Automated Support Generation for Fixed Partial Dentures and Impact of Bone Loss, Bone Quality and Support Types: Parametric Cad and Finite Element Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. CAD Modelling
2.1.1. Manual Preprocessing
2.1.2. Automatic Generation of FPD Support
- (1)
- Merge Mmesial, Mdistal and Msurface into a single mesh, MFPD.
- (2)
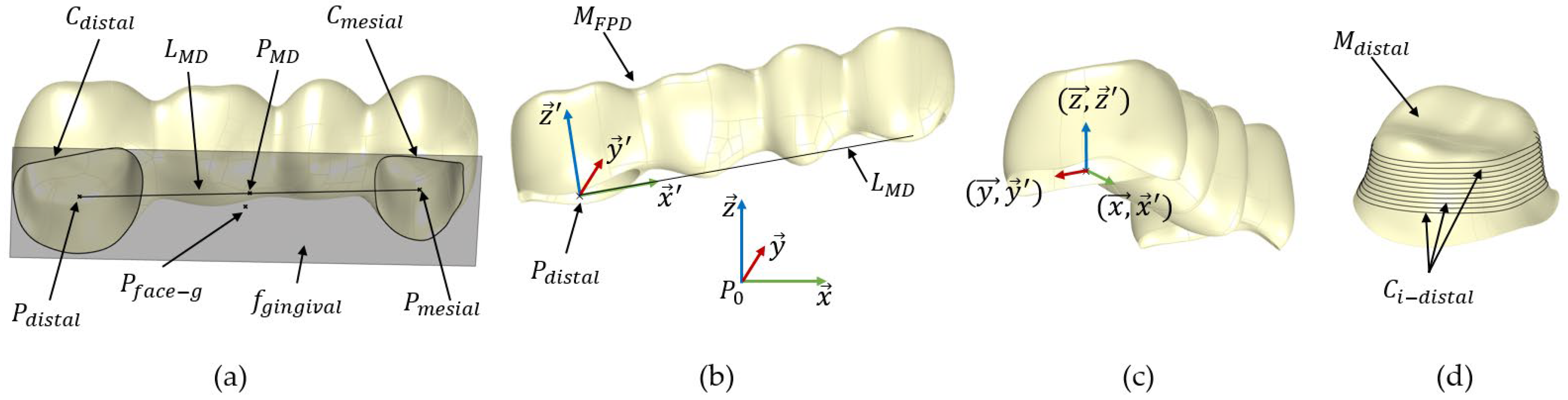
- Extract the boundary curves Cdistal and Cmesial from the open surfaces Mdistal and Mmesial using the naked edge curve function in Grasshopper, respectively; see Figure 3a. These boundary curves represent the open curves of the surfaces, which prevent the mesh from being closed.
- (3)
- Find the centre points Pdistal and Pmesial for the curves Cdistal and Cmesial.
- (4)
- Create the line LMD between Pdistal and Pmesial and compute its midpoint PMD.
- (5)
- Compute the smallest bounding box, Bmin, for the mesh MFPD using the bounding box function in Grasshopper.
- (6)
- Deconstruct the bounding box Bmin into 6 faces, fi, and compute the centre point, Pface-i, for each face.
- (7)
- Identify the gingival face, fgingival, which centre point is closest to the point PMD; see Figure 3a.
- (8)
- Create a plane, EFPD, parallel to fgingival at Pdistal.
- (9)
- Define a local coordinate system for MFPD, denoted as SFPD (). This coordinate system is centred at Pdistal. The z-axis () of SFPD is set to be perpendicular to EFPD at Pdistal. The x-axis () is defined to align with the line LMD, and the y-axis () is constructed to be orthogonal to both and ; see Figure 3b.
- (10)
- Translate MFPD so its local coordinate system’s origin Pdistal matches the global coordinate system’s origin P0 via the Grasshopper function move to point.
- (11)
- Rotate MFPD to align its local axes SFPD () with the global coordinate system’s axes Sglobal () using the function Orient; see Figure 3c.
- (1)
- Create a line Lp that connects the centre points, Pface-g and Pface-o, of the gingival face fgingival and the occlusal face focclusal, respectively.
- (2)
- Generate 100 equally spaced planes Ei along the line Lp, each perpendicular to Lp.
- (3)
- Find the intersection curves, Ci-distal, between the planes Ei and the lumen mesh Mdistal (analogue Ci-mesial with Mmesial for the mesial side). Retain only the closed curves and remove the initial and final curves to eliminate any errors; see Figure 3d.
- (4)
- Calculate the circularity index (CI) of the curves Ci-distal using the formula , where n is the number of curves, Ai represents the area of each curve and Pei represents the perimeter of each curve [21].
- (1)
- Generate the interior mesh surface, Minterior, by creating a parallel mesh to the distal lumen mesh surface, Mdistal, at a distance defined by the cement thickness, dcement. This involves creating new vertices parallel to each original vertex of Mdistal inward along its normal vectors by dcement and then using these newly calculated vertices to construct Minterior; see Figure 4a.
- (2)
- Extract the boundary curve, Cinterior, from Minterior.
- (3)
- Create a lofted mesh surface, Mloft, by interpolating between the curves Cdistal and Cinterior; see Figure 4a.
- (4)
- Join the surfaces Mdistal, Mloft and Minterior to create the geometry of the cement layer, MCement.

- (1)
- Divide Cinterior into 100 equal-length segments and identify Pmin (xmin, ymin and zmin), as the point with the lowest z-coordinate.
- (2)
- Create a point – ε, where ε prevents the intersection of Cinterior with the first projected curve to enable the loft function.
- (3)
- Create a line segment Ltooth defined by the start point Ps, a tangent vector in the direction and a user-defined length dtooth.
- (4)
- Divide Ltooth into i equally spaced points (with i = 20 in this work).
- (5)
- Create i planes parallel to the xy-plane at and project Cinterior onto each , yielding curves .
- (6)
- Create Bézier functions Bx(t) and By(t) to scale the curves to mimic the anatomy of a natural tooth, and the non-uniform scaling equation iswith Pxi/yi as the control points of the Bézier function. The control points used in this study are listed in Table 1. Here, t is a parameter ranging from 0 to 1, representing the position along the line Ltooth.
- (1)
- Compute Bx(t) and By(t) for all t values, resulting in the arrays Bx and By. Each point becomes a scaling factor in the x and y directions.
- (2)
- Scale the curves using the non-uniform scaling factors Bx for the x-direction and By for the y-direction.
- (3)
- Create a lofted mesh surface Mroot by interpolating through the set of curves and Cinterior.
- (4)
- Create a planar surface Mapex from the last curve .
- (5)
- Join Minterior, Mroot and Mapex to create the geometry of the first premolar tooth, denoted as M44.
- (1)
- Perform the steps until step 8 (First premolar).
- (2)
- Create a Bézier function BT(t) to translate the scaled curves in both the x and –x direction to mimic the anatomy of roots, using the control points PT0, PT1, PT2 and PT3, as defined in Table 1.
- (3)
- Compute BT(t) for all t values, resulting in the arrays BT.
- (4)
- Duplicate the curves , translating one set in the positive x-direction and the other set in the negative x-direction by BT × dR, yielding and . In our work, dR represents the distance between the furcation and the root apex in the x-direction.
- (5)
- Create lofted mesh surfaces Mroot-1 and Mroot-2 through the set of curves and Cinterior and and Cinterior, respectively.
- (6)
- Create planar surfaces Mapex-1 and Mapex-2 from the last curves and , respectively.
- (7)
- Join Minterior, Mroot-1 and Mapex-1 to create the geometry of the first root, denoted as MT1, and join Minterior, Mroot-2 and Mapex-2 to create the geometry of the second root, denoted as MT2.
- (8)
- Perform a mesh union on the meshes MT1 and MT2 to create the geometry of the second molar tooth, denoted as M47.
- (1)
- Join the mesh surfaces Mroot and Mapex to create the surface MPDL-in, which interfaces with the tooth geometry M44.
- (2)
- Construct new vertices parallel to each original vertex of MPDL-in outward along its normal vector by the user-defined periodontal ligament thickness dPDL. Use these newly created vertices to create MPDL-ex, which later interfaces with the bone.
- (3)
- Create a lofted mesh surface, MPDL-loft, using the boundary curves CPDL-in and CPDL-ex obtained from MPDL-in and MPDL-ex.
- (4)
- Join the surfaces MPDL-in, MPDL-loft and MPDL-ex to form the periodontal ligament geometry MPDL.
- (5)
- After insertion into bone (step 4), the part of the PDL that was not in contact with the bone was cut.
- (1)
- Create circles CA1, CA2 and CA3 on planes parallel to the xy-plane with user-defined lengths lA1, lA2 and lA3 and diameters dA1, dA2 and dA3. The plane at the distance lA1 is the implant neck plane Eimplant ().
- (2)
- Compute a lofted surface through the set of curves Cinterior, CA1, CA2 and CA3. Join this surface with Minterior to create the abutment geometry without a borehole, denoted as MA-loft.
- (3)
- Create the borehole geometry by creating circles CBA1 and CBA2, each with user defined-lengths lBA1 and lBA2 and diameters dBA1 and dBA2, respectively. Extrude CBA1 upwards to the cement layer and CBA2 downwards to the lower surface of MA-loft, forming the borehole mesh MA-bore.
- (4)
- Extract MA-bore from MA-loft using a Boolean mesh subtraction operation to create the abutment geometry for implant support, MAbutment.
- (1)
- Create a cylinder MI-loft on the plane Eimplant with user-defined diameters dI1 (upper diameter) and dI2 (bottom diameter) and length lI1. In this work, the implant is designed to be straight with dI1 = dI2.
- (2)
- Chamfer the implant body MI-loft at the upper cylinder edge curve with a radius rC and fillet the bottom edge of the cylinder with a radius rF.
- (3)
- Create the borehole geometry MI- bore using circles with diameters dBI1(= dA1), dBI2(= dA3) and dBI3 and lengths lBI1, lBI2, lBI3 and lBI4. For more geometric details, see Figure 5b.
- (4)
- Extract MI-bore from MI-loft to create the implant body geometry Mimplant.
- (5)
- Construct a helix curve, Chelix, starting lt below the implant neck plane, with the same diameter dI1 as the implant, a user-defined pitch, pt, and number of turns, nturns, along the z-axis using the helix curve function in Grasshopper.
- (6)
- Extend the curve Chelix by creating tangential segments at its end points, Pstart and Pend, to replicate the toolpath movement during the milling process.
- (7)
- Generate the surface of a revolution, Mthread, using the function sweep rail with the metric thread profile as the profile curve, the helix curve Chelix as the rail curve and the z-axis as the axis of the revolution.
- (8)
- Extract Mthread from the implant body geometry Mimplant.

| Component | User Defined Parameters for Tooth Support, in mm. |
|---|---|
| Cement layer | dcement = 0.1 mm (for mesial and distal side). |
| Tooth, first premolar | ε = 0.15 mm, dtooth = 16.3 mm, Px0(0;1), Px1(0.08;0.78), Px2(0.75;0.55), Px3(1;0.05), Py0(0;1), Py1(0.2;0.58), Py2(0.95;0.5), Py3(1;0.05) |
| Tooth, second molar | ε = 0.15 mm, dtooth = 12.8 mm, Pxi (same as first premolar), Pyi (same as first premolar), PT0(0;0), PT1(0.35;0.55), PT2(0.4;0.9), PT3(1;1) |
| PDL | dPDL = 0.3 mm (for mesial and distal side) |
| Component | User Defined Parameters for Implant Support, in mm. |
| Cement layer | dcement = 0.1 mm |
| Abutment | lA1 = 1.0 mm, lA2 = 1.5 mm, lA3 = 7.0 mm, dA1 = 3.0 mm, dA2 = 2.6 mm, dA3 = 2.6 mm, lBA1 = 2.0 mm, lBA2 = 2.2 mm, dBA1 = 1.6 mm, dBA2 = 1.3 mm, (mesial and distal side). |
| Implant body | dI1 = 5.0 mm, dI2 = 5.0 mm, lI1 = 8.0 mm, rC = 1.5 mm, rF = 0.2 mm, lBI1 = 0.5 mm, lBI2 = 4.0 mm, lBI3 = 5.0 mm, lBI4 = 5.3 mm, dBI1 = 3.0 mm, dBI2 2.6 = mm, dBI3 = 1.1 mm, rb = 0.2 mm, lt = 1.5 mm, pt = 0.5 mm, nturns = 10, (mesial and distal side). |
| Abutment screw | ls = 4.5 mm. |
- (1)
- Merge the FPD (MFPD) and the support geometries on both the mesial and distal sides. For tooth–tooth support, include Mcement-distal, Mcement-mesial, M44, M47, MPDL-distal and MPDL-mesial. For implant–implant support, include Mcement-distal, Mcement-mesial, Mabutment-distal, Mabutment-mesial, Mimplant-distal, Mimplant-mesial, Mscrew-distal and Mscrew-mesial. This step forms the mesh MFPD+S.
- (2)
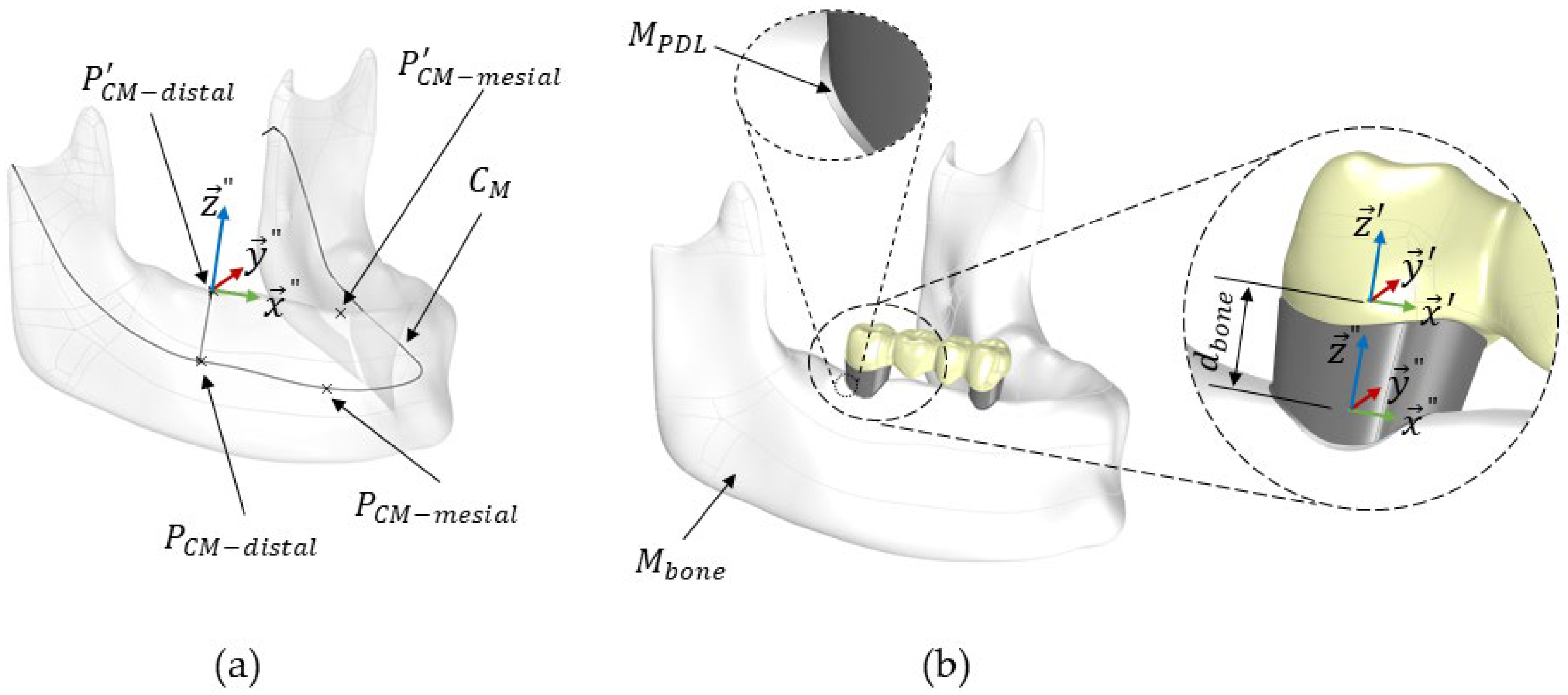
- Define the distal and mesial sides of the FPD, PCM-distal and PCM-mesial, on the mandibular centre curve, CM, as shown in Figure 6a. The distance between these points corresponds to the distance between Pmesial and Pdistal of the FPD. Further details about the construction of the centre curve CM can be found in our previous study [16].
- (3)
- Project the points PCM-distal and PCM-mesial onto the crestal mandibular surface, resulting in P’CM-distal and P’CM-mesial. The steps required the projection of a point from CM onto the crestal surface of bone were further explained in a previous work [16].
- (4)
- Define a local coordinate system for the mandibular bone Mbone, denoted as Sbone (). This system’s origin is positioned at . The z-axis () is aligned with the line connecting PCM-distal and P’CM-distal. The x-axis () aligns with the line connecting and , and the y-axis () is constructed to be orthogonal to both and ; see Figure 6a.
- (5)
- Perform the necessary rotations and translations to align MFPD+S’s local coordinate system, SFPD (), precisely with the mandibular bone’s local coordinate system, Sbone (), using the Grasshopper functions Move to point and Orient.
- (6)
- Translate MFPD+S along the z-axis () by a distance dbone, specified by the user. This distance, dbone (dbone = 2.8 mm in this study), represents the gap between Pdistal and the crestal surface of the mandibular bone; see Figure 6b.
- (7)
- After MFPD+S was positioned and aligned, it was extracted from the mandibular geometry, Mbone, using a Boolean mesh subtraction.
2.2. Finite Element Analysis
2.2.1. Material Properties, Contact Models and Mesh Size
2.2.2. Boundary Conditions and Loading
2.2.3. Evaluation
3. Results
4. Discussion
5. Conclusions
- (1)
- The proposed algorithm allows an automatic parametrised generation of support for a 4-unit fixed partial denture that can be used to predict the effect of bone loss and bone quality on stresses for patient-specific geometries.
- (2)
- The optimal treatment in terms of support type should be adapted to the patient’s specific needs to ensure long-term stability. Decision support involving automatic modelling, e.g., of support, and simulation might improve optimal treatment from a long-term perspective.
- (3)
- Further work for automated CAD and simulation still remains necessary. For example, the root generation algorithm can still be improved to adapt closer to clinically observed structures.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matta, R.E.; Eitner, S.; Stelzer, S.P.; Reich, S.; Wichmann, M.; Berger, L. Ten-year clinical performance of zirconia posterior fixed partial dentures. J. Oral Rehabil. 2022, 49, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Pieralli, S.; Kohal, R.J.; Rabel, K.; von Stein-Lausnitz, M.; Vach, K.; Spies, B.C. Clinical outcomes of partial and full-arch all-ceramic implant-supported fixed dental prostheses. A systematic review and meta-analysis. Clin. Oral Implants Res. 2018, 29, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, T.A.; Abdulmajeed, A.A.; Delgado, A.; Donovan, T.E. Fracture rate of 188695 lithium disilicate and zirconia ceramic restorations after up to 7.5 years of clinical service: A dental laboratory survey. J. Prosthet. Dent. 2020, 123, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Onodera, K.; Sato, T.; Nomoto, S.; Miho, O.; Yotsuya, M. Effect of connector design on fracture resistance of zirconia all-ceramic fixed partial dentures. Bull. Tokyo Dent. Coll. 2011, 52, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Bragger, U.; Lang, N.P.; Zwahlen, M. Comparison of survival and complication rates of tooth-supported fixed dental prostheses (FDPs) and implant-supported FDPs and single crowns (SCs). Clin. Oral Implants Res. 2007, 18 (Suppl 3), 97–113. [Google Scholar] [CrossRef]
- Tsouknidas, A.; Giannopoulos, D.; Savvakis, S.; Michailidis, N.; Lympoudi, E.; Fytanidis, D.; Pissiotis, A.; Michalakis, K. The Influence of Bone Quality on the Biomechanical Behavior of a Tooth-Implant Fixed Partial Denture: A Three-Dimensional Finite Element Analysis. Int. J. Oral Maxillofac. Implants 2016, 31, e143–e154. [Google Scholar] [CrossRef]
- Naguib, G.H.; Hashem, A.B.H.; Natto, Z.S.; Abougazia, A.O.; Mously, H.A.; Hamed, M.T. The Effect of Implant Length and Diameter on Stress Distribution of Tooth-Implant and Implant Supported Fixed Prostheses: An In Vitro Finite Element Analysis Study. J. Oral Implantol. 2023, 49, 46–54. [Google Scholar] [CrossRef]
- Chandranaik, M.B.; Thippanna, R.K. Fixed Partial Denture Failures: A Clinical Survey for Evaluation of the Factors Responsible. CODS J. Dent. 2019, 9, 41–45. [Google Scholar] [CrossRef]
- Campaner, L.M.; Silveira, M.P.M.; de Andrade, G.S.; Borges, A.L.S.; Bottino, M.A.; Dal Piva, A.M.D.; Lo Giudice, R.; Ausiello, P.; Tribst, J.P.M. Influence of Polymeric Restorative Materials on the Stress Distribution in Posterior Fixed Partial Dentures: 3D Finite Element Analysis. Polymers 2021, 13, 758. [Google Scholar] [CrossRef]
- Zarnpelis, A.; Rangert, B.; Heiji, L. Tilting of splinted implants for improved prosthodontic support: A two dimensional finite element analysis. J. Prosthet. Dent. 2007, 97, S35–S43. [Google Scholar] [CrossRef]
- Larsson, C.; Holm, L.; Lovgren, N.; Kokubo, Y.; von Steyern, P.V. Fracture strength of four-unit Y-TZP FPD cores designed with varying connector diameter: An in-vitro study. J. Oral Rehabil. 2007, 34, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Muddugangadhar, B.C.; Amarnath, G.S.; Sonika, R.; Chheda, P.S.; Garg, A. Meta-analysis of Failure and Survival Rate of Implant-supported Single Crowns, Fixed Partial Denture, and Implant Tooth-supported Prostheses. J. Int. Oral Health 2015, 7, 11–17. [Google Scholar] [PubMed]
- Lang, N.P.; Pjetursson, B.E.; Tan, K.; Brägger, U.; Egger, M.; Zwahlen, M. A systematic review of the survival and complication rates of fixed partial dentures (FPDs) after an observation period of at least 5 years: II.: Combined tooth-implant-supported FPDs. Clin. Oral Implants Res. 2004, 15, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Tan, K.; Lang, N.P.; Brägger, U.; Egger, M.; Zwahlen, M. A systematic review of the survival and complication rates of fixed partial dentures (FPDs) after an observation period of at least 5 years: I.: Implant-supported FPDs. Clin. Oral Implants Res. 2004, 15, 625–642. [Google Scholar] [CrossRef]
- Tan, K.; Pjetursson, B.E.; Lang, N.P.; Chan, E.S.Y. A systematic review of the survival and complication rates of fixed partial dentures (FPDs) after an observation period of at least 5 years: III.: Conventional FPDs. Clin. Oral Implants Res. 2004, 15, 654–666. [Google Scholar] [CrossRef]
- Jemaa, H.; Eisenburger, M.; Greuling, A. Semi-automated generation of bone loss defects around dental implants and its application in finite element analysis. Comput. Methods Biomech. Biomed. Engin. 2023, 13, 1756–1767. [Google Scholar] [CrossRef]
- Demoly, F.; Roth, S. Knowledge-based parametric CAD models of configurable biomechanical structures using geometric skeletons. Comput. Ind. 2017, 92–93, 104–117. [Google Scholar] [CrossRef]
- Jemaa, H.; Eisenburger, M.; Greuling, A. Automated Remodelling of Connectors in Fixed Partial Dentures. Dent. J. 2023, 11, 252. [Google Scholar] [CrossRef]
- Dittmer, M.P.; Borchers, L.; Stiesch, M.; Kohorst, P. Stresses and distortions within zirconia-fixed dental prostheses due to the veneering process. Acta Biomater. 2009, 5, 3231–3239. [Google Scholar] [CrossRef]
- Rand, A.; Kohorst, P.; Greuling, A.; Borchers, L.; Stiesch, M. Stress Distribution in All-Ceramic Posterior 4-Unit Fixed Dental Prostheses Supported in Different Ways: Finite Element Analysis. Implant Dent. 2016, 25, 485–491. [Google Scholar] [CrossRef]
- Choi, J.; Kwon, J.; Shin, J.W.; Lee, J.; Lee, S.; Kook, M.S. Quantitative optical coherence tomography angiography of macular vascular structure and foveal avascular zone in glaucoma. PLoS ONE 2017, 12, e0184948. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Akbari, A.; Jiang, F.; Liu, Y.; Chen, J. The effects of different types of periodontal ligament material models on stresses computed using finite element models. Am. J. Orthod. Dentofac. Orthop. 2022, 162, e328–e336. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa Castolo, G.; Guevara Perez, S.V.; Arnoux, P.J.; Badih, L.; Bonnet, F.; Behr, M. Mechanical strength and fracture point of a dental implant under certification conditions: A numerical approach by finite element analysis. J. Prosthet. Dent. 2018, 119, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Denisova, L. The use of acoustic microscopy to study the mechanical properties of glass-ionomer cement. Dent. Mater. 2004, 20, 358–363. [Google Scholar] [CrossRef]
- Dittmer, M.P.; Kohorst, P.; Borchers, L.; Stiesch-Scholz, M. Finite element analysis of a four-unit all-ceramic fixed partial denture. Acta Biomater. 2009, 5, 1349–1355. [Google Scholar] [CrossRef]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Ko, C.C.; Chu, C.S.; Chung, K.H.; Lee, M.C. Effects of posts on dentin stress distribution in pulpless teeth. J. Prosthet. Dent. 1992, 68, 421–427. [Google Scholar] [CrossRef]
- Roffmann, O.; Stiesch, M.; Greuling, A. Preventing stress singularities in peri-implant bone—A finite element analysis using a graded bone. Comput. Methods Biomech. Biomed. Engin. 2023, 27, 547–557. [Google Scholar] [CrossRef]
- Azcarate-Velázquez, F.; Castillo-Oyagüe, R.; Oliveros-López, L.-G.; Torres-Lagares, D.; Martínez-González, Á.-J.; Pérez-Velasco, A.; Lynch, C.D.; Gutiérrez-Pérez, J.-L.; Serrera-Figallo, M.-Á. Influence of bone quality on the mechanical interaction between implant and bone: A finite element analysis. J. Dent. 2019, 88, 103161. [Google Scholar] [CrossRef]
- Misch, C.E.; Dietsh-Misch, F.; Hoar, J.; Beck, G.; Hazen, R.; Misch, C.M. A bone quality-based implant system: First year of prosthetic loading. J. Oral Implantol. 1999, 25, 185–197. [Google Scholar] [CrossRef]
- Burkhart, T.A.; Andrews, D.M.; Dunning, C.E. Finite element modeling mesh quality, energy balance and validation methods: A review with recommendations associated with the modeling of bone tissue. J. Biomech. 2013, 46, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, M.P.; Kohorst, P.; Borchers, L.; Stiesch, M. Influence of the supporting structure on stress distribution in all-ceramic FPDs. Int. J. Prosthodont. 2010, 23, 63–68. [Google Scholar] [PubMed]
- Mahmood, D.J.; Linderoth, E.H.; Vult Von Steyern, P. The influence of support properties and complexity on fracture strength and fracture mode of all-ceramic fixed dental prostheses. Acta Odontol. Scand. 2011, 69, 229–237. [Google Scholar] [CrossRef]
- Brune, A.; Stiesch, M.; Eisenburger, M.; Greuling, A. The effect of different occlusal contact situations on peri-implant bone stress—A contact finite element analysis of indirect axial loading. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 367–373. [Google Scholar] [CrossRef]
- Rodriguez-Ferrer, J.D.; Lizcano-Prada, I.A.; Diosa-Peña, J.G. Linear, bilinear, and hyperelastic comparison for the periodontal ligament modeling. J. Phys. Conf. Ser. 2021, 2046, 012018. [Google Scholar] [CrossRef]
- Schindler, H.J.; Stengel, E.; Spiess, W.E.L. Feedback control during mastication of solid food textures—A clinical-experimental study. J. Prosthet. Dent. 1998, 80, 330–336. [Google Scholar] [CrossRef]
- Van der Bilt, A. Assessment of mastication with implications for oral rehabilitation: A review: Mastication and oral rehabilitation. J. Oral Rehabil. 2011, 38, 754–780. [Google Scholar] [CrossRef]




| Component | Material | Young’s Modulus E [GPa] | Poisson’s Ratio |
|---|---|---|---|
| Implant 1 | Titanium grade 4 | 104.5 | 0.37 |
| Abutment 1 | Titanium grade 4 | 104.5 | 0.37 |
| Implant screw 1 | Titanium grade 5 | 114.0 | 0.33 |
| Cement layer 2 | Glass ionomer cement | 15.9 | 0.33 |
| Abutment tooth 3 | Polyurethane | 3.525 | 0.33 |
| FPD 4 | Zirconium dioxide | 210 | 0.27 |
| Cortical bone 5 | - | 13.7 | 0.3 |
| Transition zone 6 | - | graded | 0.3 |
| Cancellous bone 7 | - | 9.5 (D1 quality) | 0.3 |
| - | 5.5 (D2 quality) | 0.3 | |
| - | 1.6 (D3 quality) | 0.3 | |
| - | 0.69 (D4 quality) | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jemaa, H.; Eisenburger, M.; Greuling, A. Automated Support Generation for Fixed Partial Dentures and Impact of Bone Loss, Bone Quality and Support Types: Parametric Cad and Finite Element Analysis. Dent. J. 2024, 12, 394. https://doi.org/10.3390/dj12120394
Jemaa H, Eisenburger M, Greuling A. Automated Support Generation for Fixed Partial Dentures and Impact of Bone Loss, Bone Quality and Support Types: Parametric Cad and Finite Element Analysis. Dentistry Journal. 2024; 12(12):394. https://doi.org/10.3390/dj12120394
Chicago/Turabian StyleJemaa, Hassen, Michael Eisenburger, and Andreas Greuling. 2024. "Automated Support Generation for Fixed Partial Dentures and Impact of Bone Loss, Bone Quality and Support Types: Parametric Cad and Finite Element Analysis" Dentistry Journal 12, no. 12: 394. https://doi.org/10.3390/dj12120394
APA StyleJemaa, H., Eisenburger, M., & Greuling, A. (2024). Automated Support Generation for Fixed Partial Dentures and Impact of Bone Loss, Bone Quality and Support Types: Parametric Cad and Finite Element Analysis. Dentistry Journal, 12(12), 394. https://doi.org/10.3390/dj12120394









