116 | 507,329 | 509,377 | 514,210 | 515,172 | 520,005 | 524,938 |
| Aaa | 500,329 | 504,265 | 510,794 | 510,960 | 513,583 | 530,871 | 503,189 | 506,050 | 510,544 | 512,296 | 516,790 | 524,905 |
| C. coli | 499,754 | 506,396 | 513,786 | 511,665 | 516,312 | 521,797 | 510,199 | 510,906 | 513,822 | 515,704 | 518,620 | 528,620 |
| L. casei | 496,075 | 507,173 | 509,809 | 510,831 | 515,268 | 523,264 | 502,363 | 508,030 | 511,699 | 513,208 | 516,876 | 527,070 |
| Thermomechanical Loading | Thermocycling | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | 1st Quartile | Median | Mean | 3rd Quartile | Max | Min | 1st Quartile | Median | Mean | 3rd Quartile | Max | |
| Candida tropicalis | 217,282 | 341,234 | 385,222 | 378,850 | 439,730 | 465,562 | 219,254 | 254,462 | 352,510 | 348,994 | 447,041 | 471,700 |
| Candida glabrata | 225,313 | 246,065 | 262,678 | 268,364 | 290,845 | 327,597 | 184,674 | 227,776 | 250,716 | 242,263 | 265,203 | 282,946 |
| Candida dubliniensis | 285,913 | 327,206 | 331,453 | 348,002 | 345,835 | 478,154 | 327,498 | 385,702 | 411,369 | 434,964 | 460,630 | 589,619 |
| Candida albicans | 110,375 | 224,104 | 288,444 | 272,661 | 316,789 | 380,726 | 301,789 | 358,219 | 384,111 | 378,853 | 404,745 | 445,398 |
| S. pneumoniae | 109,486 | 143,622 | 259,004 | 233,199 | 287,371 | 383,126 | 100,000 | 212,150 | 365,828 | 359,290 | 512,968 | 605,506 |
| S. gallolyticus | 217,038 | 233,505 | 290,082 | 313,556 | 333,302 | 586,911 | 141,288 | 193,417 | 258,145 | 260,751 | 325,479 | 385,428 |
| V. parvula | 143,606 | 278,304 | 338,548 | 309,169 | 369,346 | 383,873 | 124,813 | 209,168 | 261,543 | 248,175 | 300,550 | 344,799 |
| T. denticola | 109,853 | 224,276 | 271,285 | 260,355 | 327,284 | 362,279 | 192,606 | 202,036 | 221,284 | 223,541 | 242,789 | 258,989 |
| T. forsythia | 116,516 | 228,361 | 263,827 | 262,679 | 296,038 | 402,735 | 193,887 | 202,409 | 235,870 | 239,400 | 272,860 | 291,973 |
| S. sobrinus | 110,479 | 228,074 | 277,931 | 275,089 | 336,756 | 397,246 | 262,909 | 288,476 | 299,820 | 301,256 | 312,601 | 342,476 |
| S. sanguinis | 186,712 | 260,753 | 319,200 | 311,340 | 372,454 | 413,536 | 158,952 | 265,727 | 369,944 | 424,695 | 439,865 | 439,895 |
| S. salivarius | 183,190 | 261,160 | 289,836 | 281,151 | 298,268 | 368,143 | 220,188 | 250,046 | 279,214 | 274,872 | 304,040 | 320,874 |
| S. pasteuri | 252,142 | 287,569 | 293,603 | 301,347 | 300,692 | 396,385 | 144,374 | 170,950 | 199,114 | 230,808 | 258,973 | 380,631 |
| S. parasanguinis | 112,858 | 210,692 | 301,458 | 281,694 | 341,119 | 399,906 | 243,447 | 275,141 | 303,708 | 295,559 | 324,126 | 331,372 |
| S. oralis | 280,561 | 351,924 | 391,391 | 390,849 | 409,087 | 528,500 | 297,603 | 305,743 | 328,975 | 360,780 | 384,012 | 487,569 |
| S. mutans | 227,850 | 290,089 | 313,220 | 311,207 | 376,317 | 407,205 | 188,905 | 277,723 | 322,566 | 301,035 | 345,877 | 370,103 |
| S. moorei | 109,937 | 189,142 | 243,573 | 228,104 | 268,104 | 306,521 | 223,630 | 258,245 | 289,429 | 283,920 | 315,104 | 333,192 |
| S. mitis | 150,640 | 185,939 | 215,018 | 243,150 | 313,548 | 376,590 | 154,386 | 206,231 | 262,503 | 252,076 | 308,348 | 328,913 |
| S. constellatus | 145,954 | 188,560 | 226,072 | 250,585 | 328,127 | 365,342 | 193,170 | 216,380 | 225,202 | 227,024 | 235,847 | 264,521 |
| S. aureus | 187,880 | 262,263 | 280,057 | 291,265 | 302,379 | 408,494 | 226,065 | 308,542 | 370,618 | 363,374 | 425,451 | 486,196 |
| P. putida | 143,161 | 253,023 | 322,618 | 309,755 | 368,265 | 455,233 | 297,895 | 306,702 | 363,186 | 366,827 | 423,311 | 443,041 |
| P. nigrescens | 177,730 | 207,023 | 266,496 | 277,380 | 353,889 | 397,399 | 321,529 | 362,934 | 386,017 | 442,321 | 465,404 | 675,723 |
| P. micra | 189,217 | 190,844 | 207,689 | 224,872 | 243,638 | 334,089 | 185,569 | 265,603 | 299,005 | 277,407 | 310,809 | 326,048 |
| P. melaninogenica | 448,694 | 514,857 | 561,123 | 591,295 | 652,611 | 880,154 | 566,416 | 582,489 | 605,760 | 632,014 | 655,285 | 750,119 |
| P. intermedia | 156,879 | 225,608 | 287,838 | 340,493 | 383,084 | 812,934 | 207,091 | 244,818 | 320,549 | 314,632 | 390,363 | 410,339 |
| P. gingivalis | 117,124 | 264,842 | 272,863 | 298,062 | 369,196 | 496,776 | 263,035 | 268,326 | 281,348 | 302,660 | 315,681 | 384,908 |
| P. endodontalis | 110,453 | 233,541 | 284,995 | 268,700 | 331,040 | 345,795 | 147,782 | 176,117 | 204,091 | 242,392 | 270,366 | 413,606 |
| P. anareobius | 151,960 | 225,042 | 265,177 | 263,778 | 293,364 | 405,937 | 146,384 | 230,859 | 280,924 | 279,409 | 329,475 | 409,404 |
| P. aeruginosa | 227,437 | 239,907 | 290,105 | 308,114 | 377,696 | 413,180 | 225,416 | 225,727 | 246,442 | 273,559 | 294,274 | 375,936 |
| M. salivarium | 187,273 | 247,973 | 341,173 | 333,144 | 415,076 | 469,885 | 309,088 | 333,366 | 364,032 | 365,248 | 395,914 | 423,843 |
| K. pneumoniae | 195,112 | 282,004 | 404,784 | 402,078 | 450,658 | 783,747 | 232,059 | 260,969 | 348,822 | 358,690 | 446,544 | 505,059 |
| F. nucleatum | 150,975 | 272,399 | 305,683 | 303,963 | 341,448 | 454,396 | 235,202 | 263,872 | 306,925 | 317,749 | 368,002 | 421,946 |
| E.faecalis | 186,020 | 274,720 | 341,316 | 325,884 | 396,734 | 420,810 | 309,552 | 331,993 | 360,781 | 353,452 | 382,240 | 382,694 |
| E. corrodens | 231,374 | 281,719 | 336,372 | 366,000 | 409,572 | 574,781 | 230,021 | 282,075 | 343,949 | 333,581 | 395,455 | 416,406 |
| C. rectus | 328,256 | 351,452 | 426,916 | 477,979 | 581,944 | 767,913 | 115,174 | 209,127 | 361,902 | 343,182 | 495,957 | 533,751 |
| C. gengivalis | 233,038 | 310,492 | 342,198 | 425,489 | 541,128 | 748,697 | 236,263 | 260,985 | 287,295 | 320,936 | 347,246 | 472,891 |
| B. fragilis | 277,918 | 292,194 | 355,0285 | 408,566 | 432,525 | 792,708 | 348,069 | 370,687 | 402,494 | 470,874 | 502,680 | 730,439 |
| Aaa | 110,516 | 268,966 | 287,284 | 296,592 | 338,523 | 427,716 | 148,299 | 178,126 | 229,103 | 244,087 | 295,064 | 369,845 |
| C. coli | 147,005 | 230,240 | 268,391 | 279,505 | 346,504 | 389,418 | 268,353 | 293,782 | 306,211 | 329,242 | 335,372 | 410,996 |
| L. casei | 147,514 | 282,125 | 345,747 | 319,614 | 377,600 | 387,103 | 147,246 | 233,982 | 322,463 | 322,463 | 346,930 | 595,550 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, R.F.; Mata, V.B.d.; Tomaselli, L.d.O.; Simionato, A.A.; Santos, E.d.S.; Faria, A.C.L.; Rodrigues, R.C.S.; do Nascimento, C. Microbial Leakage through Three Different Implant–Abutment Interfaces on Morse Taper Implants In Vitro. Dent. J. 2024, 12, 226. https://doi.org/10.3390/dj12070226
Ribeiro RF, Mata VBd, Tomaselli LdO, Simionato AA, Santos EdS, Faria ACL, Rodrigues RCS, do Nascimento C. Microbial Leakage through Three Different Implant–Abutment Interfaces on Morse Taper Implants In Vitro. Dentistry Journal. 2024; 12(7):226. https://doi.org/10.3390/dj12070226
Chicago/Turabian StyleRibeiro, Ricardo Faria, Victor Barboza da Mata, Lucas de Oliveira Tomaselli, Anselmo Agostinho Simionato, Emerson de Souza Santos, Adriana Cláudia Lapria Faria, Renata Cristina Silveira Rodrigues, and Cássio do Nascimento. 2024. "Microbial Leakage through Three Different Implant–Abutment Interfaces on Morse Taper Implants In Vitro" Dentistry Journal 12, no. 7: 226. https://doi.org/10.3390/dj12070226
APA StyleRibeiro, R. F., Mata, V. B. d., Tomaselli, L. d. O., Simionato, A. A., Santos, E. d. S., Faria, A. C. L., Rodrigues, R. C. S., & do Nascimento, C. (2024). Microbial Leakage through Three Different Implant–Abutment Interfaces on Morse Taper Implants In Vitro. Dentistry Journal, 12(7), 226. https://doi.org/10.3390/dj12070226









