Surface Characterization of Bone-Level and Tissue-Level PEEK and Titanium Dental Implant Scan Bodies After Repeated Autoclave Sterilization Cycles
Abstract
1. Introduction
2. Materials and Methods
2.1. Autoclave Sterilizaiton
2.2. Fourier Transform Infrared Spectroscopy
2.3. X-Ray Photo Electron Spectroscopy
2.4. Optical Profilometery
3. Results
3.1. FTIR
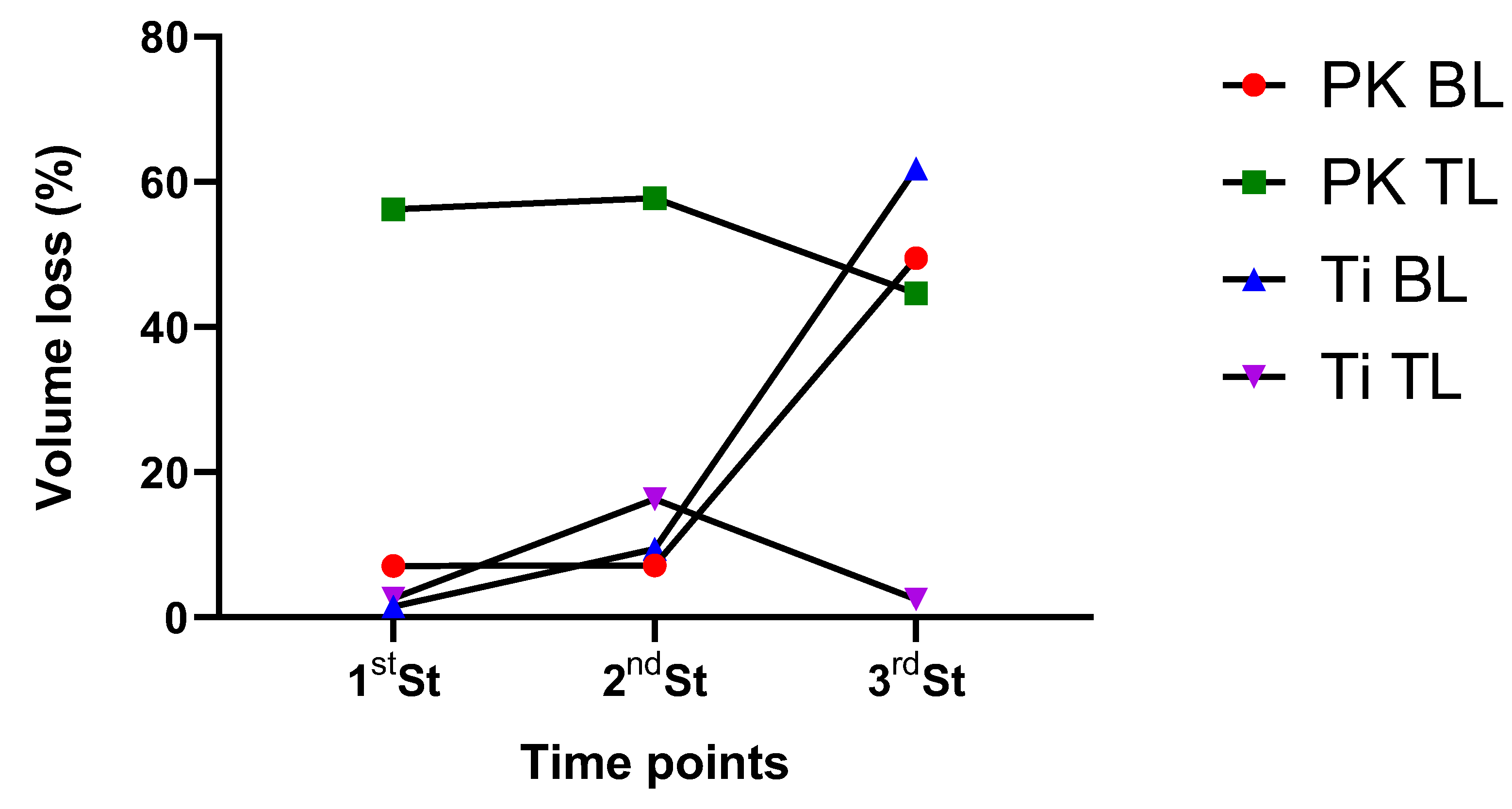
3.2. XPS
3.3. Surface Roughness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diker, E.; Terzioglu, H.; Gouveia, D.N.M.; Donmez, M.B.; Seidt, J.; Yilmaz, B. Effect of material type, torque value, and sterilization on linear displacements of a scan body: An in vitro study. Clin. Implant. Dent. Relat. Res. 2023, 25, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Son, K.; Lee, K.B. Displacement of scan body during screw tightening: A comparative in vitro study. J. Adv. Prosthodont. 2020, 12, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Bae, J.-H.; Lee, S.Y. Trueness of digital implant impressions based on implant angulation and scan body materials. Sci. Rep. 2021, 11, 21892. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Yasunami, N.; Furuhashi, A.; Sanda, K.; Ayukawa, Y. Effects of Autoclave Sterilization and Multiple Use on Implant Scanbody Deformation In Vitro. Materials 2022, 15, 7717. [Google Scholar] [CrossRef]
- Tan, J.Z.H.; Tan, M.Y.; See Toh, Y.L.; Wong, K.Y.; Tan, K.B.C. Three-dimensional positional accuracy of intraoral and laboratory implant scan bodies. J. Prosthet. Dent. 2022, 128, 735–744. [Google Scholar] [CrossRef]
- Thiruchitrambalam, M.; Bubesh Kumar, D.; Shanmugam, D.; Jawaid, M. A review on PEEK composites—Manufacturing methods, properties and applications. Mater. Today Proc. 2020, 33, 1085–1092. [Google Scholar] [CrossRef]
- Sawyers, J.; Baig, M.R.; El-Masoud, B. Effect of Multiple Use of Impression Copings and Scanbodies on Implant Cast Accuracy. Int. J. Oral. Maxillofac. Implants 2019, 34, 891–898. [Google Scholar] [CrossRef]
- Sussman, E.M.; Oktem, B.; Isayeva, I.S.; Liu, J.; Wickramasekara, S.; Chandrasekar, V.; Nahan, K.; Shin, H.Y.; Zheng, J. Chemical Characterization and Non-targeted Analysis of Medical Device Extracts: A Review of Current Approaches, Gaps, and Emerging Practices. ACS Biomater. Sci. Eng. 2022, 8, 939–963. [Google Scholar] [CrossRef]
- Mylläri, V.; Ruoko, T.-P.; Vuorinen, J.; Lemmetyinen, H. Characterization of thermally aged polyetheretherketone fibres—Mechanical, thermal, rheological and chemical property changes. Polym. Degrad. Stab. 2015, 120, 419–426. [Google Scholar] [CrossRef]
- Pascual, A.; Toma, M.; Tsotra, P.; Grob, M.C. On the stability of PEEK for short processing cycles at high temperatures and oxygen-containing atmosphere. Polym. Degrad. Stab. 2019, 165, 161–169. [Google Scholar] [CrossRef]
- Phillips, R.; Glauser, T.; Månson, J.A.E. Thermal stability of PEEK/carbon fiber in air and its influence on consolidation. Polym. Compos. 1997, 18, 500–508. [Google Scholar] [CrossRef]
- Al Lafi, A.G. FTIR spectroscopic analysis of ion irradiated poly (ether ether ketone). Polym. Degrad. Stab. 2014, 105, 122–133. [Google Scholar] [CrossRef]
- Gaitanelis, D.; Worrall, C.; Kazilas, M. Detecting, characterising and assessing PEEK’s and CF-PEEK’s thermal degradation in rapid high-temperature processing. Polym. Degrad. Stab. 2022, 204, 110096. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Adhikari, R. 2—Nondegradable synthetic polymers for medical devices and implants. In Biosynthetic Polymers for Medical Applications; Poole-Warren, L., Martens, P., Green, R., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 33–62. [Google Scholar]
- Majchrowicz, K.; Sotniczuk, A.; Malicka, J.; Choinska, E.; Garbacz, H. Thermal Stability and Mechanical Behavior of Ultrafine-Grained Titanium with Different Impurity Content. Materials 2023, 16, 1339. [Google Scholar] [CrossRef] [PubMed]
- Krishna, D.N.G.; Philip, J. Review on surface-characterization applications of X-ray photoelectron spectroscopy (XPS): Recent developments and challenges. Appl. Surf. Sci. 2022, 12, 100332. [Google Scholar] [CrossRef]
- Nakamura, H.; Nakamura, T.; Noguchi, T.; Imagawa, K. Photodegradation of PEEK sheets under tensile stress. Polym. Degrad. Stab. 2006, 91, 740–746. [Google Scholar] [CrossRef]
- Patel, K.; Doyle, C.S.; James, B.J.; Hyland, M.M. Valence band XPS and FT-IR evaluation of thermal degradation of HVAF thermally sprayed PEEK coatings. Polym. Degrad. Stab. 2010, 95, 792–797. [Google Scholar] [CrossRef]
- Louette, P.; Bodino, F.; Pireaux, J.-J. Poly(ether ether ketone) (PEEK) XPS Reference Core Level and Energy Loss Spectra. Surf. Sci. Spectra 2007, 12, 149–153. [Google Scholar] [CrossRef]
- Yang, L.; Ohki, Y.; Hirai, N.; Hanada, S. Aging of poly(ether ether ketone) by heat and gamma rays—Its degradation mechanism and effects on mechanical, dielectric and thermal properties. Polym. Degrad. Stab. 2017, 142, 117–128. [Google Scholar] [CrossRef]
- Massaro, C.; Rotolo, P.; De Riccardis, F.; Milella, E.; Napoli, A.; Wieland, M.; Textor, M.; Spencer, N.D.; Brunette, D.M. Comparative investigation of the surface properties of commercial titanium dental implants. Part I: Chemical composition. J. Mater. Sci. Mater. Med. 2002, 13, 535–548. [Google Scholar] [CrossRef]
- Batak, B.; Çakmak, G.; Johnston, W.M.; Yilmaz, B. Surface roughness of high-performance polymers used for fixed implant-supported prostheses. J. Prosthet. Dent. 2021, 126, 254.e251–254.e256. [Google Scholar] [CrossRef] [PubMed]
- Çulhaoğlu, A.K.; Özkır, S.E.; Şahin, V.; Yılmaz, B.; Kılıçarslan, M.A. Effect of Various Treatment Modalities on Surface Characteristics and Shear Bond Strengths of Polyetheretherketone-Based Core Materials. J. Prosthodont. 2020, 29, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Heimer, S.; Schmidlin, P.R.; Roos, M.; Stawarczyk, B. Surface properties of polyetheretherketone after different laboratory and chairside polishing protocols. J. Prosthet. Dent. 2017, 117, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Doumeng, M.; Makhlouf, L.; Berthet, F.; Marsan, O.; Delbé, K.; Denape, J.; Chabert, F. A comparative study of the crystallinity of polyetheretherketone by using density, DSC, XRD, and Raman spectroscopy techniques. Polym. Test. 2021, 93, 106878. [Google Scholar] [CrossRef]
- Barba, D.; Arias, A.; Garcia-Gonzalez, D. Temperature and strain rate dependences on hardening and softening behaviours in semi-crystalline polymers: Application to PEEK. I J. Solids Struct. 2020, 182–183, 205–217. [Google Scholar] [CrossRef]
- Park, J.H.; Olivares-Navarrete, R.; Baier, R.E.; Meyer, A.E.; Tannenbaum, R.; Boyan, B.D.; Schwartz, Z. Effect of cleaning and sterilization on titanium implant surface properties and cellular response. Acta Biomater. 2012, 8, 1966–1975. [Google Scholar] [CrossRef]
- Smith, D.C.; Pilliar, R.M.; Chernecky, R. Dental implant materials. I. Some effects of preparative procedures on surface topography. J. Biomed. Mater. Res. 1991, 25, 1045–1068. [Google Scholar] [CrossRef]
- Xin, H.; Shepherd, D.E.T.; Dearn, K.D. Strength of poly-ether-ether-ketone: Effects of sterilisation and thermal ageing. Polym. Test. 2013, 32, 1001–1005. [Google Scholar] [CrossRef]





| Material | PEEK | PEEK | Titanium | Titanium |
|---|---|---|---|---|
| Manufacturer | Straumann® Basel Switzerland | Straumann® Basel Switzerland | Straumann® Basel Switzerland | Straumann® Basel Switzerland |
| Type | Tissue Level | Bone Level | Tissue Level | Bone Level |
| Wavenumber (cm−1) | Spectral Changes and Identification |
|---|---|
| 1741 | Fluorenone peak formation |
| 1277 and 1305 | Two bands pertaining to high frequency of the ether peak at 1219 cm−1 |
| 1100 | Alterations in the diphenyl ether bonds |
| 1185 | Disappearance of peak of diphenyl ether group |
| 837 | Changes in the aromatic rings |
| 926 | Increased intensity after sterilization |
| Neat (Pristine) | 1st ST | |||
|---|---|---|---|---|
| Elements | Ti BL | Ti TL | Ti BL | Ti TL |
| C1s | 284.66, (1.3), 76.66 | 284.67, (1.11), 79.67 | 284.63, (1.09), 77.02 | 284.67, (1.11), 79.67 |
| O1s | 531.96, (1.76), 15.79 | 530.88, (1.61), 14.66 | 531.78, (1.89), 17.21 | 531.88, (1.61), 14.66 |
| Si2p | 101.87, (1.64), 7.24 | 101.9, (1.49), 4.84 | 101.9, (1.52), 3.9 | 101.9, (1.49), 4.84 |
| Zr3d | 183.5, (1.68), 0.31 | 182.58, (1.24), 0.83 | 182.26, (1.24), 1.87 | 182.58, (1.24), 0.83 |
| Ca2p | - | - | - | - |
| 2nd ST | 3rd ST | |||
| Ti BL | Ti TL | Ti BL | Ti TL | |
| C1s | 284.62, (1.49), 71.03 | 284.6, (1.32), 79.28 | 284.62, (1.49), 81.95 | 284.60, (1.32), 79.28 |
| O1s | 531.92, (1.87), 17.9 | 531.8, (1.71), 13.95 | 531.92, (1.87), 11.50 | 531.80, (1.71), 13.95 |
| Si2p | 101.87, (1.79), 9.2 | 101.76, (1.57), 5.2 | 101.87, (1.79), 5.33 | 101.76, (1.57), 5.20 |
| Zr3d | 182.84, (1.47), 0.53 | 182.84, (1.25), 0.22 | 182.84, (1.47), 0.53 | 182.84, (1.25), 0.22 |
| Ca2p | 347.02, (2.06), 1.34 | 347.02, (1.6), 1.36 | 347.29, (2.06), 1.22 | 347.02, (1.60), 1.36 |
| Neat (Pristine) | 1st ST | |||
|---|---|---|---|---|
| Elements | PK BL | PK TL | PK BL | PK TL |
| C1s | 284.61, (1.11), 81.05 | 284.65, (1.1), 83.08 | 284.61, (1.11), 81.05 | 284.69, (1.04), 78.3 |
| O1s | 531.6, (1.79), 13.15 | 531.69, (1.7), 12.56 | 531.6, (1.79), 13.15 | 1531.88, (1.71), 5.11 |
| Si2p | 101.79, (1.55), 2.29 | 101.83, (1.36), 2.10 | 101.79, (1.55), 2.29 | 101.94, (1.39), 5.03 |
| Na1s | 1071.29, (1.71), 0.75 | 1071.42, (1.73), 0.21 | 1071.29, (1.71), 0.75 | 1071.13, (1.26), 0.68 |
| Ca2p | 347.04, (1.46), 1.59 | 347.12, (1.44), 1.78 | 347.04, (1.46), 1.59 | 347.02, (1.41), 0.87 |
| Zn2p | 1021.7, (1.61), 0.39 | - | 284.61, (1.11), 81.05 | - |
| 2nd ST | 3rd ST | |||
| PK BL | PK TL | PK BL | PK TL | |
| C1s | 284.68, (1.28), 79.81 | 284.67, (1.14), 84.4 | 284.68, (1.28), 79.81 | 284.67, (1.1.4), 84.40 |
| O1s | 531.98, (2.06), 15.83 | 531.61, (1.80), 11.56 | 531.98, (2.06), 15.83 | 531.61, (1.80), 11.56 |
| Si2p | 102.03, (1.73), 2.36 | 101.86, (1.80), 1.9 | 102.03, (1.63), 2.36 | 101.86, (1.43), 1.90 |
| Na1s | 1071.10, (1.73), 0.78 | 1071.22, (1.94), 0.73 | 1071.10, (1.73), 0.78 | 1071.22, (1.94), 0.73 |
| Ca2p | 346.98, (1.68), 1.21 | 347.06, (1.53), 1.41 | 346.98, (1.68), 1.21 | 347.06, (1.53), 1.41 |
| Zn2p | - | - | - | - |
| T1 | T2 | T3 | T4 | |
|---|---|---|---|---|
| PK BL | 3.40 ± 1.00 | 3.16 ± 1.38 (0.70) | 3.66 ± 0.68 (0.43) | 1.71 ± 0.33 (0.006) |
| PK TL | 3.52 ± 0.72 | 1.54 ± 0.56 (<0.001) | 1.48 ± 0.18 (<0.001) | 1.95 ± 0.68 (<0.001) |
| Ti BL | 4.39 ± 0.80 | 4.46 ± 0.61 (0.83) | 3.97 ± 0.70 (0.36) | 1.67 ± 0.49 (<0.001) |
| Ti TL | 2.64 ± 0.70 | 2.76 ± 0.51 (0.68) | 2.21 ± 0.62 (0.25) | 2.57 ± 0.32 (0.82) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qasim, S.S.B.; Akbar, A.A.; Sadeqi, H.A.; Baig, M.R. Surface Characterization of Bone-Level and Tissue-Level PEEK and Titanium Dental Implant Scan Bodies After Repeated Autoclave Sterilization Cycles. Dent. J. 2024, 12, 392. https://doi.org/10.3390/dj12120392
Qasim SSB, Akbar AA, Sadeqi HA, Baig MR. Surface Characterization of Bone-Level and Tissue-Level PEEK and Titanium Dental Implant Scan Bodies After Repeated Autoclave Sterilization Cycles. Dentistry Journal. 2024; 12(12):392. https://doi.org/10.3390/dj12120392
Chicago/Turabian StyleQasim, Syed Saad Bin, Aqdar A. Akbar, Haneen A. Sadeqi, and Mirza Rustum Baig. 2024. "Surface Characterization of Bone-Level and Tissue-Level PEEK and Titanium Dental Implant Scan Bodies After Repeated Autoclave Sterilization Cycles" Dentistry Journal 12, no. 12: 392. https://doi.org/10.3390/dj12120392
APA StyleQasim, S. S. B., Akbar, A. A., Sadeqi, H. A., & Baig, M. R. (2024). Surface Characterization of Bone-Level and Tissue-Level PEEK and Titanium Dental Implant Scan Bodies After Repeated Autoclave Sterilization Cycles. Dentistry Journal, 12(12), 392. https://doi.org/10.3390/dj12120392







