Abstract
Soft drinks may have a deleterious effect on dental health due to a high titratable acidity and a low pH that could be sufficient to induce tooth demineralization. The use of oral care products immediately after acidic challenge may diminish the erosive potential of soft drinks. We assessed the effect of oral care foams and a spray on salivary pH changes after exposure to Coca-Cola® in young adults. Thirty-three consenting eligible patients were recruited in this double-blind, randomized, crossover study performed in six visits. Baseline examination included unstimulated salivary flow rate, stimulated salivary buffer capacity, and the simplified oral hygiene index (OHI-S) assessment. Salivary pH and time for pH recovery were registered after exposure to Coca-Cola® alone or that followed by the application of each of the studied products (an oral foam containing hydroxyapatite and probiotics, an oral foam containing amino fluoride, an alkaline oral spray, and tap water). Thirty-two patients completed the entire study protocol and were included in the final analysis. The mean minimum salivary pH and the mean oral clearance rate after rinsing with Coca-Cola® were 6.3 and 27 min, respectively. Further rinsing with any one of the tested solutions, including tap water, resulted in a significant improvement in these parameters. When the pH curves were plotted, the oral care products demonstrated a lower area under the curve that differed significantly from the area under the curve for Coca-Cola®; tap water did not differ significantly from Coca-Cola® and oral care products. Minimum salivary pH correlated positively with salivary buffer capacity and salivation rate, while salivary clearance correlated with OHI-S plaque scores. In conclusion, the effect of oral care foams and a spray on minimum salivary pH and salivary clearance after exposure to Coca-Cola® did not differ significantly among the tested products and tap water. Trial registration NCT06148662. Funding: none.
1. Introduction
Soft drinks such as colas or orange sodas are extremely popular across the world [1]. Over the past decades, consumption of these beverages has substantially increased in most parts of the world [2,3,4,5]. The prevalence of soft drink consumption is especially high among young people [6,7,8,9,10]. In addition, a rise in the use of carbonated beverages was observed during the COVID-19 pandemic [11].
It has been found that soft drinks may have a deleterious effect on general [12,13,14] and dental [15,16] health. A recent review [16] reported strong evidence for an association between carbonated drink consumption and oral health problems, including increased risks of periodontitis [17], dental caries, and erosion [18,19]. Dental erosion is the irreversible loss of hard tooth tissues caused by direct contact with intrinsic or extrinsic acids without bacterial involvement [20,21]. The prevalence of erosive tooth wear was shown to be especially high in adolescents and young adults [22,23,24,25]. A meta-analysis by Salas et al. (2015) confirmed that frequent consumption of carbonated drinks increased the odds of tooth erosion [24]; therefore, the high prevalence of dental erosion in young adults may be partially explained by the high frequency of soft drink consumption [26].
The deleterious effects of soft drinks on hard tooth tissues can be related to two main factors. The first factor is organic acids produced by plaque micro-organisms when metabolising fermentable carbohydrates from the soft drinks [27,28]. The second factor is the low pH and titratable acidity of the drinks themselves [27]. Most soft drinks, excluding bottled waters, have a pH that ranges from 2.5 to 3.5 [29], as they are carbonated and commonly contain acids added to enhance flavour (phosphoric acid, malic acid, citric acid, etc.) [30,31]. As a consequence, they show a high titratable acidity and a low pH that could be sufficient to induce tooth demineralization [32]. These acids, if not neutralized, are capable of dissolving the enamel surface [27].
Saliva plays a key role in maintaining the integrity of the enamel due to its buffering ability that controls the demineralization/remineralization equilibrium at the enamel surface [33]. A recent systematic review has shown that of all potential risk factors associated with saliva, whole saliva pH showed the strongest negative association with tooth wear [34]. The role of salivary buffer systems is to maintain the salivary pH at a relatively constant level (i.e., 6.5–7) by neutralizing acids from food and drinks and those produced by bacteria, thus decreasing the tooth demineralization rate [33].
A number of studies have assessed salivary pH dynamics after consumption of various soft drinks. The existing studies vary in experimental design, namely in their saliva collection conditions, type of exposure to soft drinks, and types of soft drinks assessed; therefore, their results are difficult to compare. In general, they found that soft drink consumption was associated with a salivary pH drop of different degrees [35,36,37,38,39,40], followed by a gradual recovery to baseline pH levels due to salivary clearance and buffering [36,38]. However, these protective mechanisms may not be sufficient in cases of a decreased salivation rate [35] or frequent exposure to acidic food or beverages [36]. It has been found that daily consumption of more than four acidic units is strongly associated with enamel erosion development [41,42,43].
Several approaches have been proposed to diminish the deleterious effects of soft drinks on hard tooth tissues. Some authors have proposed increasing salivation by chewing cheese or chewing gum to counteract the erosive potential of acid beverages [44,45,46]. Others have tested modifications of various soft drinks with low concentrations of calcium or a combination of calcium, phosphate, and fluoride. It has been shown that the addition of calcium and phosphate to the experimental drinks considerably decreased their erosive potential [47,48,49]. Another approach is to use oral care products immediately after acidic exposure. Regarding anti-erosive and remineralizing potential, the most promising results have been reported for fluoride- and hydroxyapatite-containing products [20,50,51,52,53,54,55,56]. Alkaline oral care products may also be of interest due to their ability to increase salivary pH [57,58,59]. Several studies have assessed the neutralizing effect of mouthwashes containing these ingredients and have concluded that they might potentially reduce tooth erosion caused by acid exposure [57,60,61]. However, the literature is lacking regarding the anti-erosive potential of oral care products with different compositions and forms. Moreover, with the development of new oral care products with various active ingredients and characteristics, it is important to continuously update evaluations of their effects. Oral care foams and sprays may be considered an alternative to mouthwashes in cases where the use of the latter is inconvenient, as only few mouthwashes are available in portable size packages. It was found that oral foams can increase salivation [62]. To the best of our knowledge, there have been no previous studies regarding the neutralizing effect of oral foams or sprays with different compositions. Therefore, we aimed to assess the effect of three oral care products (an oral foam with fluoride, an oral foam with Zn-hydroxyapatite and probiotics, and an alkaline spray) on salivary pH changes after exposure to Coca-Cola® in young adults.
2. Materials and Methods
2.1. Ethical Approval
The Ethics Committee of Sechenov University approved the study (Protocol no. 04-23, 2 March 2023). The study registration number on clinicaltrials.gov is NCT06148662.
2.2. Study Design
This double-blind, randomized, crossover study was conducted from November 2023 to December 2023 at the Therapeutic Dentistry Department, Sechenov University, Moscow, Russia. The study included six visits. After enrolment, dental examination was performed using the following indices: the Decayed, Missing, and Filled Teeth index (DMFT), the Simplified Oral Hygiene Index (OHI-S), and the Bleeding index (BI) [63,64,65]. Stimulated salivary buffer capacity, unstimulated salivary flow rate, and baseline salivary pH were measured. Salivary pH changes were registered after exposure to Coca-Cola® alone or followed by the application of each of the following products: an oral foam containing zinc hydroxyapatite and probiotics (BF), an oral foam containing amino fluoride (WF), an oral spray with alkaline thermal water (BS), and tap water as a control. The pH and titratable acidity of the solutions was measured using a digital pH meter (MILWAUKEE PH56 PRO, Rocky Mount, NC, USA). To assess the titratable acidity, 50 mL of each solution was titrated by adding 0.1 mL aliquots of NaOH (0.1 M) until the pH of 7.0 and the amount of NaOH (mmol) needed for neutralization was calculated. All measurements were repeated 3 times and the results were averaged. Table 1 shows the active ingredients and pH and titratable acidity values of the products used in the study.

Table 1.
The characteristics of the tested solutions.
2.3. Sampling Criteria
Thirty-two young adults of both genders aged 18–44 years were enrolled by two study authors (MP and AE). Participants were free to withdraw from the study at any point.
2.3.1. Inclusion Criteria
The inclusion criteria were males and females aged 18–44 years; all participants signed informed consent forms for participation in the study and publication of the data for research and education purposes.
2.3.2. Exclusion Criteria
Patients with systemic or local conditions that may affect the study results or in which the exposure to Coca-Cola is contraindicated were not enrolled in the study. Systemic conditions included diseases (e.g., diabetes mellitus, Sjögren syndrome, chronic kidney disease, arterial hypertension, etc.) and/or intake of medications (e.g., of antihypertensive, anti-acne, anticholinergic, and antiseizure medications, antidepressants, antihistamines, etc.) that may affect salivary parameters. Pregnant and breastfeeding patients were also not included due to physiologic hormonal changes that are known to affect salivary parameters. Local conditions included oral mucosa pathology as it may affect salivation rate; moreover, the presence of any pathological lesions on the oral mucosa makes the exposure to acidic beverage and any new oral care product unacceptable. Orthodontic appliance treatment was an exclusion criterion as it may alter salivation and affect oral hygiene. Finally, dental bleaching makes the exposure to acidic and coloured beverage undesirable, thus it was also considered an exclusion criterion.
2.3.3. Elimination Criteria
The patients were eliminated from the study in case of withdrawal of consent, detection of an allergic reaction to any components of the of the products used in the study; prescription of medicaments after the enrolment that may compromise the protocol, and non-compliance with the study protocol (failure to attend any of the six visits).
2.4. Randomization
The allocation concealment was performed using a randomized sequence of oral care products for each patient applied by an operator who did not participate in the study. The oral care products were in unlabelled non-transparent bottles. Neither patients nor researchers were aware of the type of the oral care product used.
2.5. Interventions and Outcomes
At the baseline visit, the following indices were assessed: DMFT, OHI-S, and BI (as described elsewhere [63,64,65]). Demographic characteristics, medical and medication history were registered. Two trained and calibrated researchers, MP and AE (intra- and inter-examiner Kappa agreement of at least 94%), performed all clinical examinations.
Unstimulated salivary flow rate, baseline salivary pH, and stimulated saliva buffer capacity were assessed. Unstimulated saliva samples were collected between 10 and 11 am. Participants abstained from food, drink, smoking, or oral hygiene procedures for at least 1.5 h before the procedure. Participants sat comfortably, did not swallow saliva, and expectorated it into a graduated tube every two minutes to assess salivary flow rate. The salivation rate (mL/min) was calculated as the saliva volume collected within the 10 min period divided by 10 min. Salivation rate was classified as follows: low (0.046–0.264 mL/min), medium (0.265–0.451 mL/min), or high (0.451–1.850 mL/min) [66]. Salivary pH was measured immediately after the collection using a pH meter (MILWAUKEE PH56 PRO, Rocky Mount, NC, USA).
To assess salivary buffer capacity, 0.5 mL of stimulated saliva was mixed with 1.5 mL of HCl (5 mM) in a plastic vial and left it open for 5 min for CO2 release. Then, the pH of the mixture was measured. Both tests (salivation rate and buffer capacity) for each participant were repeated thrice and their results were averaged.
The second visit was scheduled after 1 week. Participants’ salivary baseline pH levels were measured as described above. The participants held 20 mL of Coca-Cola® beverage in the mouth for 30 s. After that, the Coca-Cola® was eliminated (either spat out or swallowed). Then, the participants spat saliva into a sterile glass tube and salivary pH was measured within 1 min (time required to collect enough saliva and perform the measurement) after exposure to the beverage, 2 min after the exposure, and then every 5 min until the pH reached the baseline levels. The time required for pH recovery was registered.
The following visits were performed at weekly intervals. Participants’ salivary baseline pH levels were measured as described above. The participants held 20 mL of Coca-Cola® beverage in the mouth for 30 s. After that Coca-Cola® was eliminated (either spat out or swallowed). Then the participants spat saliva into a sterile glass tube and salivary pH was measured within 1 min (time required to collect enough saliva and perform the measurement). Next, one of the tested oral care products was used according to the instructions of the manufacturer and pH levels were measured immediately after application (2 min measurement) and then every 5 min until the pH reaching baseline levels. The time required for pH recovery was registered.
The tested products were used as follows:
- WF and BF were squeezed (2 pumps of the foams) into the mouth, swished for 30 s, and spat out without rinsing.
- BS was sprayed in the mouth (2 sprays) and left for 30 s; could be swallowed.
- A sip of tap water was swished in the mouth for 30 s and spat out.
Primary outcome measures included minimal pH level and salivary clearance time after exposure to Coca-Cola® alone or followed by rinsing with the studied solutions. Salivary clearance time was the time required for the salivary pH to reach the baseline level. Area under the curve was calculated as the area between the baseline pH level and the part of the curve where the pH remained below the baseline pH level [67].
2.6. Statistical Analysis
The sample size was calculated for a Wilcoxon matched-pairs signed-rank test (effect size was assumed to be medium, i.e., 0.5). G*Power (version 3.1.9.6, Institute for Experimental Psychology, Dusseldorf, Germany) was used for sample size calculation: the power was set at 80% and alpha level was set as 0.05. The target sample size was 33 participants in each group (sample size calculations yielded 28 participants and with 15% were added to compensate for potential dropout).
Means, standard deviations, and medians and interquartile ranges were used to present continuous variables. The Friedman test was used to assess statistical differences among the tested solutions, A pairwise signed ranks post hoc test with a Benjamini–Hochberg procedure was used to adjust for multiple comparisons. Kendall’s W was used to calculate the effect size for each variable. Spearman’s correlation coefficient was used to accomplish correlation analysis.
2.7. Data Management
The data were entered in the MS Excel database (Excel for Mac version 16.79.1 (23111614), Microsoft Corp., Mountain View, CA, USA) and converted to the CSV file format. Pseudonymized data were analysed in R (version 4.2.3 (15 March 2023), R Development Core Team, Columbia university, New York, NY, USA) using “rstatix” and “ellipse” packages in RStudio version 2023.03.0+386.
3. Results
Thirty-five patients aged 18 to 44 years were assessed for eligibility. Of them, two patients were ineligible due to meeting the exclusion criteria. A total of 33 patients were enrolled in the study (24 females and 9 males). One participant was lost to follow up (n = 1). A total of 32 participants (mean age = 23.8 ± 6.1 years) were included in the final analysis (Table 2, Figure 1).

Table 2.
Subject demographics.
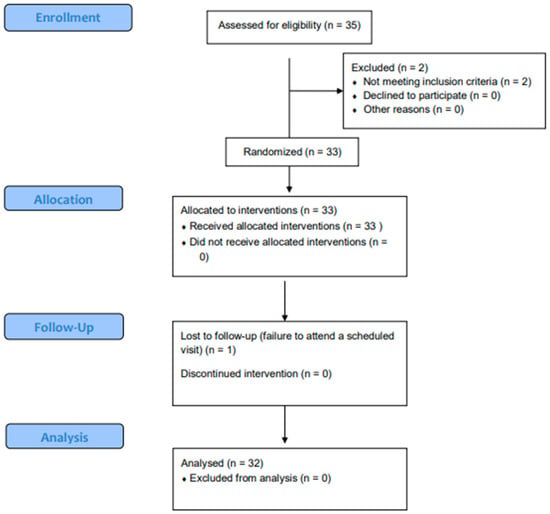
Figure 1.
Participant flow chart.
Table 3 shows the oral health status of the participants. The mean DMFT value in the study population was 7.8 teeth (low caries intensity), with the FT component being predominant (mean value 6.2). Forty-nine percent of the study participants presented with fair oral hygiene. The mean BI value was 0.14 points.

Table 3.
Oral health indicators.
The salivary parameters profile of the study participants showed that the majority of participants had a high salivation rate (58.9%) and high buffer capacity (10.7%) Table 4.

Table 4.
Salivary parameters.
Figure 2 shows the pH curves plotted using mean pH readings for all subjects after exposure to Coca-Cola® and rinsing with different liquids over time. The mean salivary pH of the subjects at baseline was 7.18 ± 0.22. The maximum drop in pH was observed within the first 2 min. Then, a gradual recovery of the salivary pH was observed in all groups. The quantitative description of the minimum pH, and time required for salivary clearance, as well as the areas under the pH curves are also presented in Table 5.
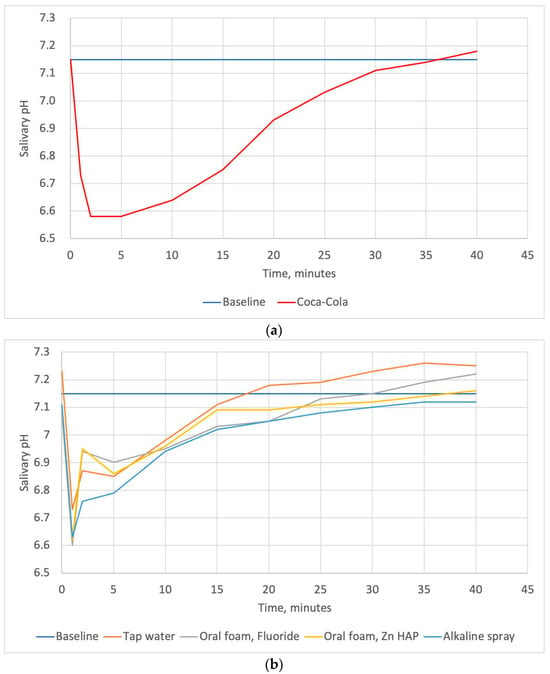
Figure 2.
The pH–time curves plotted using mean salivary pH readings for all subjects after exposure to Coca-Cola® (a) and the pH–time curves plotted using mean salivary pH readings for all subjects after exposure to Coca-Cola® followed by rinsing with oral care foams, a spray, and tap water (b); “Baseline” line represents the mean salivary pH level before exposure to the beverage.

Table 5.
The mean minimum pH, salivary clearance 1, and area under pH curve after exposure to Coca-Cola® alone or that followed by the use of oral care products.
Table 5 shows the mean minimum salivary pH and the mean oral clearance rate of Coca-Cola® after rinsing with tap water or tested oral care products. The mean minimum salivary pH after exposure to Coca-Cola® was 6.3. This parameter increased significantly after rinsing with any one of the tested solutions, with no significant differences among them. The mean oral clearance rate of Coca-Cola® without rinsing was found to be 27 min. Rinsing with any solutions resulted in a significant decrease in salivary clearance time. We found no significant differences in this parameter among the rinsing solutions. However, the salivary clearance time after the use of BS (alkaline spray) tended to be the lowest and comprised 16 min. The areas under pH curves plotted for the Zn-hydroxyapatite-containing foam, fluoride-containing foam, and alkaline spray differed significantly from that for Coca-Cola® (p = 0.002, p = 0.004, and 0.036, respectively). Tap water did not differ significantly from Coca-Cola® and oral care products in this parameter.
Figure 3 denotes the results of the correlation matrix of oral hygiene and salivary parameters after exposure to Coca-Cola®. We found a moderate but not quite statistically significant positive correlation between the minimum salivary pH and unstimulated salivation rate (r = 0.34, p = 0.05782) and salivary buffer capacity (r = 0.31, p = 0.08585). Also, a moderate positive correlation was detected between salivary buffer capacity and unstimulated salivation rate (r = 0.35, p = 0.04765). Salivary clearance values moderately correlated with OHI-S plaque scores (r = 0.42, p = 0.01555). The area under the pH curve correlated positively with salivary clearance time (r = 0.84, p < 0.0001) and OHI-S plaque scores (r = 0.49, p = 0.00467) and negatively with minimum salivary pH (r = −0.60, p = 0.000327) and unstimulated salivary flow rate (r = −0.35, p = 0.04664).
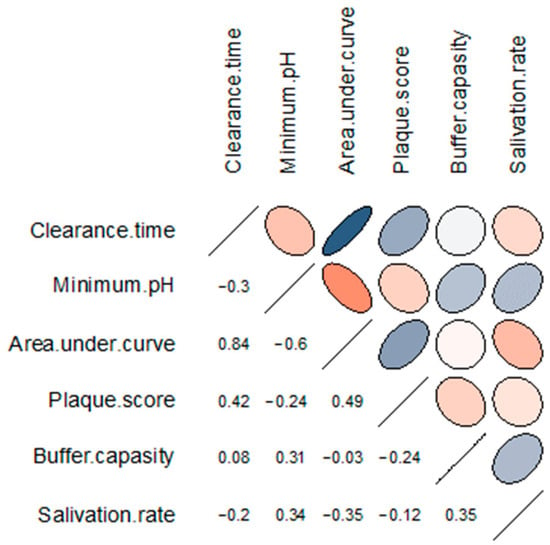
Figure 3.
Correlation matrix of the salivary parameters and oral hygiene index after exposure to Coca-Cola®. The rows and columns represent the correlating variables. In the upper triangular portion of the matrix, positive correlations are displayed in blue and negative correlations in red; the intensity of the colour is proportional to the strength of the correlation. The lower triangular portion represents the values of Spearman’s correlation coefficient between the pairs of variables.
4. Discussion
In our study, we assessed the effect of two oral care foams and a spray on salivary pH changes after exposure to Coca-Cola® in young adults. We found that rinsing with any one of the tested solutions, including tap water, resulted in a significant decrease in salivary clearance time and in a significant increase in the minimum pH. When the pH curves were plotted, the oral care products demonstrated a lower area under the curve that differed significantly from the area under the curve for Coca-Cola®; tap water did not differ significantly from Coca-Cola® and oral care products in this parameter. Salivary clearance values and the area under the pH curve moderately correlated with OHI-S plaque scores; the area under the pH curve negatively correlated with unstimulated salivary flow rate.
We did not stimulate salivary flow before the exposure to Coca-Cola® to simulate the most unfavourable situation where a soft drink is consumed in the fasted state thus exhibiting its maximum erosive potential. The exposure to Coca-Cola® in this study included holding 20 mL of the beverage in the mouth for 30 s, which is different from the way such beverages are consumed. This was performed for standardization purposes. The standard bolus size for a single swallowing in adults was found to be approximately 20–25 mL [68], while the oral transit time of liquids is about 1 s [69]. However, carbonated drinks are commonly held in the mouth for longer times (until all the bubbles have dissipated) [70]. Since the volume of a Coca-Cola® can is 330 mL, which is approximately 15 sips, each 1–2 s long, the teeth are commonly exposed to the beverage for about 30 s.
Diet plays a key role in the development of enamel erosion [11,38]. Coca-Cola® is one of the world’s most highly consumed commercial carbonated beverages [19] and has been shown to possess a high erosive potential [35,71,72]; therefore, in our study, we used rinsing with Coca-Cola® to model a dietary acidic attack. A number of studies have shown that Coca-Cola® decreased the salivary pH immediately after consumption [35,36,37,39,40], but generally it did not produce a decrease in salivary pH below 5.5 [35,36,37]. The pH value of 5.5 was found to be critical for enamel dissolution and caries development in vitro [56,61]; at the same time, in vivo this value varies over a wide range depending on the individual characteristics of the enamel and the content of mineral ions in plaque fluid and saliva [73,74,75]. In relation to erosion, critical pH is reversely proportional to the calcium and phosphate concentrations not only in the saliva and plaque fluid, but also in the foods and drinks. Therefore, it can be even lower if acidic products contain more calcium and phosphate than biofilm fluid [73,76,77,78]. In our study, the mean minimum pH after exposure to Coca-Cola® was 6.3 ± 0.5, i.e., far above the theoretical critical level. These results are in accordance with those reported by previous studies [35,36,37].
The salivary clearance of an acidic drink is the time required for the salivary pH to reach the baseline level [38,79]. According to our results, the mean salivary clearance time of Coca-Cola® was 27 min. In a study by Mojaver et al., the oral clearance rate of this beverage was found to be 30 min [71], while Barrajas-Torres et al. reported a gradual salivary pH recovery after the ingestion of Coca-Cola® that took up to 45 min [36]. According to Tenuta et al., the salivary clearance time of Coca-Cola® was 3 min [39]. Inconsistencies in the results of different studies may be explained by the differences in the ages, gender, and oral health status of the studies’ participants and differences in the studies’ designs.
Minimum salivary pH and salivary clearance may depend on drink-related factors (e.g., pH, titratable acidity, and composition [35,80]) and host-related factors (e.g., salivary buffer capacity and salivary flow rate [80]). In our study, the minimum salivary pH mainly correlated with salivary buffer capacity and salivation rate. Similar results were obtained by Tenouvo et al., who reported that the decrease in pH after consumption of the tested drinks was significantly less in subjects with a high salivary secretion rate compared to those with a low flow rate [35]. This may be explained by faster washing out of acids and the neutralizing effects of saliva buffering systems (mainly bicarbonates), which are activated with an increase in salivary secretion [37,80,81]. We found a positive correlation between salivary flow rate and buffer capacity. This finding corroborates those reported in the previous studies [82,83].
The neutralization of acids after soft drink consumption is a complex phenomenon, as salivary pH may also be influenced by organic acids produced by plaque microorganisms while metabolizing sucrose contained in these beverages [84,85]. Kumar et al. reported that the presence of plaque decreased the salivary pH at various time intervals between 0 and 60 min [86]. In our study, OHI-S plaque scores also correlated with salivary clearance of the acidic drink. The time required for acid neutralization was significantly longer in individuals with poorer oral hygiene. Interestingly, salivary clearance did not significantly correlate with salivary buffer capacity and salivation rate. This may be explained by the fact that salivary flow rate and buffer capacity mainly influence initial pH changes that occur within the first few minutes after the acidic attack, while further pH recovery may be hampered by acids produced by plaque microorganisms [27,87,88].
A number of studies have shown that the erosive potential of soft drinks may be reduced by increasing salivary flow rate [44,45,46], modification of beverages by adding calcium, phosphate, and fluoride [47,48,49], or using various oral care products immediately after the intake of soft drinks [57,60,61]. It has been shown that the use of various oral care products may result in rehardening of the enamel [60] and an increase in salivary pH [57] after an acidic attack. We found that rinsing with any one of the tested solutions resulted in a decrease in salivary clearance time and an increase in minimum pH, with no significant differences among the oral care products and tap water. Similarly, in a number of studies, water has been shown to be an effective measure to increase the salivary pH values [57,89] or even to increase enamel hardness [60] after the acidic challenge. This may be explained by the washing-out effect and eliminating acids from the oral cavity during rinsing.
On the other hand, when we plotted the pH curves, only dental foams and spray differed significantly from Coca-Cola® in terms of the areas under the pH curves, while tap water did not. This could be due to the gustatory stimulation of the salivary flow by the tested foams and spray. A number of studies have suggested that salivation might be conditioned to sensory stimulation [90,91]. Additionally, the oral care products used in our study may provide beneficial effects in patients frequently consuming soft drinks due to their compositions and properties. BS is an alkaline spray with a pH of 8.8 which can directly neutralize acids. It has been shown that alkaline oral care products may increase salivary pH [57,58,59]. In a study by Dehgan et al., an alkaline component of a two-step mouthwash was used to reduce the erosive potential of an acidic drink [57]. They concluded that this mouthwash raised pH significantly higher than Listerine and water. WF contains 250 ppm fluoride. An increase in salivary pH has been reported in previous studies assessing the effect of various fluoride-containing oral care products [58,92,93]. Moreover, a considerable amount of the literature has reported a high potential of fluoride for surface remineralization after an acidic attack and protection from demineralization in an acidic environment [20,50,51,52]. The application of fluoride results in the formation of fluorohydroxyapatite layer on the tooth surface. This layer is less soluble and less susceptible to acid erosion [20,50,51,52]. BF active ingredients include Zn-hydroxyapatite and a probiotic. Remineralizing properties of hydroxyapatite are well documented [53,54,55,56,94,95]. Biomimetic (hydroxyapatite-containing) oral care products replicate the natural structure of teeth and can promote the remineralization of hard tooth tissues [53,54,55,56]. Particularly hydroxyapatite doped with Zn ions has been found to be an effective agent in mineralizing hard tooth tissues [94,95,96]. As it was mentioned above, BF also contains probiotics. There is some evidence that topical probiotics may increase salivary flow rate [97,98]. Also, probiotics have been reported to decrease plaque formation [99,100,101,102,103,104].
However, most of these effects require prolonged usage of the tested oral care products. In our study, we assessed only an immediate effect of the foams and spray, and further research of their long-term effects is needed. This can be considered a limitation of our study. Another limitation is that we did not assess mineralization and physical characteristics of hard tooth tissues after the exposure to the tested solutions. Finally, it is advisable for further studies to include patients from populations of different areas and ages, and to compare patients with enamel erosion with healthy ones.
5. Conclusions
Within the limitations of our study, we may conclude that rinsing with oral care products or tap water is an effective measure to increase the salivary pH values and salivary clearance rate after exposure to acidic beverage. Oral care products might provide additional benefits for decreasing the erosive potential of soft drinks, when the area under the pH curve is considered.
Author Contributions
Conceptualization, K.B. and N.N.; methodology, K.B. and N.N.; software, M.P. and A.M.; validation, M.P.; formal analysis, M.P. and A.Z.; investigation, A.E.; resources, A.E.; data curation, A.E., V.D., A.Z. and A.M.; writing—original draft preparation, K.B., A.E., N.N., M.P., V.D., A.M. and A.Z.; writing—review and editing, K.B., N.N. and A.Z.; visualization, M.P., V.D. and A.M.; supervision, K.B. and N.N.; project administration, K.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. This research received no funding from the manufacturers of the products and materials used in the study. All materials used in the study were purchased by the authors of this research.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of I.M. Sechenov First Moscow State Medical University (Sechenov University) (Protocol no. 04-23, 2 March 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest or financial or personal relationships with other individuals or organizations that could inappropriately influence their actions in a way that creates bias.
References
- Ren, J.S.; Freedman, N.D.; Kamangar, F.; Dawsey, S.M.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Tea, Coffee, Carbonated Soft Drinks and Upper Gastrointestinal Tract Cancer Risk in a Large United States Prospective Cohort Study. Eur. J. Cancer 2010, 46, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.J.; Popkin, B.M. Changes in Beverage Intake between 1977 and 2001. Am. J. Prev. Med. 2004, 27, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; McKee, M.; Galea, G.; Stuckler, D. Relationship of Soft Drink Consumption to Global Overweight, Obesity, and Diabetes: A Cross-National Analysis of 75 Countries. Am. J. Public Health 2013, 103, 2071–2077. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Hawkes, C. Sweetening of the Global Diet, Particularly Beverages: Patterns, Trends, and Policy Responses. Lancet Diabetes Endocrinol. 2016, 4, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Lasater, G.; Piernas, C.; Popkin, B.M. Beverage Patterns and Trends among School-Aged Children in the US, 1989–2008. Nutr. J. 2011, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Tuan, H.; Na, X.; Yang, H.; Yang, Y.; Zhang, Y.; Xi, M.; Tan, Y.; Yang, C.; Zhang, J.; et al. The Association between Sugar-Sweetened Beverages and Male Pattern Hair Loss in Young Men. Nutrients 2023, 15, 214. [Google Scholar] [CrossRef]
- Terry-McElrath, Y.M.; O’Malley, P.M.; Johnston, L.D. Energy Drinks, Soft Drinks, and Substance Use among United States Secondary School Students. J. Addict. Med. 2014, 8, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, X.; Coday, M.; Garcia, D.O.; Li, X.; Mossavar-Rahmani, Y.; Naughton, M.J.; Lopez-Pentecost, M.; Saquib, N.; Shadyab, A.H.; et al. Sugar-Sweetened and Artificially Sweetened Beverages and Risk of Liver Cancer and Chronic Liver Disease Mortality. JAMA 2023, 330, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Khanferyan, R.A.; Vybornaya, K.V.; Radzhabkadiev, R.M.; Evstratova, V.S.; Nalivayko, N.V.; Semin, V.B.; Galstyan, A.G. Frequency of Consumption of Sweet Carbonated Drinks by the Population of Different Age Groups of the Russian Federation. Vopr. Pitan. [Probl. Nutr.] 2017, 86, 55–58. [Google Scholar]
- Petrova, M.M.; Pronina, E.A.; Yaganova, S.S.; Anonen, P.Y.; Demakova, M.Y. Investigation of Sugar-Sweetened Beverages Consumption in Students of Krasnoyarsk State Medical University Named after V.F. Voino-Yasenetsky. Vopr. Pitan. [Probl. Nutr.] 2017, 86, 93–98. [Google Scholar]
- Inchingolo, A.M.; Malcangi, G.; Ferrante, L.; Del Vecchio, G.; Viapiano, F.; Mancini, A.; Inchingolo, F.; Inchingolo, A.D.; Di Venere, D.; Dipalma, G.; et al. Damage from Carbonated Soft Drinks on Enamel: A Systematic Review. Nutrients 2023, 15, 1785. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Hu, F.B. Sugar-Sweetened Beverages and Cardiometabolic Health: An Update of the Evidence. Nutrients 2019, 11, 1840. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Pan, N.; Xu, X.; Li, H.; Lin, L.; Chen, J.; Jin, C.; Pan, S.; Jing, J.; Li, X. The Association between Sugar-Sweetened Beverages and Milk Intake with Emotional and Behavioral Problems in Children with Autism Spectrum Disorder. Front. Nutr. 2022, 9, 927212. [Google Scholar] [CrossRef]
- Kashino, I.; Kochi, T.; Imamura, F.; Eguchi, M.; Kuwahara, K.; Nanri, A.; Kurotani, K.; Akter, S.; Hu, H.; Miki, T.; et al. Prospective Association of Soft Drink Consumption with Depressive Symptoms. Nutrition 2021, 81, 110860. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, M.J.; Waterhouse, B.; Aggarwal, V.R.; Bloor, K.; Doran, T. Effect of Sugar-Sweetened Beverages on Oral Health: A Systematic Review and Meta-Analysis. Eur. J. Public Health 2021, 31, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Çetinkaya, H.; Romaniuk, P. Relationship between Consumption of Soft and Alcoholic Drinks and Oral Health Problems. Cent. Eur. J. Public Health 2020, 28, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Song, I.-S.; Han, K.; Ko, Y.; Park, Y.-G.; Ryu, J.-J.; Park, J.-B. Associations between the Consumption of Carbonated Beverages and Periodontal Disease. Medicine 2016, 95, e4253. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.M.; Vani, N.V.; Almutari, D.A.; Jafar, M.A.; Boreak, N. Analysis of Sugars and PH in Commercially Available Soft Drinks in Saudi Arabia with a Brief Review on Their Dental Implications. J. Int. Soc. Prev. Community Dent. 2016, 6, S192–S196. [Google Scholar] [CrossRef] [PubMed]
- Giacaman, R.A.; Pailahual, V.; Díaz-Garrido, N. Cariogenicity Induced by Commercial Carbonated Beverages in an Experimental Biofilm-Caries Model. Eur. J. Dent. 2018, 12, 27–35. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Gargani, A.; Parcianello, R.G.; Pezzato, L.; Bertolini, R.; Zuccon, A.; Stellini, E.; Ludovichetti, F.S. Protection against Dental Erosion and the Remineralization Capacity of Non-Fluoride Toothpaste, Fluoride Toothpaste and Fluoride Varnish. Appl. Sci. 2023, 13, 1849. [Google Scholar] [CrossRef]
- Ruiz, D.C.; Marqués Martínez, L.; García Miralles, E. Dental Erosion and Diet in Young Children and Adolescents: A Systematic Review. Appl. Sci. 2023, 13, 3519. [Google Scholar] [CrossRef]
- Vieira Pedrosa, B.R.; de Menezes, V.A. Prevalence of Erosive Tooth Wear and Related Risk Factors in Adolescents: An Integrative Review. J. Dent. Child (Chic) 2020, 87, 18–25. [Google Scholar] [PubMed]
- Salas, M.M.S.; Nascimento, G.G.; Huysmans, M.C.; Demarco, F.F. Estimated Prevalence of Erosive Tooth Wear in Permanent Teeth of Children and Adolescents: An Epidemiological Systematic Review and Meta-Regression Analysis. J. Dent. 2015, 43, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.M.S.; Nascimento, G.G.; Vargas-Ferreira, F.; Tarquinio, S.B.C.; Huysmans, M.C.D.N.J.M.; Demarco, F.F. Diet Influenced Tooth Erosion Prevalence in Children and Adolescents: Results of a Meta-Analysis and Meta-Regression. J. Dent. 2015, 43, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Rius-Bonet, O.; Roca-Obis, P.; Zamora-Olave, C.; Willaert, E.; Martinez-Gomis, J. Prevalence of Dental Attrition and Its Relationship with Dental Erosion and Salivary Function in Young Adults. Quintessence Int. 2023, 54, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Lussi, A.; Jaeggi, T. Chemical Factors. Monogr. Oral. Sci. 2006, 20, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.E.; Brandao, A.C.S.; Bícego-Pereira, E.C.; Del Bel Cury, A.A.; Cury, J.A.; Tenuta, L.M.A. Effect of PH and Titratable Acidity on Enamel and Dentine Erosion. Clin. Oral Investig. 2022, 26, 5867–5873. [Google Scholar] [CrossRef] [PubMed]
- Rios, D.; Ionta, F.-Q.; Rebelato, R.; Jordão, M.-C.; Wang, L.; Magalhães, A.-C.; Honório, H.-M. The Effect of Aspartame and PH Changes on the Erosive Potential of Cola Drinks in Bovine Enamel: An in Vitro Study. J. Clin. Exp. Dent. 2018, 10, e933–e937. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, C.R.; Shahnawaz, K.; Kumari, P.D.; Chowdhury, A.; Gootveld, M.; Lynch, E. Highly Acidic PH Values of Carbonated Sweet Drinks, Fruit Juices, Mineral Waters and Unregulated Fluoride Levels in Oral Care Products and Drinks in India: A Public Health Concern. Perspect. Public Health 2019, 139, 186–194. [Google Scholar] [CrossRef]
- Yip, H.H.Y.; Wong, R.W.K.; Hägg, U. Complications of Orthodontic Treatment: Are Soft Drinks a Risk Factor? World J. Orthod. 2009, 10, 33–40. [Google Scholar]
- González-Aragón Pineda, Á.E.; Borges-Yáñez, S.A.; Irigoyen-Camacho, M.E.; Lussi, A. Relationship between Erosive Tooth Wear and Beverage Consumption among a Group of Schoolchildren in Mexico City. Clin. Oral Investig. 2019, 23, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Dugmore, C.R.; Rock, W.P. A Multifactorial Analysis of Factors Associated with Dental Erosion. Br. Dent. J. 2004, 196, 283–286; discussion 273. [Google Scholar] [CrossRef] [PubMed]
- Lynge Pedersen, A.M.; Belstrøm, D. The Role of Natural Salivary Defences in Maintaining a Healthy Oral Microbiota. J. Dent. 2019, 80, S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Madariaga, V.I.; Pereira-Cenci, T.; Walboomers, X.F.; Loomans, B.A.C. Association between Salivary Characteristics and Tooth Wear: A Systematic Review and Meta-Analysis. J. Dent. 2023, 138, 104692. [Google Scholar] [CrossRef] [PubMed]
- Tenovuo, J.; Rekola, M. Some Effects of Sugar-Flavored Acid Beverages on the Biochemistry of Human Whole Saliva and Dental Plaque. Acta Odontol. Scand. 1977, 35, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Barajas-Torres, G.C.; Klünder-Klünder, M.; Garduño-Espinosa, J.; Parra-Ortega, I.; Franco-Hernández, M.I.; Miranda-Lora, A.L. Effects of Carbonated Beverage Consumption on Oral PH and Bacterial Proliferation in Adolescents: A Randomized Crossover Clinical Trial. Life 2022, 12, 1776. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, G.A.; Fernandez De Preliasco, M.V. Salivary pH Changes during Soft Drinks Consumption in Children. Int. J. Paediatr. Dent. 2003, 13, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Hans, R.; Thomas, S.; Garla, B.; Dagli, R.J.; Hans, M.K. Effect of Various Sugary Beverages on Salivary PH, Flow Rate, and Oral Clearance Rate amongst Adults. Scientifica 2016, 2016, 5027283. [Google Scholar] [CrossRef]
- Tenuta, L.M.A.; Fernández, C.E.; Brandão, A.C.S.; Cury, J.A. Titratable Acidity of Beverages Influences Salivary PH Recovery. Braz. Oral Res. 2015, 29, 1–6. [Google Scholar] [CrossRef]
- Alkasso, I.R.; Salih Al Qassar, S.S.; Taqa, G.A. Durability of Different Types of Mouthwashes on the Salivary Buffering System in Orthodontic Patients. Dent. 3000 2021, 9, 178–192. [Google Scholar] [CrossRef]
- Lussi, A.; Schaffner, M. Progression of and Risk Factors for Dental Erosion and Wedge-Shaped Defects over a 6-Year Period. Caries Res. 2000, 34, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Zoller, M.J.; Procopio, A.; Attin, T.; Wegehaupt, F.J. Homemade Modification of Salad Dressings to Reduce Their Erosive Potential. Oral. Health Prev. Dent. 2021, 19, 433–440. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, E.A.; Curzon, M.E. A Comparison of Acidic Dietary Factors in Children with and without Dental Erosion. ASDC J. Dent. Child. 2000, 67, 160, 186–192. [Google Scholar] [PubMed]
- Thomas, E.; Tayab, T.; Rai, K.; Kumari, V. Effect of Chewing Paneer and Cheese on Salivary Acidogenicity: A Comparative Study. Int. J. Clin. Pediatr. Dent. 2012, 5, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Rios, D.; Honório, H.M.; Magalhães, A.C.; Delbem, A.C.B.; Machado, M.A.A.M.; Silva, S.M.B.; Buzalaf, M.A.R. Effect of Salivary Stimulation on Erosion of Human and Bovine Enamel Subjected or Not to Subsequent Abrasion: An in Situ/Ex Vivo Study. Caries Res. 2006, 40, 218–223. [Google Scholar] [CrossRef] [PubMed]
- de Alencar, C.R.B.; Magalhães, A.C.; de Andrade Moreira Machado, M.A.; de Oliveira, T.M.; Honório, H.M.; Rios, D. In Situ Effect of a Commercial CPP-ACP Chewing Gum on the Human Enamel Initial Erosion. J. Dent. 2014, 42, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- Attin, T.; Weiss, K.; Becker, K.; Buchalla, W.; Wiegand, A. Impact of Modified Acidic Soft Drinks on Enamel Erosion. Oral Dis. 2005, 11, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.C.; Moraes, S.M.; Rios, D.; Buzalaf, M.A.R. Effect of Ion Supplementation of a Commercial Soft Drink on Tooth Enamel Erosion. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2009, 26, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Jensdottir, T.; Bardow, A.; Holbrook, P. Properties and Modification of Soft Drinks in Relation to Their Erosive Potential in Vitro. J. Dent. 2005, 33, 569–575. [Google Scholar] [CrossRef]
- Bologa, E.; Stoleriu, S.; Nica, I.; Tărăboanță, I.; Georgescu, A.; Matei, R.I.; Andrian, S. The Effect of Three Desensitizing Toothpastes on Dentinal Tubules Occlusion and on Dentin Hardness. Biomedicines 2023, 11, 2464. [Google Scholar] [CrossRef]
- Lussi, A.; Buzalaf, M.A.R.; Duangthip, D.; Anttonen, V.; Ganss, C.; João-Souza, S.H.; Baumann, T.; Carvalho, T.S. The Use of Fluoride for the Prevention of Dental Erosion and Erosive Tooth Wear in Children and Adolescents. Eur. Arch. Paediatr. Dent. 2019, 20, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Tulumbaci, F.; Gungormus, M. In Vitro Remineralization of Primary Teeth with a Mineralization-Promoting Peptide Containing Dental Varnish. J. Appl. Oral Sci. 2020, 28, e20200259. [Google Scholar] [CrossRef] [PubMed]
- Imran, E.; Cooper, P.R.; Ratnayake, J.; Ekambaram, M.; Mei, M.L. Potential Beneficial Effects of Hydroxyapatite Nanoparticles on Caries Lesions In Vitro-A Review of the Literature. Dent. J. 2023, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Scribante, A.; Dermenaki Farahani, M.R.; Marino, G.; Matera, C.; Rodriguez Y Baena, R.; Lanteri, V.; Butera, A. Biomimetic Effect of Nano-Hydroxyapatite in Demineralized Enamel before Orthodontic Bonding of Brackets and Attachments: Visual, Adhesion Strength, and Hardness in In Vitro Tests. BioMed Res. Int. 2020, 2020, 6747498. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Montasser, M.A.; Abd El Latief, M.H.; Modica, G.G.; Scribante, A. Home Oral Care with Biomimetic Hydroxyapatite vs. Conventional Fluoridated Toothpaste for the Remineralization and Desensitizing of White Spot Lesions: Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 8676. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.D.; Pop, L.C.; Benea, H.R.C.; Tomoaia, G.; Racz, C.P.; Mocanu, A.; Dobrota, C.T.; Balint, R.; Soritau, O.; Tomoaia-Cotisel, M. Remineralization Induced by Biomimetic Hydroxyapatite Toothpastes on Human Enamel. Biomimetics 2023, 8, 450. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, M.; Tantbirojn, D.; Kymer-Davis, E.; Stewart, C.W.; Zhang, Y.H.; Versluis, A.; Garcia-Godoy, F. Neutralizing Salivary PH by Mouthwashes after an Acidic Challenge. J. Investig. Clin. Dent. 2017, 8, e12198. [Google Scholar] [CrossRef]
- Novozhilova, N.; Andreeva, E.; Polyakova, M.; Makeeva, I.; Sokhova, I.; Doroshina, V.; Zaytsev, A.; Babina, K. Antigingivitis, Desensitizing, and Antiplaque Effects of Alkaline Toothpastes: A Randomized Clinical Trial. Dent. J. 2023, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Kanenaga, R.; Tanaka, Y.; Hotta, K.; Arakawa, S. The Neutralizing Effect of Mouth Rinsing with Alkaline Electrolyzed Water on Different Regions of the Oral Cavity Acidified by Acidic Beverages. J. Oral Sci. 2022, 64, 17–21. [Google Scholar] [CrossRef]
- Wiegand, A.; Müller, I.; Schnapp, J.D.; Werner, C.; Attin, T. Impact of Fluoride, Milk and Water Rinsing on Surface Rehardening of Acid Softened Enamel. An in Situ Study. Am. J. Dent. 2008, 21, 113–118. [Google Scholar]
- Belardinelli, P.A.; Morelatto, R.A.; Benavidez, T.E.; Baruzzi, A.M.; López de Blanc, S.A. Effect of Two Mouthwashes on Salivary Ph. Acta Odontol. Latinoam. 2014, 27, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Arakelyan, M.G.; Polyakova, M.A.; Babina, K.S.; Novozhilova, N.E.; Margaryan, E.G.; Doroshina, V.Y.; Arzukanyan, A.V.; Makeeva, M.K. Qualitative and Quantitative Evaluation of the Efficiency of the Application of Foams with False Xerostomia. J. Int. Soc. Prev. Community Dent. 2019, 9, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.E.; Baez, R.J. (Eds.) Oral Health Surveys: Basic Methods, 5th ed.; WHO Press: Geneve, Switzerland, 2013; ISBN 9783642204784. [Google Scholar]
- Greene, J.G.; Vermillion, J.R. The Simplified Oral Hygiene Index. J. Am. Dent. Assoc. 1964, 68, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Ainamo, J.; Bay, I. Periodontal indexes for and in practice. Tandlaegebladet 1976, 80, 149–152. [Google Scholar] [PubMed]
- Foglio-Bonda, P.L.; Brilli, K.; Pattarino, F.; Foglio-Bonda, A. Salivary Flow Rate and PH in Patients with Oral Pathologies. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 369–374. [Google Scholar] [PubMed]
- Bowen, W.H. The Stephan Curve Revisited. Odontology 2013, 101, 2–8. [Google Scholar] [CrossRef]
- Shahmoon, R.; Tamir, Y.; Beiderman, Y.; Agdarov, S.; Beiderman, Y.; Zalevsky, Z. Analysis of Swallowing in Infants and Adults Using Speckle Pattern Analysis. Sci. Rep. 2022, 12, 3847. [Google Scholar] [CrossRef] [PubMed]
- Cassiani, R.A.; Santos, C.M.; Parreira, L.C.; Dantas, R.O. The Relationship between the Oral and Pharyngeal Phases of Swallowing. Clinics 2011, 66, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Sales-Peres, S.H.; Magalhães, A.C.; de Andrade Moreira Machado, M.A.; Buzalaf, M.A.R. Evaluation of the Erosive Potential of Soft Drinks. Eur. J. Dent. 2007, 1, 10–13. [Google Scholar] [CrossRef]
- Mojaver, Y.N.; Javidi, N.; Manshaee, K. Influence of Soft Drink on Salivary PH. Chin. J. Dent. Res. 2008, 11, 52–55. [Google Scholar]
- Barac, R.; Gasic, J.; Trutic, N.; Sunaric, S.; Popovic, J.; Djekic, P.; Radenkovic, G.; Mitic, A. Erosive Effect of Different Soft Drinks on Enamel Surface in Vitro: Application of Stylus Profilometry. Med. Princ. Pract. 2015, 24, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Dawes, C. What Is the Critical PH and Why Does a Tooth Dissolve in Acid? J. Can. Dent. Assoc. 2003, 69, 722–724. [Google Scholar] [PubMed]
- Lussi, A.; Schlueter, N.; Rakhmatullina, E.; Ganss, C. Dental Erosion—An Overview with Emphasis on Chemical and Histopathological Aspects. Caries Res. 2011, 45 (Suppl. S1), 2–12. [Google Scholar] [CrossRef] [PubMed]
- Epple, M.; Enax, J.; Meyer, F. Prevention of Caries and Dental Erosion by Fluorides—A Critical Discussion Based on Physico-Chemical Data and Principles. Dent. J. 2022, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Benjakul, P.; Chuenarrom, C. Association of Dental Enamel Loss with the PH and Titratable Acidity of Beverages. J. Dent. Sci. 2011, 6, 129–133. [Google Scholar] [CrossRef]
- Barbour, M.E.; Lussi, A. Erosion in Relation to Nutrition and the Environment. Monogr. Oral. Sci. 2014, 25, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.-H.; Son, H.-H.; Yi, K.; Chang, J. Elemental Analysis of Caries-Affected Root Dentin and Artificially Demineralized Dentin. Restor. Dent. Endod. 2016, 41, 255. [Google Scholar] [CrossRef] [PubMed]
- Widodo, G.; Wilson, R.; Bartlett, D. Oral Clearance of an Acidic Drink in Patients with Erosive Tooth Wear Compared with That in Control Subjects. Int. J. Prosthodont. 2005, 18, 323–327. [Google Scholar] [PubMed]
- Banan, L.K.; Hegde, A.M. Plaque and Salivary PH Changes after Consumption of Fresh Fruit Juices. J. Clin. Pediatr. Dent. 2005, 30, 9–13. [Google Scholar] [CrossRef]
- Dodds, M.; Roland, S.; Edgar, M.; Thornhill, M. Saliva A Review of Its Role in Maintaining Oral Health and Preventing Dental Disease. BDJ Team 2015, 2, 15123. [Google Scholar] [CrossRef]
- Hara, A.T.; Zero, D.T. The Potential of Saliva in Protecting against Dental Erosion. Monogr. Oral. Sci. 2014, 25, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Fenoll-Palomares, C.; Muñoz Montagud, J.V.; Sanchiz, V.; Herreros, B.; Hernández, V.; Mínguez, M.; Benages, A. Unstimulated Salivary Flow Rate, PH and Buffer Capacity of Saliva in Healthy Volunteers. Rev. Esp. Enfermedades Dig. 2004, 96, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Mokeem, L.S.; Willis, L.H.; Jack Windsor, L.; Blaine Cook, N.; Eckert, G.; Gregory, R.L. Combined Effects of Soft Drinks and Nicotine on Streptococcus Mutans Metabolic Activity and Biofilm Formation. J. Oral Sci. 2021, 63, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.K.; Lingström, P.; Birkhed, D. Effect of Soft Drinks on Proximal Plaque PH at Normal and Low Salivary Secretion Rates. Acta Odontol. Scand. 2007, 65, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Hedge, R.; Dixit, U. Role of Plaque in the Clearance of Salivary Sucrose and Its Influence on Salivary Ph. J. Indian. Soc. Pedod. Prev. Dent. 2011, 29, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Shaw, L. Baby Fruit Juices and Tooth Erosion. Br. Dent. J. 1987, 162, 65–67. [Google Scholar] [CrossRef]
- Grenby, T.H.; Mistry, M.; Desai, T. Potential Dental Effects of Infants’ Fruit Drinks Studied in Vitro. Br. J. Nutr. 1990, 64, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Lussi, A.; von Salis-Marincek, M.; Ganss, C.; Hellwig, E.; Cheaib, Z.; Jaeggi, T. Clinical Study Monitoring the PH on Tooth Surfaces in Patients with and without Erosion. Caries Res. 2012, 46, 507–512. [Google Scholar] [CrossRef]
- Kershaw, J.C.; Running, C.A. Conditioning of Human Salivary Flow Using a Visual Cue for Sour Candy. Arch. Oral. Biol. 2018, 92, 90–95. [Google Scholar] [CrossRef]
- Morquecho-Campos, P.; Bikker, F.J.; Nazmi, K.; de Graaf, K.; Laine, M.L.; Boesveldt, S. A Stepwise Approach Investigating Salivary Responses upon Multisensory Food Cues. Physiol. Behav. 2020, 226, 113116. [Google Scholar] [CrossRef]
- Fibryanto, E.; Widyastuti, W. Effect of Brushing the Teeth before and after Meals on Salivary PH: A Quasi-Experimental Study. J. Int. Oral Health 2022, 14, 163. [Google Scholar] [CrossRef]
- Setiawan, S.; Haroen, E.R.; Hadidjah, D. The Difference in Saliva PH before and after Brushing with Fluoride Containing Toothpaste and without Toothpaste. Padjadjaran J. Dent. 2008, 20, 139. [Google Scholar] [CrossRef]
- Al Asmari, D.; Khan, M. Evaluate Efficacy of Desensitizing Toothpaste Containing Zinc-Carbonate Hydroxyapatite Nanocrystals: Non-Comparative Eight-Week Clinical Study. J. Int. Soc. Prev. Community Dent. 2019, 9, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Steinert, S.; Zwanzig, K.; Doenges, H.; Kuchenbecker, J.; Meyer, F.; Enax, J. Daily Application of a Toothpaste with Biomimetic Hydroxyapatite and Its Subjective Impact on Dentin Hypersensitivity, Tooth Smoothness, Tooth Whitening, Gum Bleeding, and Feeling of Freshness. Biomimetics 2020, 5, 17. [Google Scholar] [CrossRef]
- Polyakova, M.; Sokhova, I.; Doroshina, V.; Arakelyan, M.; Novozhilova, N.; Babina, K. The Effect of Toothpastes Containing Hydroxyapatite, Fluoroapatite, and Zn-Mg-Hydroxyapatite Nanocrystals on Dentin Hypersensitivity: A Randomized Clinical Trial. J. Int. Soc. Prev. Community Dent. 2022, 12, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Saruta, J.; Takahashi, T.; To, M.; Shimizu, T.; Hayashi, T.; Morozumi, T.; Kubota, N.; Kamata, Y.; Makino, S.; et al. Effect of Ingesting Yogurt Fermented with Lactobacillus Delbrueckii Ssp. Bulgaricus OLL1073R-1 on Influenza Virus-Bound Salivary IgA in Elderly Residents of Nursing Homes: A Randomized Controlled Trial. Acta Odontol. Scand. 2019, 77, 517–524. [Google Scholar] [CrossRef]
- Sanghvi, U.; Chhabra, T.; Sethuraman, R. Effect of Probiotics on the Amount and PH of Saliva in Edentulous Patients: A Prospective Study. J. Indian Prosthodont. Soc. 2018, 18, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.D.; López-López, A.; Nicolescu, T.; Perez-Vilaplana, S.; Boix-Amorós, A.; Dzidic, M.; Garcia, S.; Artacho, A.; Llena, C.; Mira, A. Topic Application of the Probiotic Streptococcus Dentisani Improves Clinical and Microbiological Parameters Associated With Oral Health. Front. Cell Infect. Microbiol. 2020, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Babina, K.; Salikhova, D.; Doroshina, V.; Makeeva, I.; Zaytsev, A.; Uvarichev, M.; Polyakova, M.; Novozhilova, N. Antigingivitis and Antiplaque Effects of Oral Probiotic Containing the Streptococcus Salivarius M18 Strain: A Randomized Clinical Trial. Nutrients 2023, 15, 3882. [Google Scholar] [CrossRef]
- Kuru, B.E.; Laleman, I.; Yalnızoğlu, T.; Kuru, L.; Teughels, W. The Influence of a Bifidobacterium Animalis Probiotic on Gingival Health: A Randomized Controlled Clinical Trial. J. Periodontol. 2017, 88, 1115–1123. [Google Scholar] [CrossRef]
- Schlagenhauf, U.; Rehder, J.; Gelbrich, G.; Jockel-Schneider, Y. Consumption of Lactobacillus Reuteri-Containing Lozenges Improves Periodontal Health in Navy Sailors at Sea: A Randomized Controlled Trial. J. Periodontol. 2020, 91, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Hambire, C.; Hambire, U. Evaluation of Effect of Consumption of Probiotics on the Gingival and Periodontal Health Status in Children Undergoing Chemotherapy. Indian J. Cancer 2023, 60, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Babina, K.; Salikhova, D.; Polyakova, M.; Svitich, O.; Samoylikov, R.; Ahmad El-Abed, S.; Zaytsev, A.; Novozhilova, N. The Effect of Oral Probiotics (Streptococcus Salivarius K12) on the Salivary Level of Secretory Immunoglobulin A, Salivation Rate, and Oral Biofilm: A Pilot Randomized Clinical Trial. Nutrients 2022, 14, 1124. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).