Direct Immunofluorescence in Oral Lichen Planus and Related Lesions: Sensitivity, Specificity, and Diagnostic Accuracy in a Single Diagnostic Center in Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Histopathology
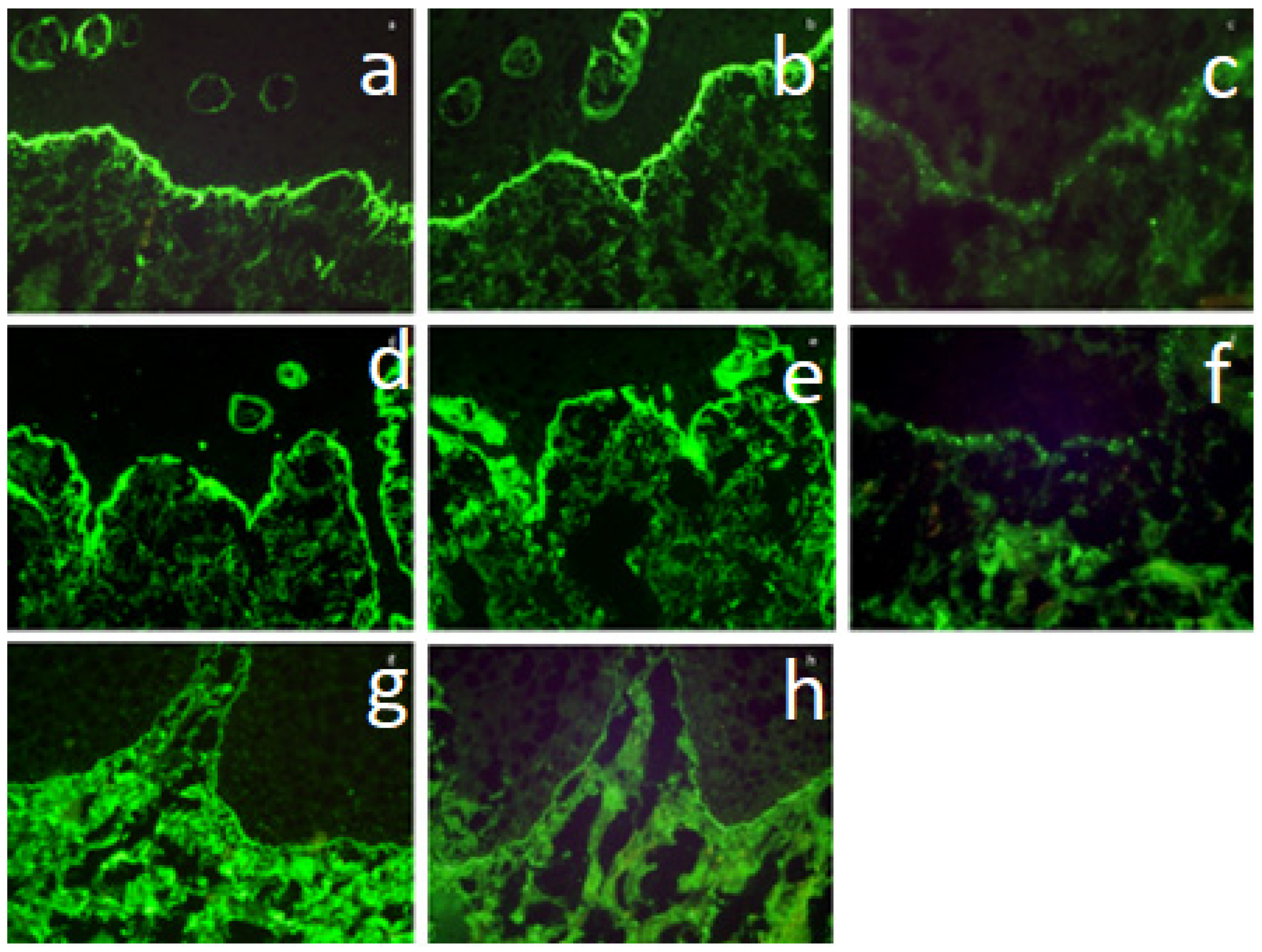
2.3. Direct Immunofluorescence
2.4. ELISA for IgG Antibodies Directed Against the NC16a Epitope of the BP180 Antigen
2.5. Statistical Analysis
3. Results
3.1. Group 1—Contains Patients in Whom HP Was Characteristic of LP
3.2. Group 2—Contains Patients in Whom HP Did Not Detect LP Features
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- González-Moles, M.Á.; Warnakulasuriya, S.; González-Ruiz, I.; González-Ruiz, L.; Ayén, Á.; Lenouvel, D. Worldwide prevalence of oral lichen planus: A systematic review and meta-analysis. Oral Dis. 2021, 27, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Van der Meij, E.H.; van der Waal, I. Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J. Oral Pathol. Med. 2003, 32, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Buajeeb, W.; Okuma, N.; Thanakun, S.; Laothumthut, T. Direct immunofluorescence in oral lichen planus. J. Clin. Diagn. Res. 2015, 9, ZC34–ZC37. [Google Scholar] [CrossRef] [PubMed]
- Laskaris, G.; Sklavounou, A.; Angelopoulos, A. Direct immunofluorescence in oral lichen planus. Oral Surg. Oral Med. Oral Pathol. 1982, 53, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Firth, N.A.; Rich, A.M.; Radden, B.G.; Reade, P.C. Assessment of the value of immunofluorescence microscopy in the diagnosis of oral mucosal lichen planus. J. Oral Pathol. Med. 1990, 19, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Helander, S.D.; Rogers, R.S., III. The sensitivity and specificity of direct immunofluorescence testing in disorders of mucous membranes. J. Am. Acad. Dermatol. 1994, 30, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Boisnic, S.; Frances, C.; Branchet, M.C.; Szpirglas, H.; Le Charpentier, Y. Immunohistochemical study of oral lesions of lichen planus: Diagnostic and pathophysiologic aspects. Oral Surg. Oral Med. Oral Pathol. 1990, 70, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Kolde, G.; Wesendahl, C.; Stein, H.; Reichart, P.A. Oral lichen planus: Diagnostic immunofluorescence testing on routine histological material. Br. J. Dermatol. 2003, 148, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Ioannides, D.; Vakirlis, E.; Kemeny, L.; Marinovic, B.; Massone, C.; Murphy, R.; Nast, A.; Ronnevig, J.; Ruzicka, T.; Cooper, S.M.; et al. European S1 guidelines on the management of lichen planus: A cooperation of the European Dermatology Forum with the European Academy of Dermatology and Venereology. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.B.; Porter, S.R.; Smoller, B.R.; Sitaru, C. Oral mucosal manifestations of autoimmune skin diseases. Autoimmun. Rev. 2015, 14, 930–951. [Google Scholar] [CrossRef] [PubMed]
- Korkitpoonpol, N.; Kanjanabuch, P. Direct immunofluorescence cannot be used solely to differentiate among oral lichen planus, oral lichenoid lesion, and oral epithelial dysplasia. J. Dent. Sci. 2023, 18, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Montague, L.J.; Bhattacharyya, I.; Islam, M.N.; Cohen, D.M.; Fitzpatrick, S.G. Direct immunofluorescence testing results in cases of premalignant and malignant oral lesions. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2015, 119, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, Y.D.; Yamashita, M.; Innocentini, L.M.A.D.; Macedo, L.D.D.; Chahud, F.; Ribeiro-Silva, A.; Roselino, A.M.; Rocha, M.J.A.; Motta, A.C.D. Direct Immunofluorescence as a Helpful Tool for the Differential Diagnosis of Oral Lichen Planus and Oral Lichenoid Lesions. Am. J. Dermatopathol. 2018, 40, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Jakubowska, B.; Pietrzyk, E.; Maciejewicz, P.; Kowalewski, C.; Wozniak, K. Diagnostic and prognostic values of conjunctival and oral biopsies analyzed by direct immunofluorescence in patients with mucous membrane pemphigoid. Front. Med. 2023, 10, 1257288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, R.; Tseng, F.; Cheng, C.; E Van Dyke, T.; Sung, C.; You, J.; Weng, P.; Shieh, Y.; Cheng, W. Complement components C3b and C4b as potential reliable site-specific diagnostic biomarkers for periodontitis. J. Periodontal. Res. 2023, 58, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Hasturk, H.; Lambris, J.D. Contributing authors. C3-targeted therapy in periodontal disease: Moving closer to the clinic. Trends Immunol. 2021, 42, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Carrozzo, M.; Porter, S.; Mercadante, V.; Fedele, S. Oral lichen planus: A disease or a spectrum of tissue reactions? Types, causes, diagnostic algorhythms, prognosis, management strategies. Periodontology 2000 2019, 80, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Bresler, S.C.; Bavarian, R.; Granter, S.R.; Woo, S.B. Direct immunofluorescence is of limited utility in patients with low clinical suspicion for an oral autoimmune bullous disorder. Oral Dis. 2020, 26, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Reimann, J.D.R.; Moynihan, S.P.; Horn, T.D. Assessment of Clinical and Laboratory Use of the Cutaneous Direct Immunofluorescence Assay. JAMA Dermatol. 2021, 157, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Carey, B.; Joshi, S.; Abdelghani, A.; Mee, J.; Andiappan, M.; Setterfield, J. The optimal oral biopsy site for diagnosis of mucous membrane pemphigoid and pemphigus vulgaris. Br. J. Dermatol. 2020, 182, 747–753. [Google Scholar] [CrossRef] [PubMed]
| Feature | Yes (%) | No (%) |
|---|---|---|
| Sex: male | 17 (25.8) | 49 (74.2) |
| Erosions | 41 (62.1) | 25 (37.9) |
| Bilateral erosions | 36 (54.5) | 30 (45.5) |
| Ulcerations | 51 (77.3) | 15 (22.7) |
| White patches | 39 (59.1) | 27 (40.9) |
| Histological confirmation of OLP | 33 (50.0) | 33 (50.0) |
| Antibody Class | DIF Result | Type of Fluorescence | Histopathology | p-Value | |
|---|---|---|---|---|---|
| Positive N = 33 | Negative N = 33 | ||||
| DIF IgG | Positive (n = 2) | Linear (n = 2) | 0 (0.0) 0 (0.0) 33 (100.0) | 2 (6.1) 0 (0.0) 31 (93.9) | 0.4923 |
| Granular (n = 0) | |||||
| Negative (n = 64) | |||||
| DIF IgA | Positive (n = 1) | Linear (n = 1) | 0 (0.0) 0 (0.0) 33 (100.0) | 1 (3.0) 0 (0.0) 32 (97.0) | 1 |
| Granular (n = 0) | |||||
| Negative (n = 65) | |||||
| DIF IgM | Positive (n = 2) | Linear (n = 2) | 1 (3.0) 0 (0) 32 (97.0) | 1 (3.0) 0 (0) 32 (97.0) | 1 |
| Granular (n = 0) | |||||
| Negative (n = 64) | |||||
| DIF C3 | Positive (n = 12) | Linear (n = 2) | 0 (0.0) 6 (100.0) 27 (81.8) | 2 (33.3) 4 (66.6) 27 (81.8) | 1 |
| Granular (n = 10) | |||||
| Negative (n = 54) | |||||
| DIF F1 | Positive (n = 21) | Shaggy (n = 21) | 14 (42.4) 0 (0) 19 (57.6) | 7 (21.2) 0 (0) 26 (78.8) | 0.118 |
| Granular (n = 0) | |||||
| Negative (n = 45) | |||||
| DIF F2 | Positive (n = 18) | Shaggy (n = 18) | 12 (36.4) 0 (0) 21 (63.6) | 6 (18.2) 0 (0) 27 (81.8) | 0.0973 |
| Granular (n = 0) | |||||
| Negative (n = 48) | |||||
| ELISA BP180 | Positive (n = 2) | Linear (n = 2) | 0 (0.0) 0 (0.0) 32 (100.0) | 2 (6.1) 0 (0.0) 31 (93.9) | 0.1571 |
| Granular (n = 0) | |||||
| Negative (n = 63) | |||||
| Statistic | DIF IgG | DIF IgA | DIF IgM | DIF C3 | DIF F1 | DIF F2 | DIF F1 + DIF F2 | ELISA |
|---|---|---|---|---|---|---|---|---|
| Sensitivity | 0.0 0.0–10.6 | 0.0 0.0–10.6 | 3.0 0.1–15.8 | 18.2 7.0–35.5 | 42.4 25.5–60.8 | 36.4 20.4–54.9 | 38.7 21.8–57.8 | 0.0 0.0–10.9 |
| Specificity | 93.9 79.7–99.3 | 97.0 84.2–99.9 | 97.0 84.2–99.9 | 81.8 64.5–93.0 | 78.8 61.1–91.0 | 81.8 64.5–93.0 | 81.2 63.6–92.8 | 93.9 79.8–99.5 |
| Positive Predictive Value | 0 | 0 | 50.0 6.1–93.9 | 50.0 26.4–73.6 | 66.7 48.1–81.2 | 66.7 46.0–82.4 | 66.7 46.2- 82.3 | 0 |
| Negative Predictive Value | 48.4 46.3–50.6 | 49.2 47.7–50.7 | 50.0 47.9–52.1 | 50.0 44.3–55.7 | 57.8 49.3–65.8 | 56.2 48.7–63.5 | 57.8 49.7–65.5 | 49.2 47.0–51.4 |
| Accuracy | 47.0 34.6–59.7 | 48.5 36.0–61.1 | 50.0 37.4–62.6 | 50.0 37.4–62.6 | 60.6 47.8–72.4 | 59.1 46.3–71.0 | 60.3 47.2–72.4 | 47.7 35.1–60.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osipowicz, K.; Szymański, K.; Pietrzyk, E.; Milczarek, E.; Kowalewski, C.; Górska, R.; Woźniak, K. Direct Immunofluorescence in Oral Lichen Planus and Related Lesions: Sensitivity, Specificity, and Diagnostic Accuracy in a Single Diagnostic Center in Poland. Dent. J. 2024, 12, 396. https://doi.org/10.3390/dj12120396
Osipowicz K, Szymański K, Pietrzyk E, Milczarek E, Kowalewski C, Górska R, Woźniak K. Direct Immunofluorescence in Oral Lichen Planus and Related Lesions: Sensitivity, Specificity, and Diagnostic Accuracy in a Single Diagnostic Center in Poland. Dentistry Journal. 2024; 12(12):396. https://doi.org/10.3390/dj12120396
Chicago/Turabian StyleOsipowicz, Katarzyna, Konrad Szymański, Ewelina Pietrzyk, Emilia Milczarek, Cezary Kowalewski, Renata Górska, and Katarzyna Woźniak. 2024. "Direct Immunofluorescence in Oral Lichen Planus and Related Lesions: Sensitivity, Specificity, and Diagnostic Accuracy in a Single Diagnostic Center in Poland" Dentistry Journal 12, no. 12: 396. https://doi.org/10.3390/dj12120396
APA StyleOsipowicz, K., Szymański, K., Pietrzyk, E., Milczarek, E., Kowalewski, C., Górska, R., & Woźniak, K. (2024). Direct Immunofluorescence in Oral Lichen Planus and Related Lesions: Sensitivity, Specificity, and Diagnostic Accuracy in a Single Diagnostic Center in Poland. Dentistry Journal, 12(12), 396. https://doi.org/10.3390/dj12120396





