Dental Developmental Defects: A Pilot Study to Examine the Prevalence and Etiology in a Population of Children between 2 and 15 Years of Age
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of the Sample
2.2. Sample Size and Sampling Approach
2.3. Evaluation Criteria
2.4. Statistical Analysis
3. Results
3.1. Prevalence of DDDs in Patients of the Master’s in Pediatric Dentistry of the Complutense University of Madrid
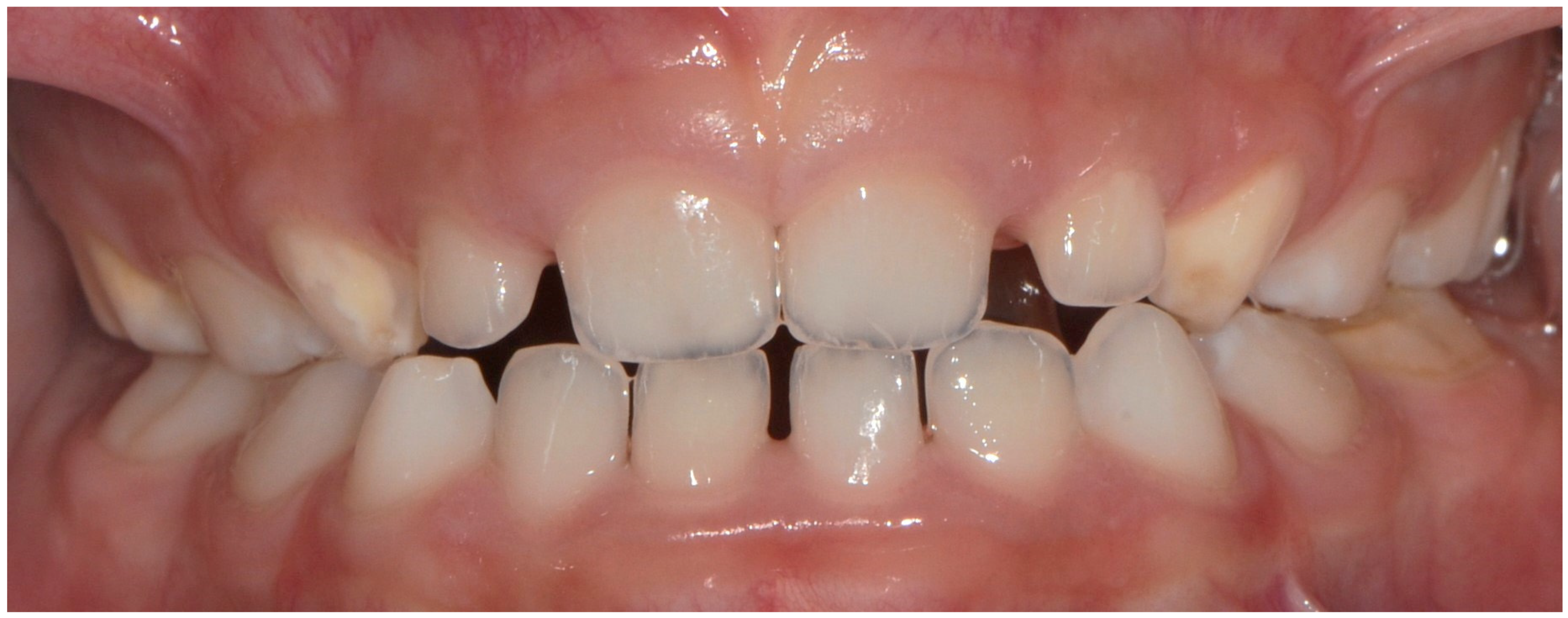
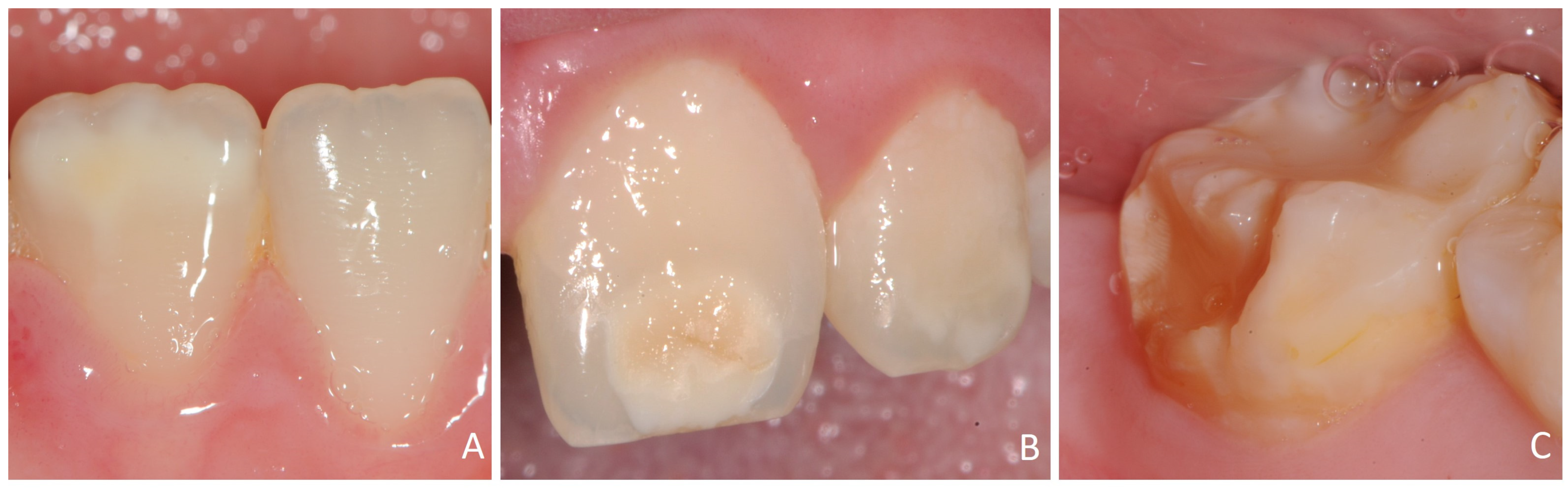
3.2. Distribution, Location, and Extent of DDDs
3.3. Aetiological Factors Present in the Sample
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
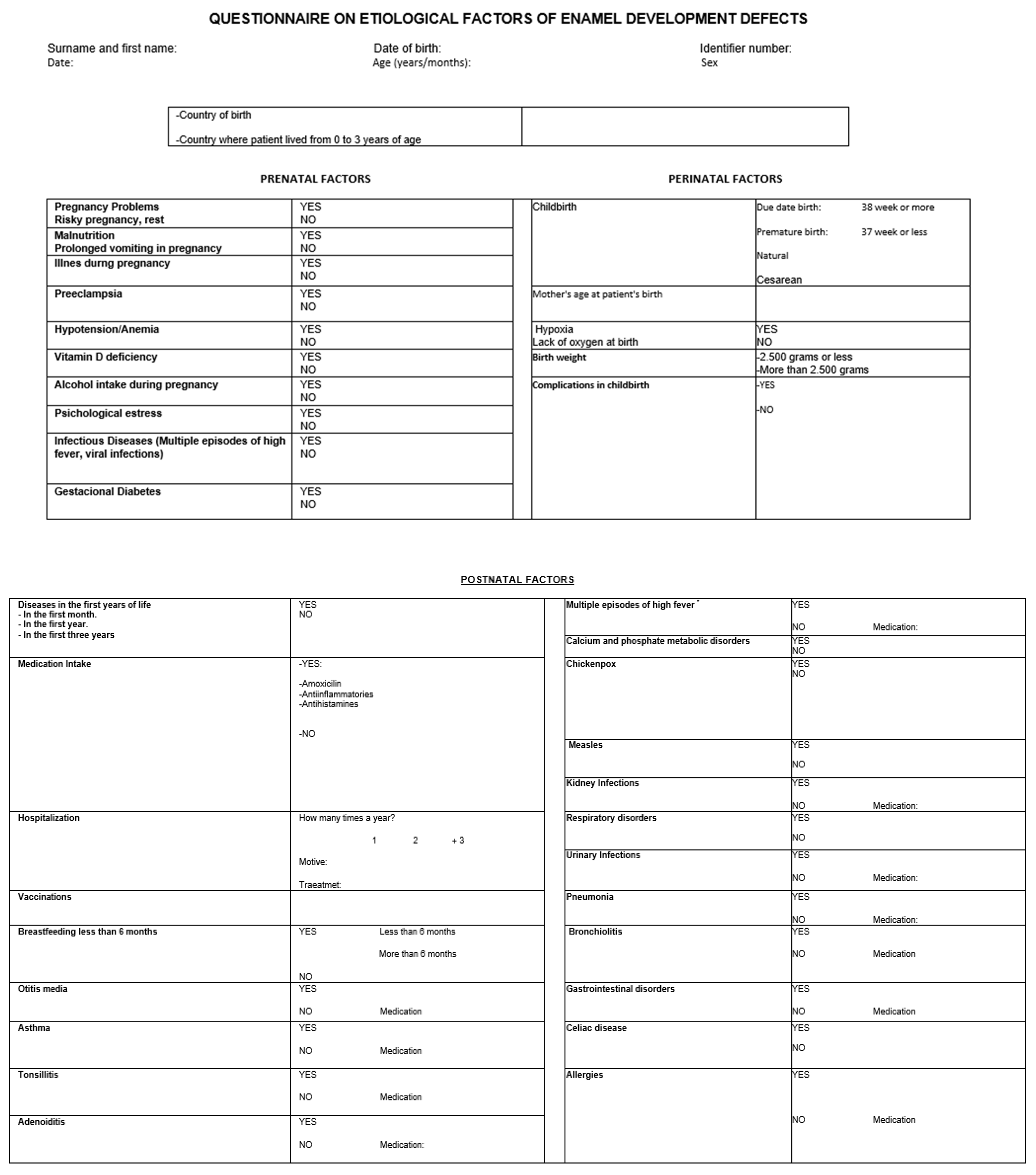
Appendix A

References
- Biondi, A.M.; Cortese, S.G.; Ortolani, A.M.; Ienco, M.; Angetieri, A.B. Prevalencia de hipomineralización molar incisiva en niños con y sin demanda de atención. Rev. Asoc. Odontol. Argen. 2013, 101, 140–146. [Google Scholar]
- Garg, N.; Jain, A.K.; Saha, S.; Singh, J. Essentiality of early diagnosis of molar incisor hypomineralization in children and review of its clinical presentation, etiology and management. Int. J. Clin. Pediatr. Dent. 2012, 5, 190–196. [Google Scholar]
- Simmer, J.P.; Papagerakis, P.; Smith, C.E.; Fisher, D.C.; Rountrey, A.N.; Zheng, L.; Hu, J.C. Regulation of dental enamel shape and hardness. J. Dent. Res. 2010, 89, 1024–1038. [Google Scholar] [CrossRef]
- Clarkson, J.; O’Mullane, D. A modified DDE Index for use in epidemiological studies of enamel defects. J. Dent. Res. 1989, 68, 445–450. [Google Scholar] [CrossRef] [PubMed]
- International Dental Federation; Commission on Oral Health; Research and Epidemiology. A review of developmental defects of enamel index (DDE Index). Int. Dent. J. 1992, 42, 411–426. [Google Scholar]
- Weerheijm, K.L.; Duggal, M.; Mejàre, I.; Papagiannoulis, L.; Koch, G.; Martens, L.C.; Hallonsten, A.-L. Judgment criteria for Molar Incisor Hipomineralisation (HIM) in epidemiologic studies: A summary of the European meeting on MIH held in Athens. Eur. J. Paediatr. Dent. 2003, 4, 110–113. [Google Scholar] [PubMed]
- Hernández, M.; Boj, J.R.; Espasa, E.; Peretz, B. First permanent molars and permanent incisors teeth by tooth prevalence of molar-incisor hypomineralization in a group of Spanish school children. Acta Stomatol. Croat. 2018, 52, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, J.; Li, Y.H.; Yang, Z.Y.; Zhou, Z. Association of molar incisor hypomineralization with premature birth or low birth weight: Systematic review and meta-analysis. J. Matern. Fetal Neonatal. Med. 2018, 33, 1700–1708. [Google Scholar] [CrossRef] [PubMed]
- da Cunha Coelho, A.S.E.; Mata, P.C.M.; Lino, C.A.; Macho, V.M.P.; Areias, C.M.F.G.P.; Norton, A.P.M.A.P.; Augusto, A.P.C.M. Dental hypomineralization treatment: A systematic review. J. Esthet. Restor. Dent. 2018, 31, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Elfrink, M.E.; Moll, H.A.; Kiefte-de Jong, J.C.; Jaddoe, V.W.; Hofman, A.; ten Cate, J.M.; Veerkamp, J.S. Pre and postnatal determinants of deciduous molar hypomineralization in 6-year-old children. The generation R study. PLoS ONE 2014, 9, e91057. [Google Scholar] [CrossRef]
- Fagrell, T.G.; Ludvigsson, J.; Ullbro, C.; Lundin, S.A.; Koch, G. Aetiology of severe demarcated enamel opacities an evaluation based on prospective medical and social data from 17,000 children. Swed. Dent. J. 2011, 35, 57–67. [Google Scholar] [PubMed]
- Cruvinel, V.R.N.; Gravina, D.B.L.; Azevedo, T.D.P.L.; Rezende, C.S.D.; Bezerra, A.C.B.; Toledo, O.A.D. Prevalence of enamel defects and associated risk factors in both dentitions in preterm and full term born children. J. Appl. Oral Sci. 2012, 20, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Correa-Faria, P.; Martins Junior, P.A.; Viera-Andrade, R.G.; Oliveira-Ferreira, F.; Marques, L.S.; Ramos-Jorge, M.L. Developmental defects of enamel in primary teeth: Prevalence and associated factors. Int. J. Paediatr. Dent. 2013, 23, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Kusku, O.; Caglar, E.; Sandalli, N. The prevalence and aetiology of molar incisor hypomineralisation in a group of children in Istanbul. EAPD 2008, 9, 139–144. [Google Scholar]
- Negre-Barber, A.; Montiel-Company, J.M.; Boronat-Catalá, M.; Catalá-Pizarro, M.; Almerich-Silla, J.M. Hypomineralized second primary molars as predictor of molar incisor hypomineralization. Sci. Rep. 2016, 6, 31929. [Google Scholar] [CrossRef] [PubMed]
- Tourino, L.F.P.; Correa-Faria, P.; Ferreira, R.C.; Bendo, C.B.; Zanzar, P.M.; Vale, M.P. Association between molar incisor hyponimeralization in school children and both prenatal and postnatal factors: A population-based study. PLoS ONE 2016, 11, e0156332. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, S.; Ivanovic, M.; Davidovic, B.; Lecic, J. Aetiological factors of molar incisor hypomineralization. Serbian Dent. J. 2013, 60, 69–75. [Google Scholar] [CrossRef]
- Farah, R.; Drummond, B.; Swain, M.; Williams, S. Linking the clinical presentation of molar incisor hypomineralization to its mineral density. Int. J. Paediatr. Dent. 2010, 20, 353–360. [Google Scholar] [CrossRef]
- Da Costa-Silva, C.M.; Ambrosano, G.M.B.; Jeremias, F.; De Souza, J.F.; Mialhe, F.L. Increase in severity of molar–incisor hypomineralization and its relationship with the colour of enamel opacity: A prospective cohort study. Int. J. Paediatr. Dent. 2011, 21, 333–341. [Google Scholar] [CrossRef]
- Machiulskiene, V.; Baelum, V.; Fejerskov, O.; Nyvad, B. Prevalence and extent of dental caries, dental fluorosis and developmental enamel defects in Lithuanian teenage populations with different fluoride exposures. Eur. J. Oral Sci. 2009, 117, 154–160. [Google Scholar] [CrossRef]
- Jälevik, B.; Klingberg, G.A. Dental treatment, dental fear and behaviour management problems in children with severe enamel hypomineralization of their permanent first molars. Int. J. Paediatr. Dent. 2002, 12, 24–32. [Google Scholar] [CrossRef]
- Jälevik, B.; Klingberg, G.A. Treatment outcomes and dental anxiety in 18-year-olds with MIH, comparisons with healthy controls—A longitudinal study. Int. J. Paediatr. Dent. 2012, 22, 85–91. [Google Scholar] [CrossRef]
- Wong, H.M.; Peng, S.M.; King, N.M.; McGrath, C. Infant growth and the occurrence of developmental defects of enamel in 12-years-olds. Caries Res. 2015, 49, 575–582. [Google Scholar] [CrossRef]
- Jälevik, B.; Szigyarto-Matei, A.; Robertson, A. The prevalence of developmental defects of enamel, aprospective cohort study of adolescents in Western Sweden: A Barn I TAnadvarden (BITA, children in dental care) study. Eur. Arch. Paediatr. Dent. 2018, 19, 187–195. [Google Scholar] [CrossRef]
- Seow, W.K. Comparison of enamel defects in the primary and permanent dentitions of children from a low-fluoride district in Australia. Paediatr. Dent. 2011, 33, 207–212. [Google Scholar]
- Vélez-León Albadalejo-Martínez, A.; Pacheco-Quito, E.M.; Armas-Vega, A.; Delgado-Gaete, A.; Pesántez-Ochoa, D.; Melo, M. Developmental Enamel Defects in Children from the Southern Region of Ecuator. Children 2022, 9, 1755. [Google Scholar] [CrossRef]
- Jacobsen, P.E.; Haubek, D.; Henriksen, T.B.; Ostergaard, J.R.; Poulsen, S. Developmental enamel defects in children born preterm: A systematic review. Eur. J. Oral Sci. 2014, 122, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Alaluusua, S.; Lukinmaa, P.L.; Koskimies, M.; Pirinen, S.; Hölttä, P.; Kallio, M.; Holttinen, T.; Salmenperä, L. Developmental dental defects associated with long breast feeding. Eur. J. Oral Sci. 1996, 104, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Tapias-Ledesma, M.A.; Jiménez, R.; Lamas, F.; González, A.; Carrasco, P.; Gíl de Miguel, A. Factors associated with first molar dental enamel defects: A multivariate epidemiological approach. J. Dent. Child. Chic. Ill. 2003, 70, 215–220. [Google Scholar]
- Velló, M.A.; Martínez-Costa, C.; Catalá, M.; Fons, J.; Brines, J.; Guijarro-Martínez, R. Prenatal and neonatal risk factors for the development of enamel defects in low birth weight children. Oral Dis. 2010, 16, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Navia, J.M.; Bian, J.Y. Prevalence and distribution of developmental enamel defects in primary dentition of Chinese children 3–5 years old. Commun. Dent. Oral Epidemiol. 1995, 23, 72–79. [Google Scholar] [CrossRef]
- García, M.; Monserrat, C.; Almerich, J. Epidemiologic study of molar incisor hipomineralization in 8-year-old Spanish children. Int. J. Peadiatr Dent. 2014, 24, 14–22. [Google Scholar] [CrossRef]
- Weerheijm, K.L.; Groen, H.J.; Beentjes, V.E.; Poorterman, J.H. Prevalence of cheese molars in eleven-years-old Dutch children. ASDC J. Dent. Child. 2001, 68, 259–262. [Google Scholar]
- Alaluusua, S.; Lukinmaa, P.L.; Vartiainen, T.; Partanen, M.; Torppa, J.; Tuomisto, J. Polychlorinated dibenzo-p-dioxins and dibenzofurans via mother’s milk may cause developmental defects in the child’s teeth. Environ. Toxicol. Pharmacol. 1996, 1, 1937. [Google Scholar] [CrossRef]
- Kemoli, A.M. Prevalence of molar incisor hyponimeralization in six to eight years old in two rural divisions in Kenya. East. Afr. Med. J. 2008, 85, 514–519. [Google Scholar]
- Jälevik, B.; Klingberg, G.; Barregard, L.; Noren, J.G. The prevalence of demarcated opacities in first molars in a group of Swedish children. Acta Odontol. Scand. 2001, 59, 255–260. [Google Scholar] [CrossRef]
- Balmer, R.; Toumba, J.; Godson, J. The prevalence of molar incisor hypomineralization in Northern England and its relationship to socioeconomic status and water fluoridation. J. Paediatr. Dent. 2012, 22, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Martínez, T.; Jimeno, F.; Luis, J. Prevalence of molar-incisor hypomineralization observed using transillumination in a group of children from Barcelona (Spain). Int. J. Paediatr. Dent. 2012, 22, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Pandey, R.K. Molar incisor hypomineralization: An Epidemiological study with prevalence and etiological factors in Indian pediatric population. Int. J. Clin. Pediatr. Dent. 2016, 9, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Preusser, S.E.; Ferring, V.; Wleklinsky, C.; Wetzel, W.E. Prevalence and severity of molar incisor hypomineralization in a region of Germany. J. Public Health Dent. 2007, 67, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Soviero, V.; Haubek, D.; Trindade, C.; Da Matta, T.; Poulsen, S. Prevalence and distribution of demarcated opacities and their sequelae in permanent 1st molars and incisors in 7 to 13 years old Brazilian Children. Acta Odontol. Scand. 2009, 67, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Zawaidh, F.I.; Al-Jundi, S.H.; Al-Jaljoli, M.H. Molar incisor hypomineralization: Prevalence in Jordanian children and clinical characteristics. Eur. Arch. Paediatr. Dent. 2011, 12, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Allazzam, S.M.; Alaki, S.M.; El Meligy, O.A.S. Molar incisor hypomineralization, prevalence and etiology. Int. J. Dent. 2014, 2014, 234508. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, S.A.; Hegde, S. Molar incisor hypomineralization: Prevalence, severity and clinical characteristics in 8 to 13 years old children of Udaipur, India. J. Indian Soc. Pedod. Prev. Dent. 2014, 32, 322–329. [Google Scholar] [CrossRef]
- Lunardelli, S.E.; Peres, M.A. Prevalence and distribution of developmental enamel defects in the primary dentition of preschool children. Braz. Oral Res. 2005, 19, 144–149. [Google Scholar] [CrossRef]
- Ghanim, A.; Manton, D.; Marino, R.; Morgan, M.; Bailey, D. Prevalence of demarcated hypomineralization defects in second primary molars in Iraqi children. Int. J. Paediatr. Dent. 2013, 23, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Costa Silva, C.M.; Jeremias, F.; Souza, J.F.; Cordeiro Rde, C.; Santos Pinto, L.; Zuanon, A.C. Molar incisor hypomineralization: Prevalence, severity and clinical consequences in Brazilian children. Int. J. Paediatr. Dent. 2010, 20, 426–434. [Google Scholar] [CrossRef]
- Cho, S.Y.; Ki, Y.; Chu, V. Molar incisor hypomineralization in Hong Kong Chinese children. Int. J. Paediatr. Dent. 2008, 18, 348–352. [Google Scholar] [CrossRef]
- Masumo, R.; Bardsen, A.; Astrom, A.N. Developmental defects of enamel in primary teeth and association with early life course events: A study of 6–36 month old children in Manyara, Tanzania. BMC Oral Health 2013, 13, 21–32. [Google Scholar] [CrossRef]
- Özgül, B.M.; Sakaryali, D.; Tirali, R.E. Does MIH affects preoperative and intraoperative hypersensitivity? J. Clin. Pediatr. Dent. 2022, 46, 204–210. [Google Scholar] [CrossRef]
- Beentjes, V.E.; Weerheijm, K.L.; Groen, H.J. Factors involved in the aetiology of molar-incisor hypomineralisation (MIH). Eur. J. Paediatr. Dent. 2002, 3, 9–13. [Google Scholar] [PubMed]
- Alaluusua, S. Aetiology of molar incisor hipomineralization: A systematic review. Eur. Arch. Paediatr. Dent. 2010, 11, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.F.; Costa Silva, C.M.; Jeremias, F.; Santos Pinto, L.; Zuanon, A.C.; Cordeiro, R.C. Molar incisor hypomineralization: Possible aetiolofical factos in children from urban and rural areas. Eur. Arch. Paediatr. Dent. 2012, 13, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Luengo, S.; Feijóo-Garcia, G.; Miegimolle-Herrero, M.; Gallardo-López, N.E.; Caleya-Zambrano, A.M. Prevalence and clinical presentation of molar incisor hypomineralisation among a population of children in the community of Madrid. BMC Oral Health 2024, 24, 229. [Google Scholar] [CrossRef]
- The D3 Group. Available online: https://www.thed3groups.org (accessed on 21 December 2023).
| Teeth | Frequency | Percentage |
|---|---|---|
| 11 | 28 | 7.5% |
| 12 | 11 | 2.9% |
| 13 | 2 | 0.5% |
| 14 | 3 | 0.8% |
| 15 | 1 | 0.3% |
| 16 | 21 | 5.6% |
| 17 | 1 | 0.3% |
| 21 | 26 | 7.0% |
| 22 | 9 | 2.4% |
| 23 | 2 | 0.5% |
| 24 | 4 | 1.1% |
| 25 | 2 | 0.5% |
| 26 | 26 | 7.0% |
| 31 | 14 | 38.0% |
| 32 | 9 | 2.4% |
| 33 | 2 | 0.5% |
| 34 | 3 | 0.8% |
| 35 | 1 | 0.3% |
| 36 | 27 | 7.2% |
| 37 | 1 | 0.3% |
| 41 | 8 | 2.1% |
| 42 | 11 | 2.9% |
| 43 | 4 | 1.1% |
| 44 | 5 | 1.3% |
| 45 | 1 | 0.3% |
| 46 | 27 | 7.2% |
| 47 | 1 | 0.3% |
| Total | 250 | 67.0% |
| Teeth | Frequency | Percentage |
|---|---|---|
| 51 | 3 | 0.8% |
| 52 | 3 | 0.8% |
| 53 | 8 | 2.1% |
| 54 | 4 | 1.1% |
| 55 | 16 | 4.3% |
| 61 | 3 | 0.8% |
| 62 | 3 | 0.8% |
| 63 | 7 | 1.9% |
| 64 | 7 | 1.9% |
| 65 | 14 | 3.8% |
| 73 | 6 | 1.6% |
| 74 | 4 | 1.1% |
| 75 | 14 | 3.8% |
| 83 | 7 | 1.9% |
| 84 | 4 | 1.1% |
| 85 | 20 | 5.4% |
| Total | 123 | 33.0% |
| Characteristics | Frequency (Permanent/Primary) | Percentage (Permanent/Primary) | |
|---|---|---|---|
| Surfaces | Occlusal | 116/81 | 46.4%/65.9% |
| Vestibular/Buccal | 230/113 | 92.0%/91.9% | |
| Lingual/Palatal | 62/43 | 24.8%/35.0% | |
| Mesial | 45/16 | 18.0%/13.0% | |
| Distal | 41/16 | 16.4%/13% | |
| Thirds | 1/3 | 239/111 | 95.6%/90.2% |
| 2/3 | 103/66 | 41.2%/53.7% | |
| 3/3 | 41/27 | 16.4%/22.0% | |
| Índex DDD | White/Creamy demarcated | 189/99 | 75.6%/80.5% |
| Yellow/Brown demarcated opacities | 70/36 | 28.0%/29.3% | |
| Hypoplasia (points) | 113/60 | 45.2%/48.8% | |
| Hypoplasia (horizontal grooves) | 113/51 | 45.2%41.5% | |
| Hypoplasia (vertical grooves) | 26/6 | 10.4%/4.9% | |
| Hypoplasia (non-enamel) | 26/16 | 10.4%/13% | |
| Discolored enamel (no opacity) | 0/0 | 0%/0% | |
| Other defects (no MIH) | 67/59 | 26.8%/48.8% | |
| Frequency | Percentage | Valid Percentage | Cumulative Percentage | |
|---|---|---|---|---|
| Primary DDDs | 15 | 25 | 25 | 25 |
| Permanent DDDs | 27 | 45 | 45 | 70 |
| Primary and Permanent DDDs | 18 | 30 | 30 | 100 |
| TOTAL | 60 | 100 | 100 |
| Etiological Factors | Frequency Group Cases | Frequency Group Cases | Frequency Control Group | Frequency Control Group | P T.Fisher Value | P T.Chi Square Value | |
|---|---|---|---|---|---|---|---|
| Prenatal | Malnutrition | 5 | 8.3% | 11 | 18.3% | 0.178 | 0.107 |
| Pregnancy problems | 17 | 28.3% | 17 | 28.3% | 1.000 | 1.000 | |
| Illness during pregnancy | 8 | 13.3% | 11 | 18.3% | 0.618 | 0.453 | |
| Preeclampsia * | 1 | 1.7% | 10 | 16.7% | 0.008 | 0.004 | |
| Hypotension/Anemia | 8 | 1.3% | 10 | 16.7% | 0.799 | 0.609 | |
| Vitamin D deficiency | 9 | 1.0% | 7 | 11.7% | 0.789 | 0.591 | |
| Alcohol intake during pregnancy | 1 | 1.7% | 0 | 0% | 1.000 | 0.315 | |
| Psychological stress * | 12 | 20.0% | 2 | 3.3% | 0.008 | 0.004 | |
| Infectious diseases | 2 | 3.3% | 0 | 0% | 0.496 | 0.154 | |
| Gestational diabetes | 3 | 5.0% | 4 | 6.7% | 1.000 | 0.697 | |
| Perinatal | Due date birth | 53 | 88,3% | 53 | 88.3% | 1.000 | 1.000 |
| Premature birth | 7 | 11.7% | 7 | 11.7% | 1.000 | 1.000 | |
| Natural birth | 44 | 73.3% | 42 | 70.0% | 0.840 | 0.685 | |
| Cesarean birth | 16 | 26.7% | 18 | 30.0% | 0.840 | 0.685 | |
| Hipoxia | 8 | 13.3% | 3 | 5.0% | 0.204 | 0.114 | |
| Birth complications | 19 | 31.7% | 12 | 20.0% | 0.210 | 0.144 | |
| Low birth weight (<2500 g) | 9 | 15.0% | 5 | 8.3% | 0.394 | 0.255 | |
| Normal birth weight (≥2500 g) | 51 | 85.0% | 55 | 91.7% | 0.394 | 0.255 | |
| Postnatal | Diseases in the first month of life | 10 | 16.7% | 6 | 10.0% | 0.421 | 0.283 |
| Diseases in the first year of life | 15 | 25.0% | 10 | 16.7% | 0.369 | 0.261 | |
| Diseases in the first three years of life * | 14 | 23.3% | 5 | 8.3% | 0.043 | 0.024 | |
| Medication intake * (amoxicillin/anti-inflammatories/antihistamines) | 29 | 48.3% | 5 | 8.3% | -- | 0.001 | |
| Hospitalizations once a year | 16 | 26.7% | 16 | 26.7% | -- | 1.000 | |
| Hospitalizations twice a year | 3 | 5.0% | 3 | 5.0% | -- | 1.000 | |
| Vaccinations | 60 | 100% | 60 | 100% | 1.000 | 0.315 | |
| Breastfeeding less than 6 months | 20 | 33.3% | 15 | 25.0% | 0.422 | 0.315 | |
| Breastfeeding more than 6 months | 36 | 60.0% | 36 | 60.0% | 1.000 | 1.000 | |
| Otitis * | 26 | 43.3% | 5 | 8.3% | 0.001 | 0.001 | |
| Asthma | 2 | 3.3% | 5 | 8.3% | 0.439 | 0.243 | |
| Tonsillitis * | 15 | 25.0% | 3 | 5.0% | 0.004 | 0.002 | |
| Adenoiditis* | 10 | 16.7% | 0 | 0% | 0.001 | 0.001 | |
| Multiple episodes of high fever * | 16 | 26.7% | 2 | 3.3% | 0.001 | 0.001 | |
| Calcium and phosphate metabolic disorders | 0 | 0% | 0 | 0% | -- | -- | |
| Chickenpox * | 7 | 11.7% | 0 | 0% | 0.013 | 0.006 | |
| Measles | 0 | 0% | 0 | 0% | -- | -- | |
| Kidney infections | 1 | 1.7% | 0 | 0% | 1.000 | 0.315 | |
| Respiratory tract disorders * | 12 | 20.0% | 4 | 6.7% | 0.058 | 0.032 | |
| Urinary tract infections | 4 | 6.7% | 2 | 3.3% | 0.679 | 0.402 | |
| Pneumonia | 4 | 6.7% | 1 | 1.7% | 0.364 | 0.171 | |
| Bronchiolitis * | 20 | 33.3% | 8 | 13.3% | 0.017 | 0.010 | |
| Gastrointestinal disorders * | 15 | 25.0% | 2 | 3.3% | 0.001 | 0.001 | |
| Celiac disease | 0 | 0% | 0 | 0% | -- | -- | |
| Allergies | 13 | 21.7% | 8 | 13.3% | 0.337 | 0.230 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarado-Gaytán, J.; Saavedra-Marbán, G.; Velayos-Galán, L.; Gallardo-López, N.E.; de Nova-García, M.J.; Caleya, A.M. Dental Developmental Defects: A Pilot Study to Examine the Prevalence and Etiology in a Population of Children between 2 and 15 Years of Age. Dent. J. 2024, 12, 84. https://doi.org/10.3390/dj12040084
Alvarado-Gaytán J, Saavedra-Marbán G, Velayos-Galán L, Gallardo-López NE, de Nova-García MJ, Caleya AM. Dental Developmental Defects: A Pilot Study to Examine the Prevalence and Etiology in a Population of Children between 2 and 15 Years of Age. Dentistry Journal. 2024; 12(4):84. https://doi.org/10.3390/dj12040084
Chicago/Turabian StyleAlvarado-Gaytán, Jorge, Gloria Saavedra-Marbán, Laura Velayos-Galán, Nuria E. Gallardo-López, Manuel J. de Nova-García, and Antonia M. Caleya. 2024. "Dental Developmental Defects: A Pilot Study to Examine the Prevalence and Etiology in a Population of Children between 2 and 15 Years of Age" Dentistry Journal 12, no. 4: 84. https://doi.org/10.3390/dj12040084
APA StyleAlvarado-Gaytán, J., Saavedra-Marbán, G., Velayos-Galán, L., Gallardo-López, N. E., de Nova-García, M. J., & Caleya, A. M. (2024). Dental Developmental Defects: A Pilot Study to Examine the Prevalence and Etiology in a Population of Children between 2 and 15 Years of Age. Dentistry Journal, 12(4), 84. https://doi.org/10.3390/dj12040084






