The Impact of Nano- and Micro-Silica on the Setting Time and Microhardness of Conventional Glass–Ionomer Cements
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Setting Time
2.3. Microhardness
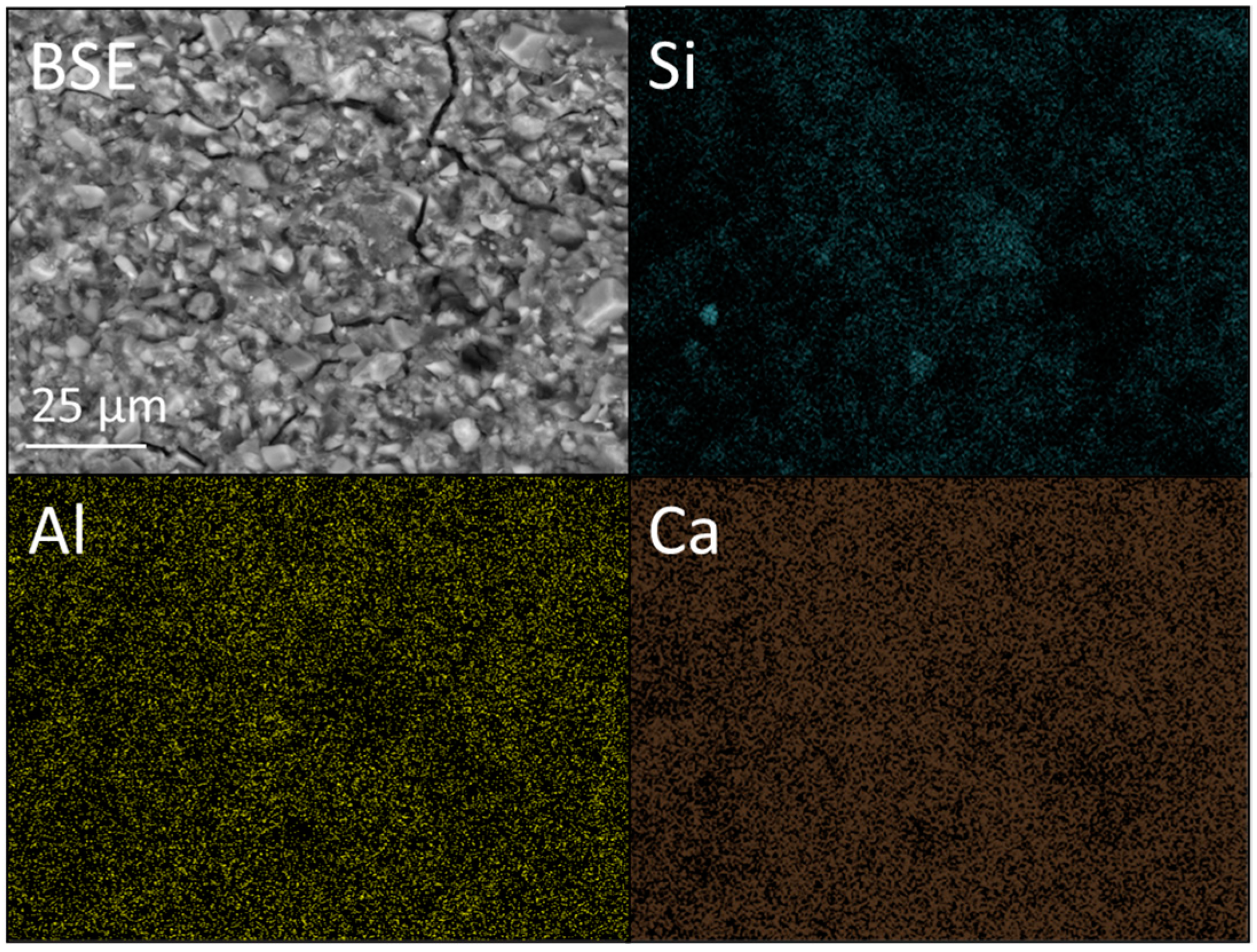
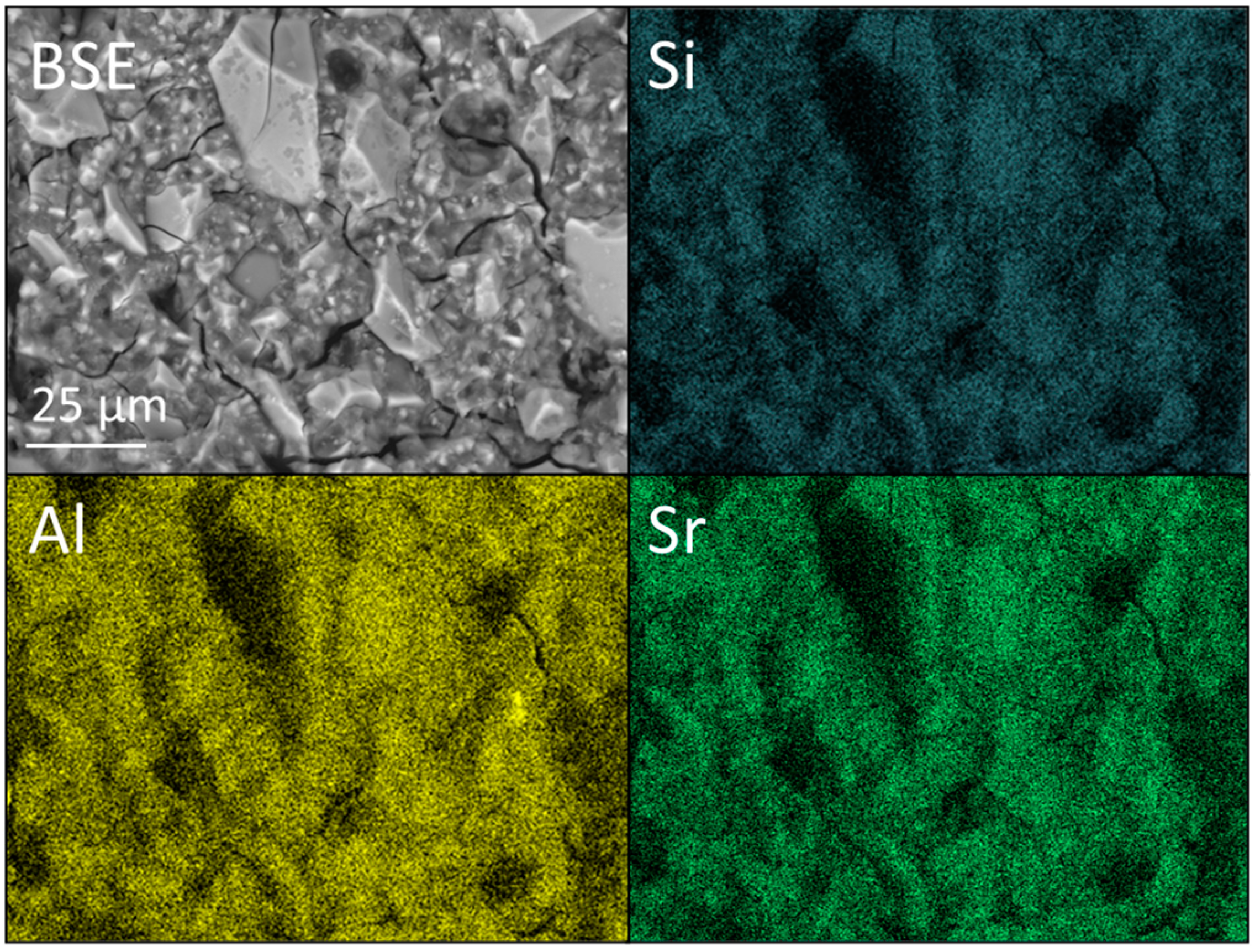
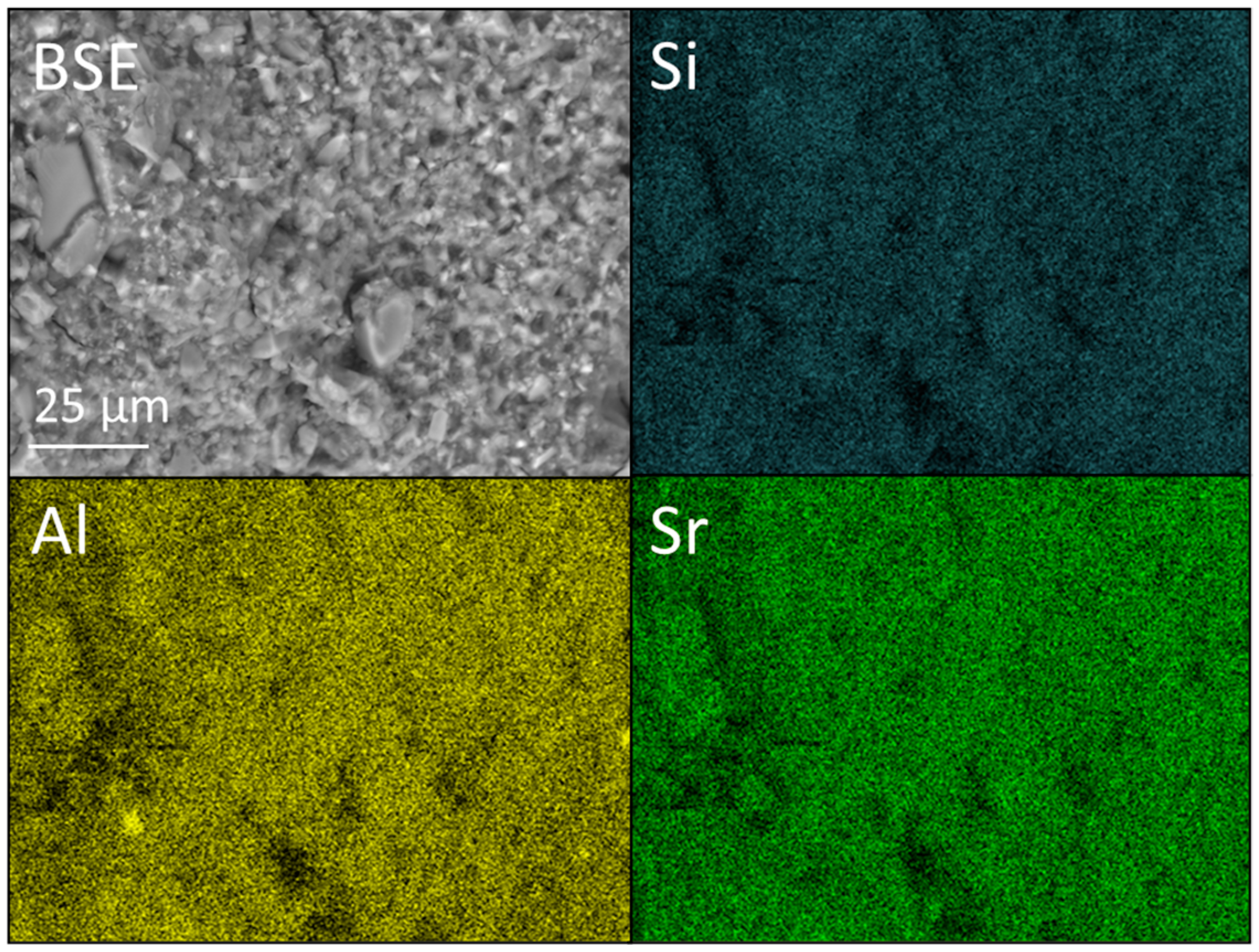
2.4. SEM and EDX Analysis
3. Results
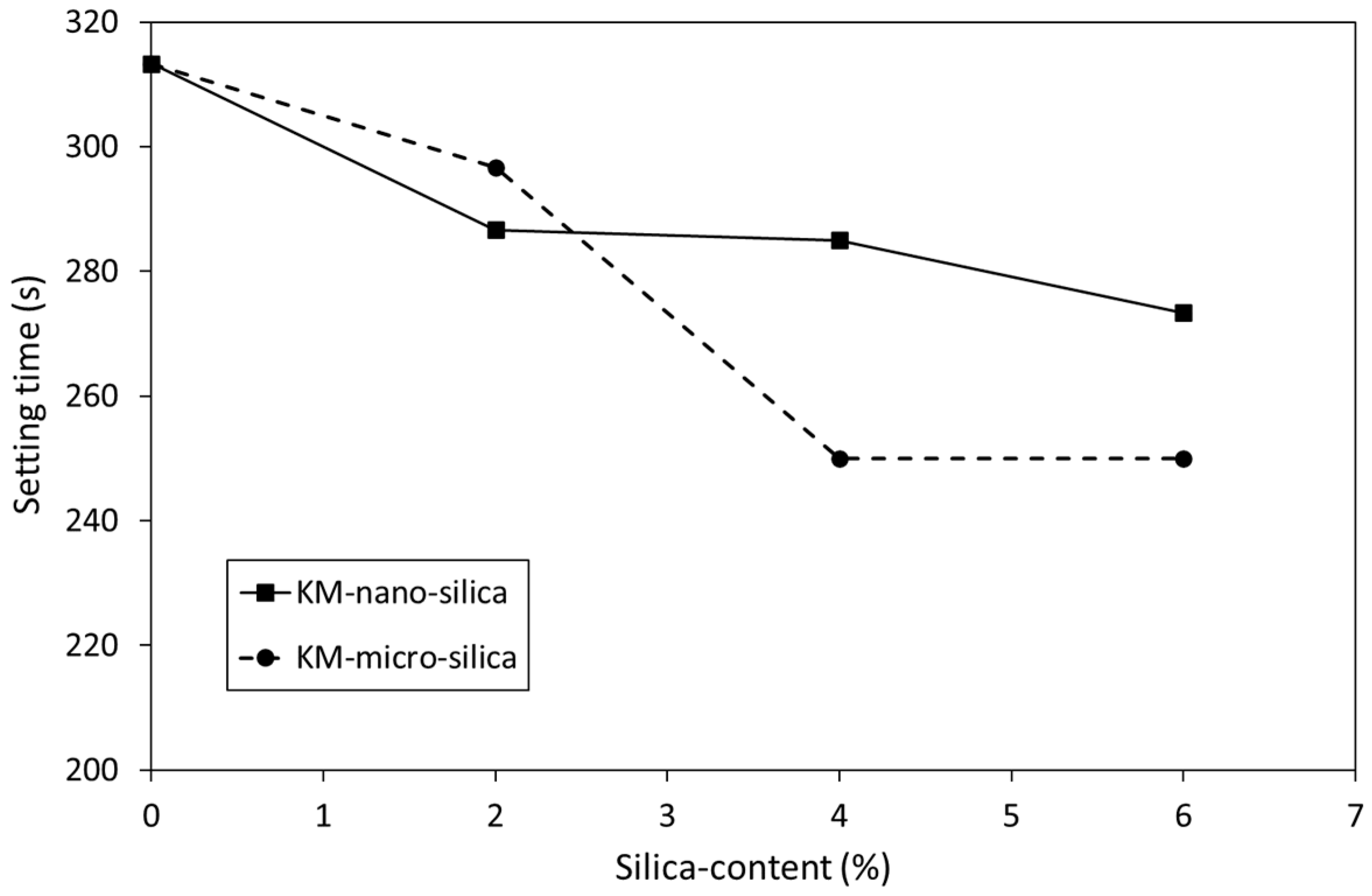
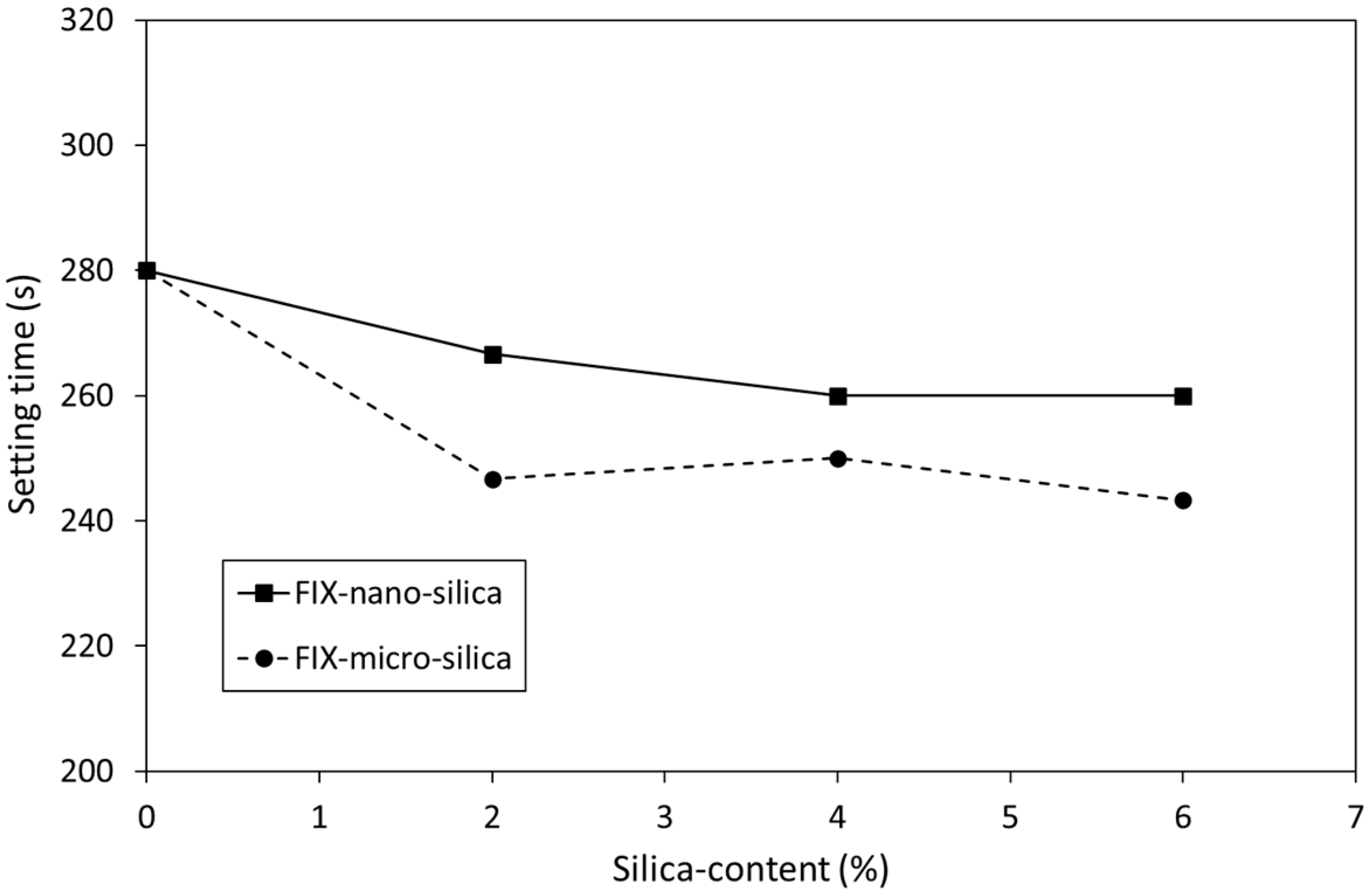
3.1. Setting Time
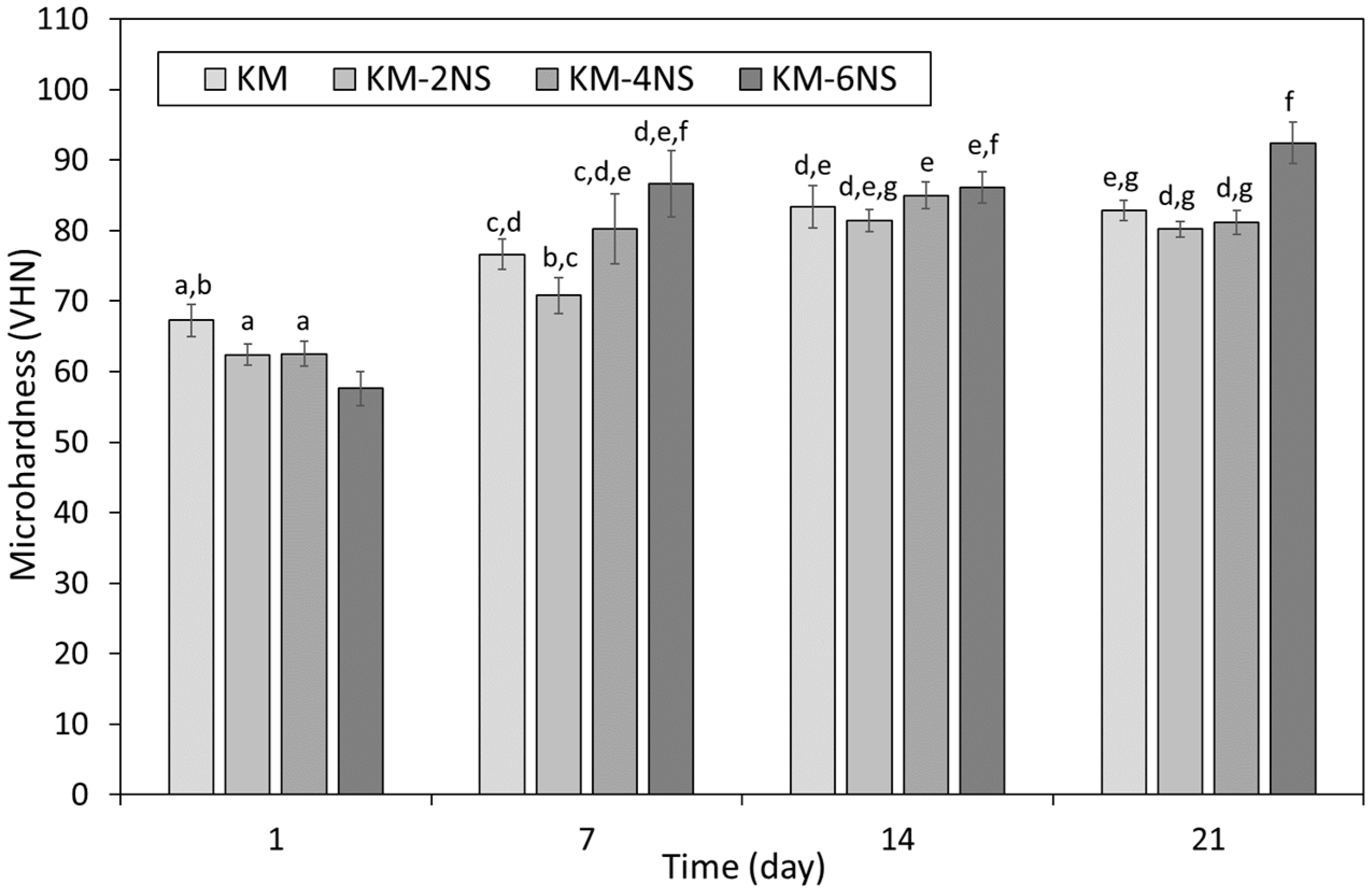
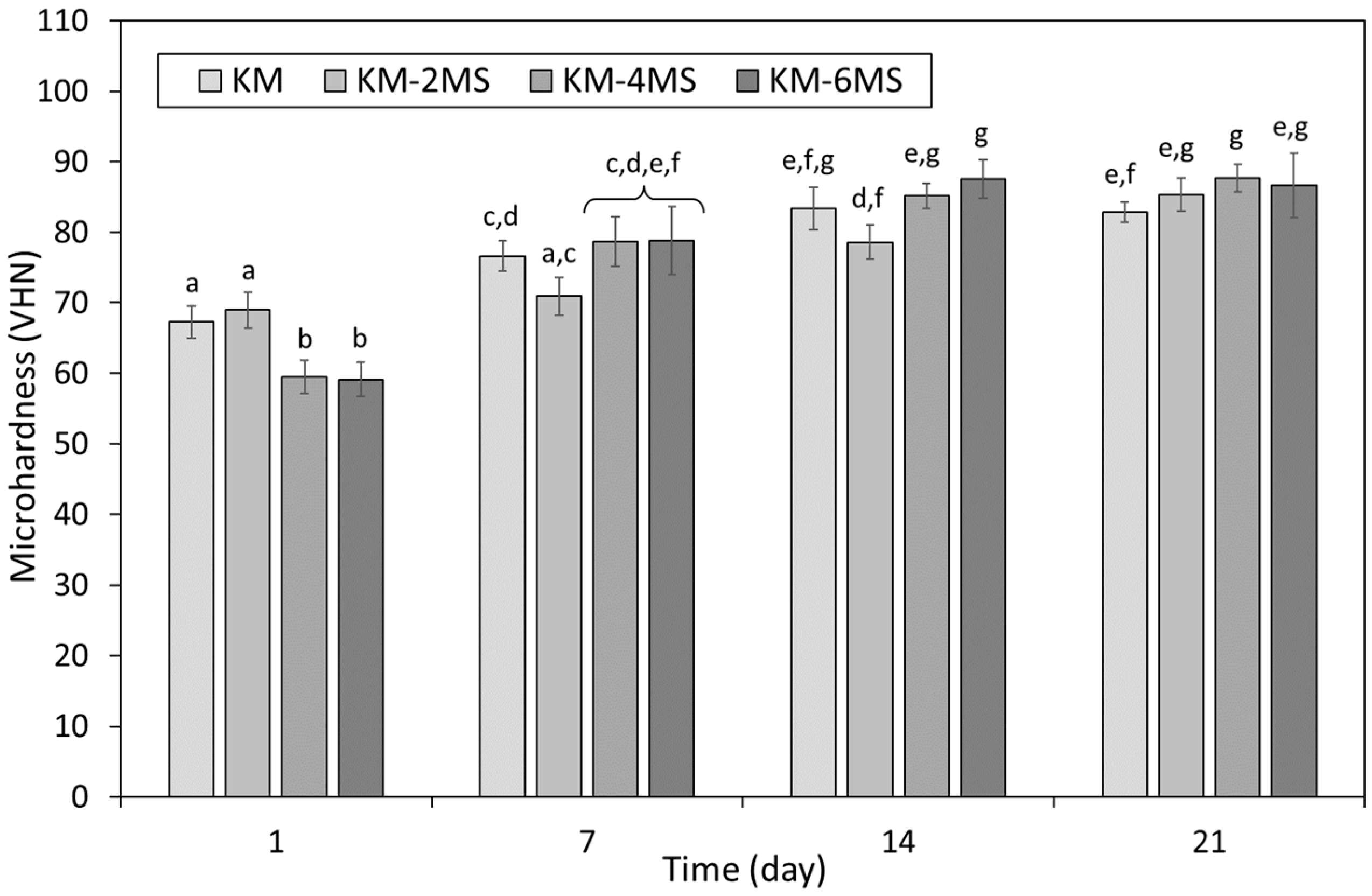
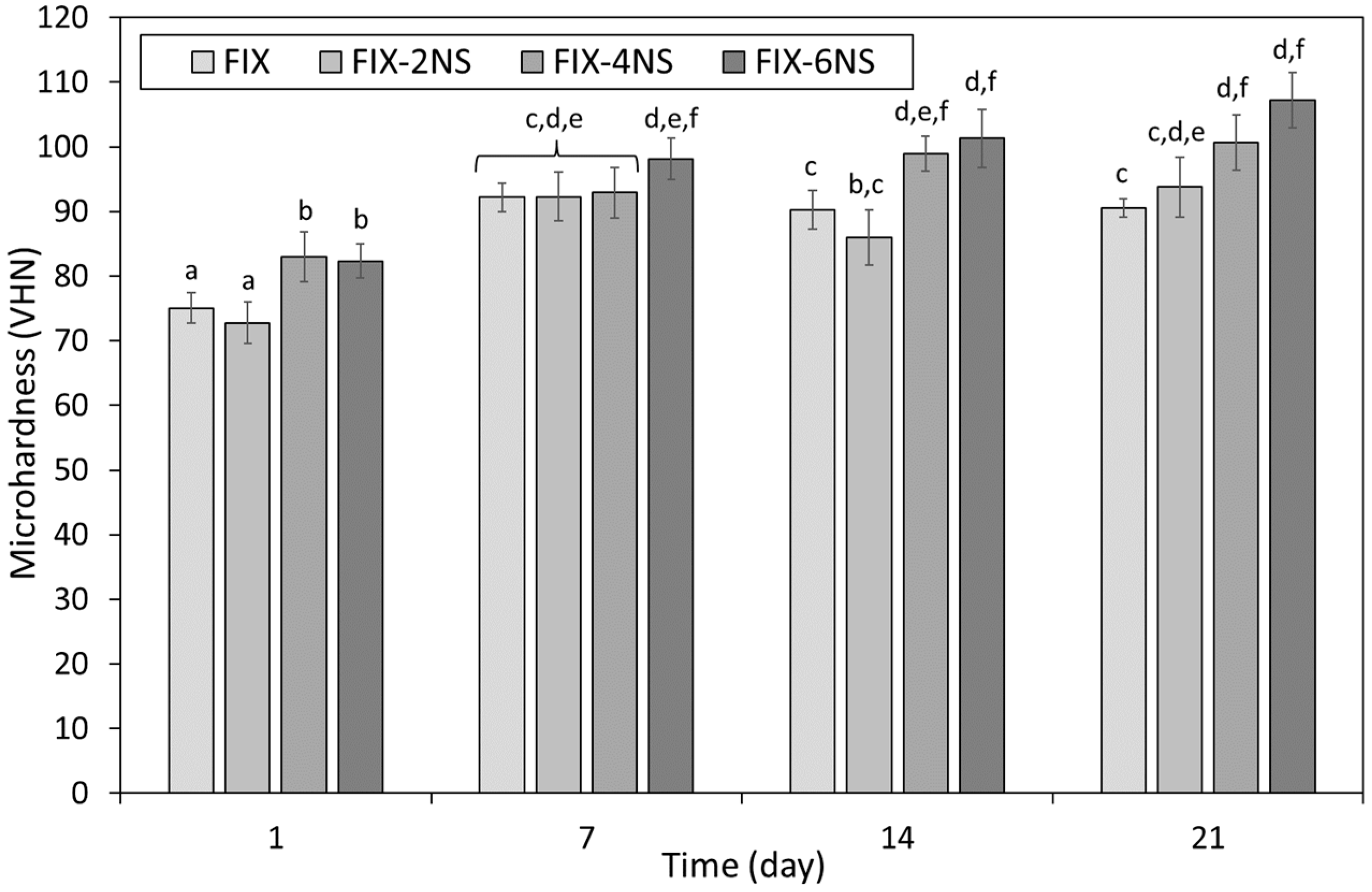
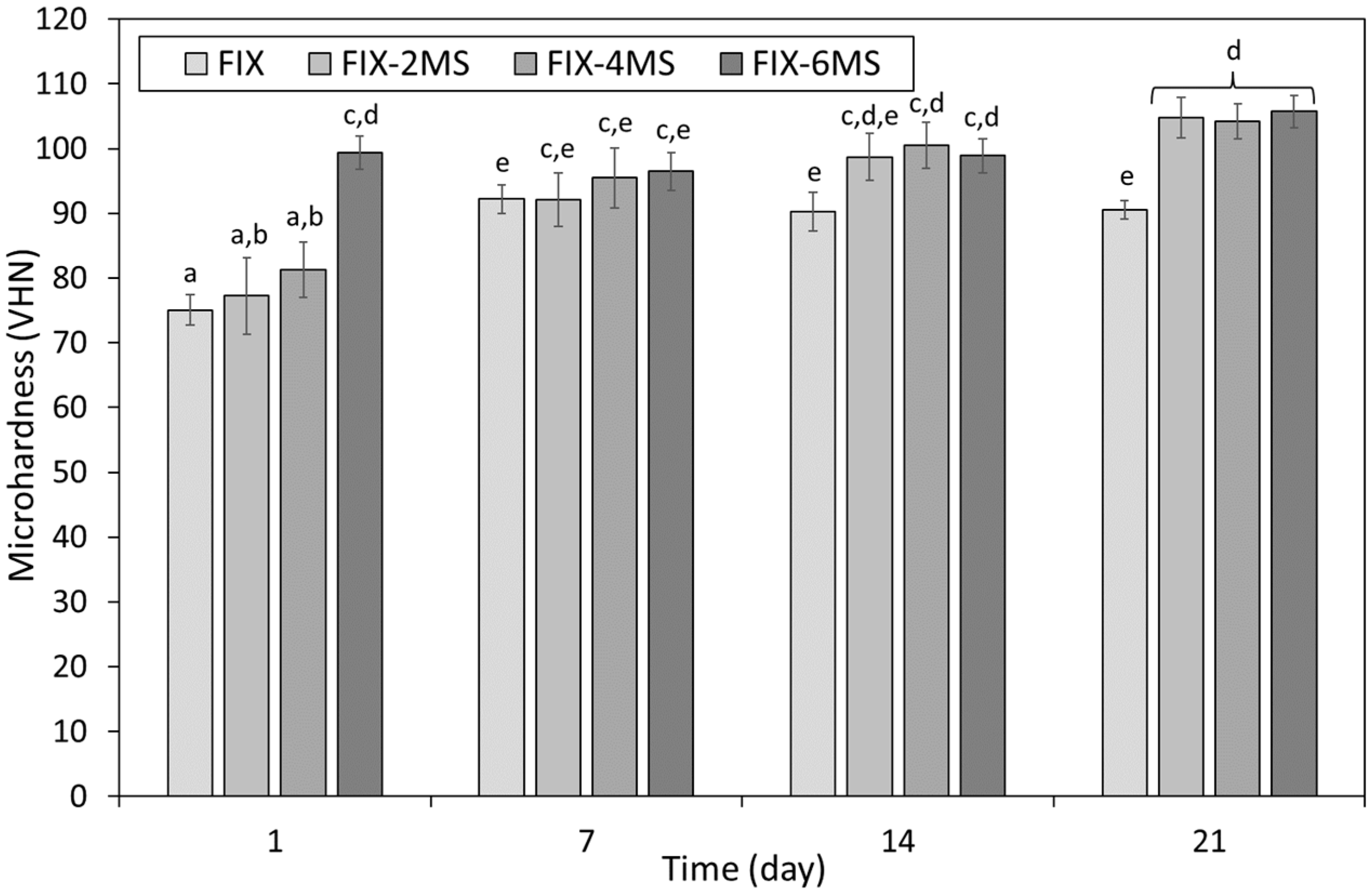
3.2. Microhardness
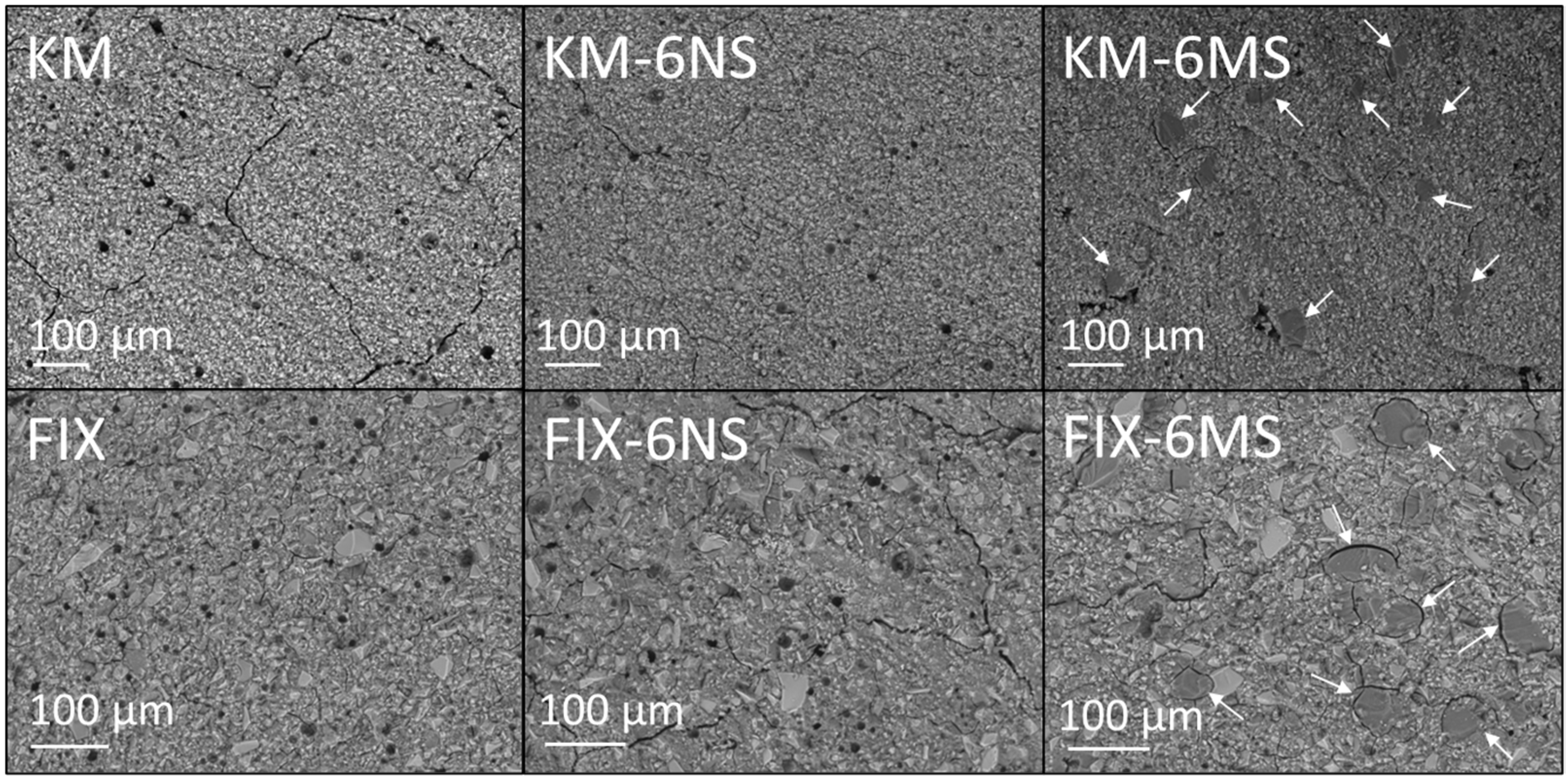
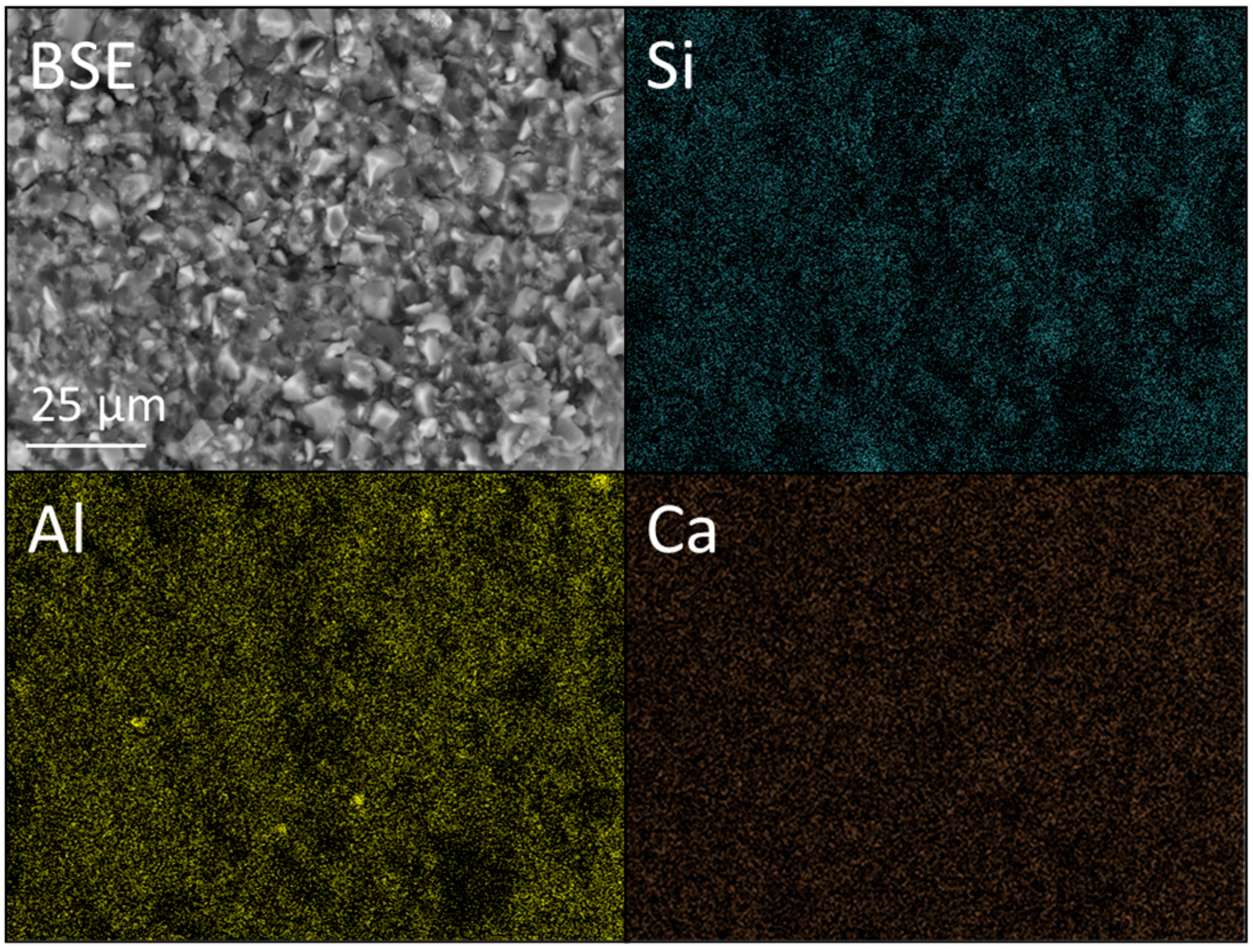
3.3. SEM and EDX Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sidhu, S.K.; Nicholson, J.W. A review of glass-ionomer cements for clinical dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef]
- Frencken, J.; Liang, S.; Zhang, Q. Survival estimates of atraumatic restorative treatment versus traditional restorative treatment: A systematic review with meta-analyses. Br. Dent. J. 2021. [Google Scholar] [CrossRef]
- Fierascu, R.C. Incorporation of nanomaterials in glass ionomer cements—Recent developments and future perspectives: A narrative review. Nanomaterials 2022, 12, 3827. [Google Scholar] [CrossRef]
- Naguib, G.; Maghrabi, A.A.; Mira, A.I.; Hisham, A.; Mously, M.H.; Hamed, M.T. Influence of inorganic nanoparticles on dental materials’ mechanical properties. A narrative review. BMC Oral Health 2023, 23, 89. [Google Scholar] [CrossRef]
- Fattah, Z.; Jowkar, Z.; Rezaeian, S. Microshear Bond strength of nanoparticle-incorporated conventional and resin-modified glass ionomer to caries-affected dentin. Int. J. Dent. 2021, 2021, 5565556. [Google Scholar] [CrossRef] [PubMed]
- Pooja, Y.; Chaitanya, P.; Vinay, C.; Uloopi, K.S.; Alla, R.K.; Ahalya, P. Antimicrobial and mechanical properties of GIC incorporated with silver vanadate nanoparticles: An in-vitro study. J. Clin. Diagn. Res. 2023, 17, ZC29–ZC32. [Google Scholar] [CrossRef]
- Shafaee, H.; Khosropanah, H.; Rahimi, H.; Darroudi, M.; Rangrazi, A. Effects of adding cinnamon, ZnO, and CuO nanoparticles on the antibacterial properties of a glass ionomer cement as the luting agent for orthodontic bands and their cytotoxicity. J. Compos. Sci. 2022, 6, 336. [Google Scholar] [CrossRef]
- Gjorgievska, E.; Nicholson, J.W.; Gabrić, D.; Guclu, Z.A.; Miletić, I.; Coleman, N.J. Assessment of the impact of the addition of nanoparticles on the properties of glass–ionomer cements. Materials 2020, 13, 276. [Google Scholar] [CrossRef]
- Prentice, L.H.; Tyas, M.J.; Burrow, M.F. The effect of ytterbium fluoride and barium sulphate nanoparticles on the reactivity and strength of a glass-ionomer cement. Dent. Mater. 2006, 22, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Ashour, A.A.; Felemban, M.F.; Felemban, N.H.; Enan, E.T.; Basha, S.; Hassan, M.M.; Gad El-Rab, S.M.F. Comparison and advanced antimicrobial strategies of silver and copper nanodrug-loaded glass ionomer cement against dental caries microbes. Antibiotics 2022, 11, 756. [Google Scholar] [CrossRef]
- Aguilar-Perez, D.; Vargas-Coronado, R.; Cervantes-Uc, J.M.; Rodriguez-Fuentes, N.; Aparicio, C.; Covarrubias, C.; Alvarez-Perez, M.; Garcia-Perez, V.; Martinez-Hernandez, M.; Cauich-Rodriguez, J.V. Antibacterial activity of a glass ionomer cement doped with copper nanoparticles. Dent. Mater. J. 2020, 39, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, A.; Khan, M.T.; Mehmood, M.; Khurshid, Z.; Ali, M.I.; Jamal, A. Synthesis and characterization of titanium oxide nanoparticles with a novel biogenic process for dental application. Nanomaterials 2022, 12, 1078. [Google Scholar] [CrossRef]
- de Souza Araújo, I.J.; Ricardo, M.G.; Gomes, O.P.; Giovani, P.A.; Puppin-Rontani, J.; Pecorari, V.A.; Martinez, E.F.; Napimoga, M.H.; Nociti Junior, F.H.; Puppin-Rontani, R.M.; et al. Titanium dioxide nanotubes added to glass ionomer cements affect S. mutans viability and mechanisms of virulence. Braz. Oral Res. 2021, 35, 062. [Google Scholar] [CrossRef]
- Noori, A.J.; Kareem, F.A. The effect of magnesium oxide nanoparticles on the antibacterial and antibiofilm properties of glass-ionomer cement. Heliyon 2019, 5, e02568. [Google Scholar] [CrossRef] [PubMed]
- Nojehdehi, A.M.; Moghaddam, F.; Hamedani, M.T. Mechanical properties of glass ionomer cement incorporating forsterite nanoparticles synthesized by the sol-gel method. J. Sol-Gel. Sci. Technol. 2023, 107, 161–169. [Google Scholar] [CrossRef]
- Ahalya, N.; Dhamodhar, P.; Vaishnavi, A.D. Green Synthesis, Characterization of zinc oxide nanoparticles and their incorporation into glass ionomer cement for inhibition of Streptococcus mutans. Asian J. Chem. 2021, 33, 515–520. [Google Scholar] [CrossRef]
- Fareed, M.A.; Stamboulis, A. Effect of nanoclay dispersion on the properties of a commercial glass ionomer cement. Int. J. Biomater. 2014, 2014, 685389. [Google Scholar] [CrossRef]
- Mabrouk, M.; Selim, M.M.; Beherei, H.; El-Gohary, M.I. Effect of incorporation of nano bioactive silica into commercial glass ionomer cement (GIC). J. Genet. Eng. Biotechnol. 2012, 10, 113–119. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, D. Effect of nanoparticles on wear resistance and surface hardness of a dental glass-ionomer cement. J. Compos. Mater. 2009, 43, 2739–2752. [Google Scholar] [CrossRef]
- Masaeli, R.; Ketabat, F.; Zandsalimi, K. Microhardness and wear resistance of glass ionomer cements modified by chitosan and nano-hydroxyapatite. J. Dentomaxillofacial Sci. 2019, 8, 7–14. [Google Scholar] [CrossRef]
- Pagano, S.; Chieruzzi, M.; Balloni, S.; Lombardo, G.; Torre, L.; Bodo, M.; Cianetti, S.; Marinucci, L. Biological, thermal and mechanical characterization of modified glass ionomer cements: The role of nanohydroxyapatite, ciprofloxacin and zinc L-carnosine. Mater. Sci. Eng. C 2019, 94, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Golkar, P.; Omrani, L.R.; Zohourinia, S.; Ahmadi, E.; Asadian, F. Cytotoxic effect of addition of different concentrations of nanohydroxyapatite to resin modified and conventional glass ionomer cements on L929 murine fibroblasts. Front. Dent. 2021, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-A.; Lee, J.-H.; Juna, S.-K.; Kim, H.-W.; Eltohamy, M.; Lee, H.-H. Sol–gel-derived bioactive glass nanoparticle-incorporated glass ionomer cement with or without chitosan for enhanced mechanical and biomineralization properties. Dent. Mater. 2017, 33, 805–817. [Google Scholar] [CrossRef]
- Murugan, R.; Yazid, F.; Nasruddin, N.S.; Anuar, N.N.M. Effects of nanohydroxyapatite incorporation into glass ionomer cement (GIC). Minerals 2022, 12, 9. [Google Scholar] [CrossRef]
- Amin, F.; Rahman, S.; Khurshid, Z.; Zafar, M.S.; Sefat, F.; Kumar, N. Effect of nanostructures on the properties of glass ionomer dental restoratives/cements: A comprehensive narrative review. Materials 2021, 14, 6260. [Google Scholar] [CrossRef]
- 3M™ Ketac™. Available online: https://www.3m.co.uk/3M/en_GB/p/d/v000200041/ (accessed on 22 December 2023).
- FUJI IX GP. Available online: https://www.gc.dental/europe/en/products/fuji9gp (accessed on 22 December 2023).
- Aerosil® OX 50 Product Information Sheet, Evonik Resource Efficiency GmbH, July 2015. Available online: https://products.evonik.com/assets/or/ld/AEROSIL_OX_50_TDS_EN_EN_TDS_PV_52043865_en_GB_WORLD.pdf (accessed on 7 February 2024).
- Aeroperl® 300 Pharma Product Information Sheet, Evonik Resource Efficiency GmbH, April 2021. Available online: https://products.evonik.com/assets/43/52/AEROPERL_300_Pharma_EN_EN_Asset_364352.pdf (accessed on 7 February 2024).
- ISO 9917-1; Dentistry—Water-Based Cements—Part 1: Powder/Liquid Acid-Base Cements. International Organization for Standardization: Geneva, Switzerland, 2007.
- Raggio, D.P.; Bonifácio, C.C.; Bönecker, M.; Imparato, J.C.; Gee, A.J.; Amerongen, W.E. Effect of insertion method on Knoop hardness of high viscous glass ionomer cements. Braz. Dent. J. 2010, 21, 439–445. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spinola, M.; Dal Piva, A.M.O.; Barbosa, P.U.; Torres, C.R.G.; Bresciani, E. Mechanical assessment of glass ionomer cements incorporated with multi-walled carbon nanotubes for dental applications. Oral 2021, 1, 190–198. [Google Scholar] [CrossRef]
- Wren, A.W.; Coughlan, A.; Laffir, F.R.; Towler, M.R. Comparison of a SiO(2)-CaO-ZnO-SrO glass polyalkenoate cement to commercial dental materials: Glass structure and physical properties. J. Mater. Sci. Mater. Med. 2013, 24, 271–280. [Google Scholar] [CrossRef]
- Joshi, A.C.; Rufus, A.L.; Suresh, S.; Chandramohan, P.; Rangarajan, S.; Velmurugan, S. Characterization of the oxide formed in the presence of poly acrylic acid over the steam generator structural materials of nuclear power plants. J. Nucl. Mater. 2013, 437, 139–148. [Google Scholar] [CrossRef]
- Joshi, A.C.; Rufus, A.L.; Velmurugan, S. Poly(acrylic acid-co-maleic acid), a polymer dispersant for the control of oxide deposition over nuclear steam generator surfaces. J. Nucl. Mater. 2018, 498, 421–429. [Google Scholar] [CrossRef]
- John, I.; Bangi, M.R.; Lawrence, M. Effect of filler and binder contents on air voids in hot-mix asphalt for road pavement construction. Open J. Civ. Eng. 2012, 11, 255–289. [Google Scholar] [CrossRef]
- Gjorgievska, E.; Van Tendeloo, G.; Nicholson, J.W.; Coleman, N.J.; Slipper, I.J.; Booth, S. The incorporation of nanoparticles into conventional glass-ionomer dental restorative cements. Microsc. Microanal. 2015, 21, 392–406. [Google Scholar] [CrossRef]
- Kantovitz, K.R.; Carlos, N.R.; Silva, I.A.P.S.; Braido, C.; Costa, B.C.; Kitagawa, I.L.; Nociti-Jr, F.H.; Basting, R.B.; de Figueiredo, F.K.P.; Lisboa-Filho, P.N. TiO2 nanotube-based nanotechnology applied to high-viscosity conventional glass-ionomer cement: Ultrastructural analyses and physicochemical characterization. Odontology 2023, 111, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, G.d.A.P.; Thomson, J.J.; Smoczer, C.; Young, L.A.; Matos, A.O.; Pacheco, R.R.; Souza, M.T.; Zanotto, E.D.; Puppin Rontani, R.M. Effect of Biosilicate® addition on physical–mechanical and biological properties of dental glass ionomer cements. J. Funct. Biomater. 2023, 14, 302. [Google Scholar] [CrossRef]
- Paiva, L.; Fidalgo, T.K.S.; da Costa, L.P.; Maia, L.C.; Balan, L.; Anselme, K.; Ploux, L.; Thiré, R.M.S.M. Antibacterial properties and compressive strength of new one-step preparation silver nanoparticles in glass ionomer cements (NanoAg-GIC). J. Dent. 2018, 69, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Noori, A.J.; Kareem, F.A. Setting time, mechanical and adhesive properties of magnesium oxide nanoparticles modified glass-ionomer cement. J. Mater. Res. Technol. 2020, 9, 1809–1818. [Google Scholar] [CrossRef]
- Agarwal, P.; Nayak, R.; Upadhya, P.N.; Ginjupalli, K.; Gupta, L. Evaluation of properties of glass ionomer cement reinforced with zinc oxide nanoparticles—An in vitro study. Mater. Today Proc. 2018, 5, 16065–16072. [Google Scholar] [CrossRef]
- Elsaka, S.E.; Hamouda, I.M.; Swain, M.V. Titanium dioxide nanoparticles addition to a conventional glass-ionomer restorative: Influence on physical and antibacterial properties. J. Dent. 2011, 39, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Simonsson, I.; Sögaard, C.; Rambaran, M.; Abbas, Z. The specific co-ion effect on gelling and surface charging of silica nanoparticles: Speculation or reality? Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 334–341. [Google Scholar] [CrossRef]
- Goyne, K.W.; Zimmerman, A.R.; Newalkar, B.L.; Komarneni, S.; Brantley, S.L.; Chorover, J. Charge of variable porosity Al2O3(s) and SiO2(s) adsorbents. J. Porous Mater. 2002, 9, 243–256. [Google Scholar] [CrossRef]
- Mylonas, P.; Zhang, J.; Banerjee, A. Conventional glass-ionomer cements: A guide for practitioners. Dental Update 2021, 48, 643–650. [Google Scholar] [CrossRef]
- Yanıkoğlu, N.D.; Rüştü Ersoy Sakarya, R.E. Test methods used in the evaluation of the structure features of the restorative materials: A literature review. J. Mater. Res. Technol. 2020, 9, 9720–9734. [Google Scholar] [CrossRef]
- Assery, M.K.A.; Abdulrahman, A.; AlWaleed, A.; Nawaf, L.; Mohamed, H. Nanoparticles as void fillers in glass ionomer cement for enhanced physicomechanical properties. Mater. Express 2020, 10, 1960–1964. [Google Scholar] [CrossRef]
- Jowkar, Z.; Jowkar, M.; Shafiei, F. The Mechanical and dentin bond strength properties of the nanosilver enriched glass ionomer cement. J. Clin. Exp. Dent. 2019, 11, 275–281. [Google Scholar] [CrossRef]
- Garcia-Contreras, R.; Scougall-Vilchis, R.J.; Contreras-Bulnes, R.; Sakagami, H.; Morales-Luckie, R.A.; Nakajima, H. Mechanical, antibacterial and bond strength properties of nano-titanium-enriched glass ionomer cement. J. Appl. Oral. Sci. 2015, 23, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Barandehfard, F.; Rad, M.K.; Hosseinnia, A.; Khoshroo, K.; Tahriri, M.; Jazayeri, H.E.; Moharamzadeh, K.; Tayebi, L. The addition of synthesized hydroxyapatite and fluorapatite nanoparticles to a glass-ionomer cement for dental restoration and its effects on mechanical properties. Ceram. Int. 2016, 42, 17866–17875. [Google Scholar] [CrossRef]
- Qasim, S.S.B.; Ali, D.; Soliman, M.S.; Zafiropoulos, G.-G. The effect of chitosan derived silver nanoparticles on mechanical properties, color stability of glass ionomer luting cements. Mater. Res. Express 2021, 8, 085401. [Google Scholar] [CrossRef]
- Mahmoud, N.A.; Metwally, A.A. Fluoride release and recharging ability of glass ionomer cement incorporating hydroxyapatite nanoparticles. Egypt. Dent. J. 2021, 67, 3741–3749. [Google Scholar] [CrossRef]
- Panahandeh, N.; Torabzadeh, H.; Aghaee, M.; Hasani, E.; Safa, S. Effect of incorporation of zinc oxide nanoparticles on mechanical properties of conventional glass ionomer cements. J. Conserv. Dent. 2018, 21, 130–135. [Google Scholar] [PubMed]
- Uribe, P.; Johansson, A.; Jugdaohsingh, R.; Powell, J.J.; Magnusson, C.; Davila, M.; Westerlund, A.; Ransjö, M. Soluble silica stimulates osteogenic differentiation and gap junction communication in human dental follicle cells. Sci. Rep. 2020, 10, 9923. [Google Scholar] [CrossRef]


| GIC | Ketac™ Molar | Fuji IX GP® |
|---|---|---|
| Manufacturer | 3M ESPE, St. Paul, MN, USA | GC Corporation, Tokyo, Japan |
| Constituents | Calcium fluoroaluminosilicate glass, poly(acrylic acid-co-maleic acid), tartaric acid and water | Strontium fluoroaluminosilicate glass, poly(acrylic acid), tartaric acid and water |
| Powder:liquid mass ratio | 4.5:1.0 | 3.6:1.0 |
| Silica | Aerosil® OX 50 | Aeroperl® 300 Pharma |
|---|---|---|
| Manufacturer | Evonik Operations GmbH, Essen, Germany | Evonik Operations GmbH, Essen, Germany |
| SiO2 content | >99.8% | >99.0% |
| Phase content | X-ray amorphous | X-ray amorphous |
| pH | 3.8–4.8 | 3.5–5.5 |
| Mean particle size | 40 nm | 20–60 μm |
| BET surface area 1 | 35–65 m2 g−1 | 260–320 m2 g−1 |
| Pore volume | - | 1.5–1.9 cm3 g−1 |
| Sample Code | GIC Powder (g) | GIC Solution (g) | Aerosil® OX 50 (g) | Aeroperl® 300 Pharma (g) |
|---|---|---|---|---|
| KM | 0.23 | 0.051 | - | - |
| KM-2NS | 0.23 | 0.051 | 0.0046 | - |
| KM-4NS | 0.23 | 0.051 | 0.0092 | - |
| KM-6NS | 0.23 | 0.051 | 0.0138 | - |
| KM-2MS | 0.23 | 0.051 | - | 0.0046 |
| KM-4MS | 0.23 | 0.051 | - | 0.0092 |
| KM-6MS | 0.23 | 0.051 | - | 0.0138 |
| FIX | 0.23 | 0.051 | - | - |
| FIX-2NS | 0.23 | 0.051 | 0.0046 | - |
| FIX-4NS | 0.23 | 0.051 | 0.0092 | - |
| FIX-6NS | 0.23 | 0.051 | 0.0138 | - |
| FIX-2MS | 0.23 | 0.051 | - | 0.0046 |
| FIX-4MS | 0.23 | 0.051 | - | 0.0092 |
| FIX-6MS | 0.23 | 0.051 | - | 0.0138 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Güçlü, Z.A.; Patat, Ş.; Coleman, N.J. The Impact of Nano- and Micro-Silica on the Setting Time and Microhardness of Conventional Glass–Ionomer Cements. Dent. J. 2024, 12, 54. https://doi.org/10.3390/dj12030054
Güçlü ZA, Patat Ş, Coleman NJ. The Impact of Nano- and Micro-Silica on the Setting Time and Microhardness of Conventional Glass–Ionomer Cements. Dentistry Journal. 2024; 12(3):54. https://doi.org/10.3390/dj12030054
Chicago/Turabian StyleGüçlü, Zeynep A., Şaban Patat, and Nichola J. Coleman. 2024. "The Impact of Nano- and Micro-Silica on the Setting Time and Microhardness of Conventional Glass–Ionomer Cements" Dentistry Journal 12, no. 3: 54. https://doi.org/10.3390/dj12030054
APA StyleGüçlü, Z. A., Patat, Ş., & Coleman, N. J. (2024). The Impact of Nano- and Micro-Silica on the Setting Time and Microhardness of Conventional Glass–Ionomer Cements. Dentistry Journal, 12(3), 54. https://doi.org/10.3390/dj12030054










