Changes in the Periodontal Gap After Long-Term Tooth Movement into Augmented Critical-Sized Defects in the Jaws of Beagle Dogs
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Procedure and Orthodontic Appliance
2.2. Histomorphometric Preparation, Data Collection, and Analysis
2.2.1. Periodontal Gap Measurements
2.2.2. Proportion of Bone, Osteoid, and Bone Marrow
2.3. Statistical Analysis
3. Results
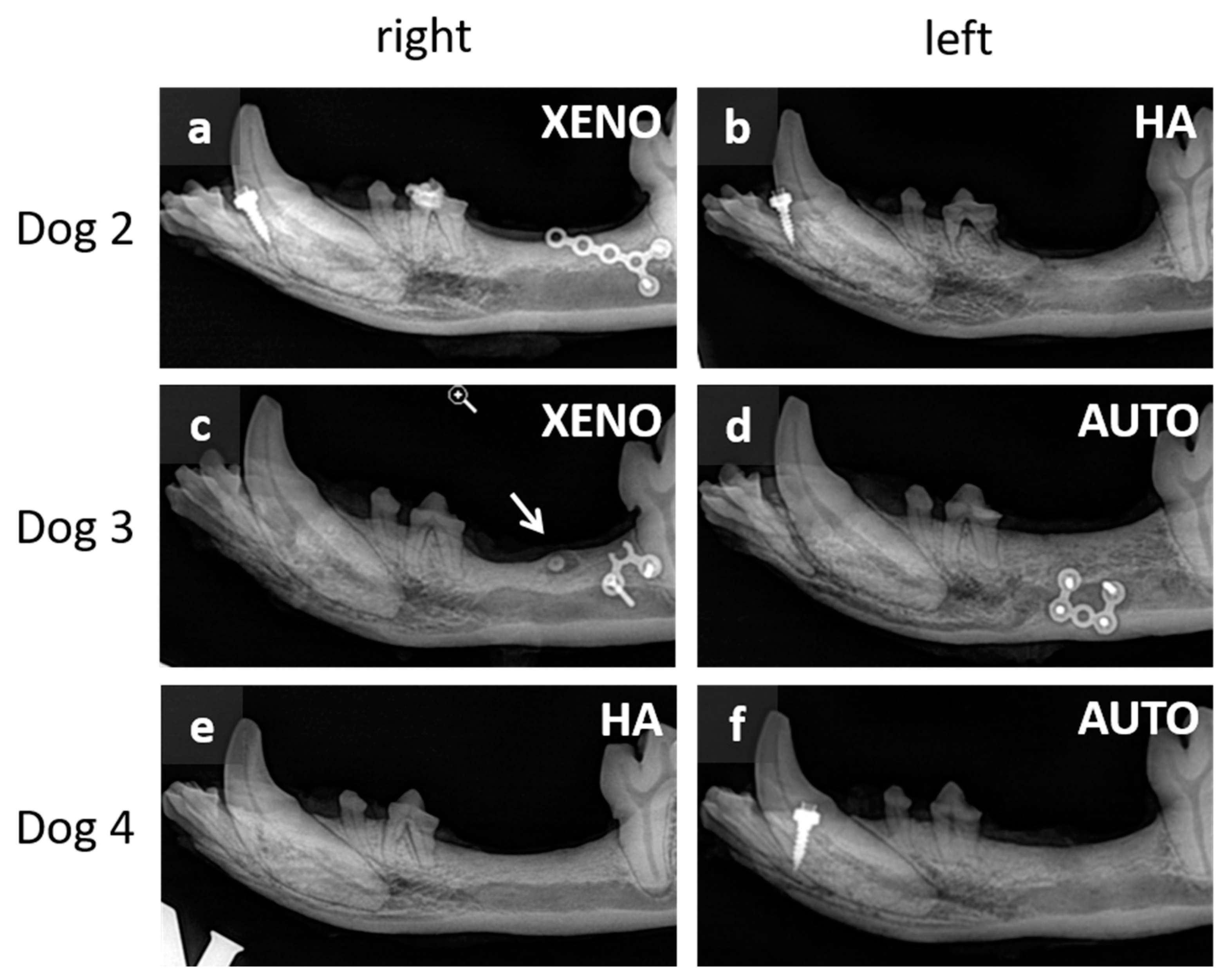
3.1. Macroscopic and Radiographic Results
3.2. Periodontal Gap Measurements
3.3. Proportion of Bone, Osteoid, and Bone Marrow
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Zhan, Q.; Bao, M.; Yi, J.; Li, Y. Biomechanical and biological responses of periodontium in orthodontic tooth movement: Up-date in a new decade. Int. J. Oral Sci. 2021, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Alikhani, M.; Sangsuwon, C.; Alansari, S.; Nervina, J.M.; Teixeira, C.C. Biphasic theory: Breakthrough understanding of tooth movement. J. World Fed. Orthod. 2018, 7, 82–88. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tanaka, T.; Noda, K.; Shimpo, S.; Oikawa, T.; Hirashita, A.; Kawamoto, T.; Kawasaki, K. Calcification of degenerating tissues in the periodontal ligament during tooth movement. J. Periodontal Res. 2003, 38, 343–350. [Google Scholar] [CrossRef]
- Krishnan, V.; Davidovitch, Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 469.e1–32. [Google Scholar] [CrossRef]
- Tsuge, A.; Noda, K.; Nakamura, Y. Early tissue reaction in the tension zone of PDL during orthodontic tooth movement. Arch. Oral Biol. 2016, 65, 17–25. [Google Scholar] [CrossRef]
- Zong, C.; van Dessel, J.; Vande Velde, G.; Willems, G.; Cadenas de Llano-Pérula, M. Dynamic changes in tooth displacement and bone morphometry induced by orthodontic force. Sci. Rep. 2022, 12, 13672. [Google Scholar] [CrossRef] [PubMed]
- Aveic, S.; Craveiro, R.B.; Wolf, M.; Fischer, H. Current Trends in In Vitro Modeling to Mimic Cellular Crosstalk in Periodontal Tissue. Adv. Healthc. Mater. 2021, 10, e2001269. [Google Scholar] [CrossRef] [PubMed]
- Meikle, M.C. The tissue, cellular, and molecular regulation of orthodontic tooth movement: 100 years after Carl Sandstedt. Eur. J. Orthod. 2006, 28, 221–240. [Google Scholar] [CrossRef]
- Rizk, M.; Niederau, C.; Florea, A.; Kiessling, F.; Morgenroth, A.; Mottaghy, F.M.; Schneider, R.K.; Wolf, M.; Craveiro, R.B. Periodontal ligament and alveolar bone remodeling during long orthodontic tooth movement analyzed by a novel user-independent 3D-methodology. Sci. Rep. 2023, 13, 19919. [Google Scholar] [CrossRef]
- Shalish, M.; Will, L.A.; Fukai, N.; Hou, B.; Olsen, B.R. Role of polycystin-1 in bone remodeling: Orthodontic tooth movement study in mutant mice. Angle Orthod. 2014, 84, 885–890. [Google Scholar] [CrossRef]
- Ru, N.; Liu, S.S.-Y.; Zhuang, L.; Li, S.; Bai, Y. In vivo microcomputed tomography evaluation of rat alveolar bone and root resorption during orthodontic tooth movement. Angle Orthod. 2013, 83, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.H.; Yang, C.-Y.; Shin, M.K.; Wang, J.; Patel, J.H.; Chung, C.-H.; Graves, D.T. Osteoblast lineage cells and periodontal ligament fibroblasts regulate orthodontic tooth movement that is dependent on Nuclear Factor-kappa B (NF-kB) activation. Angle Orthod. 2021, 91, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Mena Laura, E.E.; Cestari, T.M.; Almeida, R.; Pereira, D.S.; Taga, R.; Garlet, G.P.; Assis, G.F. Metformin as an add-on to insulin improves periodontal response during orthodontic tooth movement in type 1 diabetic rats. J. Periodontol. 2019, 90, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xia, L.; Wang, S.; Al-Balaa, M.; Liu, W.; Hua, X. The expression of extracellular matrix metalloproteinase inducer (EMMPRIN) in the compression area during orthodontic relapse. Eur. J. Orthod. 2020, 42, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Noda, K.; Shimoda, S.; Oikawa, T.; Arai, C.; Nomura, Y.; Kawasaki, K. Time-lapse observation of rat periodontal ligament during function and tooth movement, using microcomputed tomography. Eur. J. Orthod. 2008, 30, 320–326. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Li, R.; Chen, Z.; Huang, Y.; Chen, Z. Biological Effects of Orthodontic Tooth Movement Into the Grafted Alveolar Cleft. J. Oral Maxillofac. Surg. 2018, 76, 605–615. [Google Scholar] [CrossRef]
- Ru, N.; Liu, S.S.-Y.; Bai, Y.; Li, S.; Liu, Y.; Wei, X. BoneCeramic graft regenerates alveolar defects but slows orthodontic tooth movement with less root resorption. Am. J. Orthod. Dentofac. Orthop. 2016, 149, 523–532. [Google Scholar] [CrossRef]
- Möhlhenrich, S.C.; Kniha, K.; Magnuska, Z.; Chhatwani, S.; Hermanns-Sachweh, B.; Gremse, F.; Hölzle, F.; Danesh, G.; Modabber, A. Development of root resorption during orthodontic tooth movement after cleft repair using different grafting materials in rats. Clin. Oral Investig. 2022, 26, 5809–5821. [Google Scholar] [CrossRef]
- Nauth, A.; Schemitsch, E.; Norris, B.; Nollin, Z.; Watson, J.T. Critical-Size Bone Defects: Is There a Consensus for Diagnosis and Treatment? J. Orthop. Trauma 2018, 32 (Suppl. 1), S7–S11. [Google Scholar] [CrossRef]
- Cabbad, N.C.; Stalder, M.W.; Arroyave, A.; Wolfe, E.M.; Wolfe, S.A. Autogenous Bone Cranioplasty: Review of a 42-Year Experience by a Single Surgeon. Plast. Reconstr. Surg. 2019, 143, 1713–1723. [Google Scholar] [CrossRef]
- Fearon, J.A.; Griner, D.; Ditthakasem, K.; Herbert, M. Autogenous Bone Reconstruction of Large Secondary Skull Defects. Plast. Reconstr. Surg. 2017, 139, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Douglas, T.; Zamponi, C.; Becker, S.T.; Sherry, E.; Sivananthan, S.; Warnke, F.; Wiltfang, J.; Warnke, P.H. Comparison of in vitro biocompatibility of NanoBone(®) and BioOss(®) for human osteoblasts. Clin. Oral Implant. Res. 2011, 22, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Troedhan, A.; Schlichting, I.; Kurrek, A.; Wainwright, M. Primary implant stability in augmented sinuslift-sites after completed bone regeneration: A randomized controlled clinical study comparing four subantrally inserted biomaterials. Sci. Rep. 2014, 4, 5877. [Google Scholar] [CrossRef]
- Zhang, Y.; Al-Maawi, S.; Wang, X.; Sader, R.; James Kirkpatrick, C.; Ghanaati, S. Biomaterial-induced multinucleated giant cells express proinflammatory signaling molecules: A histological study in humans. J. Biomed. Mater. Res. A 2019, 107, 780–790. [Google Scholar] [CrossRef]
- Dau, M.; Kämmerer, P.W.; Henkel, K.-O.; Gerber, T.; Frerich, B.; Gundlach, K.K.H. Bone formation in mono cortical mandibular critical size defects after augmentation with two synthetic nanostructured and one xenogenous hydroxyapatite bone substitute—In vivo animal study. Clin. Oral Implant. Res. 2016, 27, 597–603. [Google Scholar] [CrossRef]
- Kijartorn, P.; Wongpairojpanich, J.; Thammarakcharoen, F.; Suwanprateeb, J.; Buranawat, B. Clinical evaluation of 3D printed nano-porous hydroxyapatite bone graft for alveolar ridge preservation: A randomized controlled trial. J. Dent. Sci. 2022, 17, 194–203. [Google Scholar] [CrossRef]
- Wähnert, D.; Koettnitz, J.; Merten, M.; Kronenberg, D.; Stange, R.; Greiner, J.F.W.; Kaltschmidt, C.; Vordemvenne, T.; Kaltschmidt, B. Spongostan™ Leads to Increased Regeneration of a Rat Calvarial Critical Size Defect Compared to NanoBone® and Actifuse. Materials 2021, 14, 1961. [Google Scholar] [CrossRef]
- Tanimoto, K.; Sumi, K.; Yoshioka, M.; Oki, N.; Tanne, Y.; Awada, T.; Kato, Y.; Sugiyama, M.; Tanne, K. Experimental Tooth Movement Into New Bone Area Regenerated by Use of Bone Marrow-Derived Mesenchymal Stem Cells. Cleft Palate Craniofac. J. 2015, 52, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.; Carmagnola, D.; Berglundh, T.; Thilander, B.; Lindhe, J. Orthodontic movement in bone defects augmented with Bio-Oss. An experimental study in dogs. J. Clin. Periodontol. 2001, 28, 73–80. [Google Scholar]
- Machibya, F.M.; Zhuang, Y.; Guo, W.; You, D.; Lin, S.; Wu, D.; Chen, J. Effects of bone regeneration materials and tooth movement timing on canine experimental orthodontic treatment. Angle Orthod. 2018, 88, 171–178. [Google Scholar] [CrossRef]
- Seifi, M.; Arayesh, A.; Shamloo, N.; Hamedi, R. Effect of nanocrystalline hydroxyapatite socket preservation on orthodontically induced inflammatory root resorption. Cell J. 2015, 16, 514–527. [Google Scholar] [PubMed]
- Abe, T.; Kunimatsu, R.; Tanimoto, K. Comparison of Orthodontic Tooth Movement of Regenerated Bone Induced by Carbonated Hydroxyapatite or Deproteinized Bovine Bone Mineral in Beagle Dogs. Materials 2023, 17, 112. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Min, Q.; Li, X.; Liu, L.; Lv, Y.; Xu, W.; Liu, X.; Wang, H. Immune System Acts on Orthodontic Tooth Movement: Cellular and Molecular Mechanisms. Biomed Res. Int. 2022, 2022, 9668610. [Google Scholar] [CrossRef] [PubMed]







| Side of the Jaw | Dog 1 | Dog 2 | Dog 3 | Dog 4 |
|---|---|---|---|---|
| Right | 29 × 9 × 10 | 26 × 9 × 8 | 24 × 9 × 10 | 26 × 9 × 8 |
| Left | 28 × 8 × 10 | 26 × 10 × 8 | 26 × 10 × 6 | 25 × 7 × 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duske, K.; Warkentin, M.; Salbach, A.; Lenz, J.-H.; Stahl, F. Changes in the Periodontal Gap After Long-Term Tooth Movement into Augmented Critical-Sized Defects in the Jaws of Beagle Dogs. Dent. J. 2024, 12, 386. https://doi.org/10.3390/dj12120386
Duske K, Warkentin M, Salbach A, Lenz J-H, Stahl F. Changes in the Periodontal Gap After Long-Term Tooth Movement into Augmented Critical-Sized Defects in the Jaws of Beagle Dogs. Dentistry Journal. 2024; 12(12):386. https://doi.org/10.3390/dj12120386
Chicago/Turabian StyleDuske, Kathrin, Mareike Warkentin, Anja Salbach, Jan-Hendrik Lenz, and Franka Stahl. 2024. "Changes in the Periodontal Gap After Long-Term Tooth Movement into Augmented Critical-Sized Defects in the Jaws of Beagle Dogs" Dentistry Journal 12, no. 12: 386. https://doi.org/10.3390/dj12120386
APA StyleDuske, K., Warkentin, M., Salbach, A., Lenz, J.-H., & Stahl, F. (2024). Changes in the Periodontal Gap After Long-Term Tooth Movement into Augmented Critical-Sized Defects in the Jaws of Beagle Dogs. Dentistry Journal, 12(12), 386. https://doi.org/10.3390/dj12120386




