Efficacy of a Herbal Toothpaste During Active Periodontal Treatment: A Clinical Study
Abstract
1. Introduction
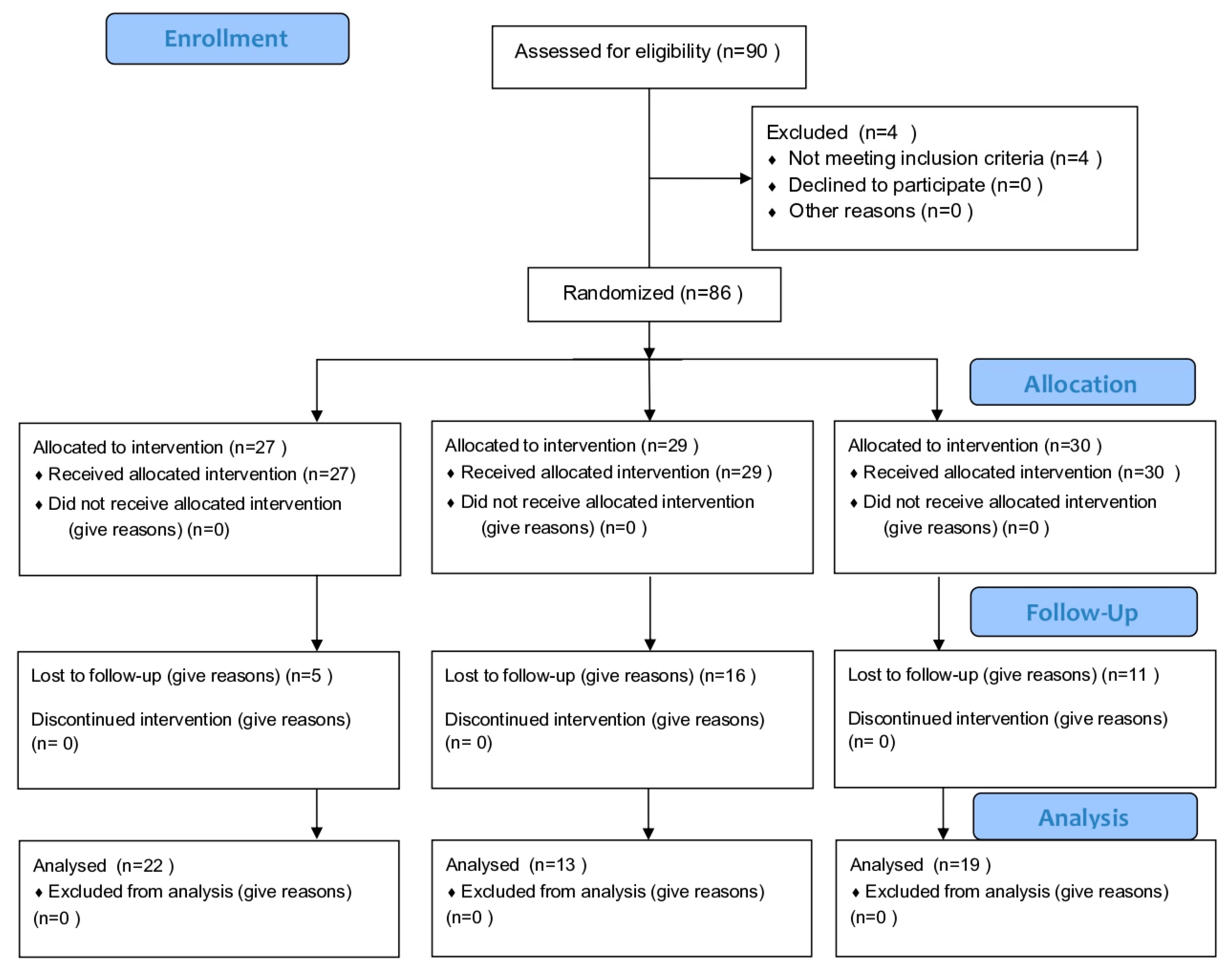
2. Materials and Methods
2.1. Study Design and Population
2.2. Intervention
2.2.1. Plaque Score (PS)
2.2.2. Bleeding on Probing (BOP)
2.2.3. Probing Depth (PD) and Clinical Attachment Level (CAL)
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Page, R.C. The etiology and pathogenesis of periodontitis. Compend. Contin. Educ. Dent. 2002, 23 (Suppl. S5), 11–14. [Google Scholar] [PubMed]
- Kornman, K.S.; Löe, H. The role of local factors in the etiology of periodontal diseases. Periodontol. 2000 1993, 2, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.M. Non-surgical pocket therapy: Mechanical. Ann. Periodontol. 1996, 1, 443–490. [Google Scholar] [CrossRef]
- Axelsson, P.; Lindhe, J. The significance of maintenance care in the treatment of periodontal disease. J. Clin. Periodontol. 1981, 8, 281–294. [Google Scholar] [CrossRef]
- Arweiler, N.B.; Auschill, T.M.; Sculean, A. Patient self-care of periodontal pocket infections. Periodontol. 2000 2018, 76, 164–179. [Google Scholar] [CrossRef]
- Davies, R.M. Toothpaste in the control of plaque/gingivitis and periodontitis. Periodontol. 2000 2008, 48, 23–30. [Google Scholar] [CrossRef]
- Atiba, A.; Ueno, H.; Uzuka, Y. The effect of Aloe vera oral administration on cutaneous wound healing in type 2 diabetic rats. J. Vet. Meol. Sci. 2011, 73, 583–589. [Google Scholar] [CrossRef]
- Pugh, N.; Ross, S.A.; ElSohly, M.A.; Pasco, D.S. Characterization of Aloeride, a new high-molecular-weight polysaccharide from Aloe vera with potent immunostimulatory activity. J. Agric. Food Chem. 2001, 49, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Zhang, W.L.Y. The antimicrobial potential of natural herbal dentrifices. Results of an in vitro diffusion method study. J. Am. Dent. Assoc. 2004, 135, 1133–1141. [Google Scholar] [CrossRef]
- Khatri, S.G.; Samuel, S.R.; Acharya, S.; Patil, S.T. Antiplaque, Antifungal Effectiveness of Aloe vera Among Intellectually Disabled Adolescents: Pilot Study. Pediatr. Dent. 2017, 39, 434–438. [Google Scholar]
- Villalobos, O.J.; Salazar, C.R.; Sánchez, G.R. Effect of a compound mouthwash Aloe vera in plaque and gingival inflammation. Acta. Odontol. Venez. 2001, 39, 16–24. [Google Scholar]
- Rajasekaran, S.; Sivagnanam, K.; Subramanian, S. Anti-oxidant effect of Aloe vera gel extract in streptozotocin-induced diabetes in rats. Pharmacol. Rep. 2005, 57, 90–96. [Google Scholar] [PubMed]
- Vajrabhaya, L.O.; Korsuwannawong, S.; Ruangsawasdi, N.; Phruksaniyom, C.; Srichan, R. The efficiency of natural wound healing and bacterial biofilm inhibition of Aloe vera and Sodium Chloride toothpaste preparation. BMC Complement. Med. Ther. 2022, 22, 66. [Google Scholar] [CrossRef]
- Pardo, A.; Fiorini, V.; Zangani, A.; Faccioni, P.; Signoriello, A.; Albanese, M.; Lombardo, G. Topical Agents in Biofilm Disaggregation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 10, 2179. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, C.; Venkitachalam, R.; Fontelo, P.; Iafolla, T.J.; Dye, B.A. Effectiveness of herbal oral care products in reducing dental plaque & gingivitis—A systematic review and meta-analysis. BMC Complement. Med. Ther. 2020, 20, 43. [Google Scholar]
- Sayar, F.; Farahmand, A.H.; Rezazadeh, M. Clinical Efficacy of Aloe Vera Toothpaste on Periodontal Parameters of Patients with Gingivitis—A Randomized, Controlled, Single-masked Clinical Trial. J. Contemp. Dent. Pract. 2021, 22, 242–247. [Google Scholar]
- Kaldahl, W.B.; Kalkwarf, K.L.; Patil, K.D.; Dyer, J.K.; Bates, R.E., Jr. Evaluation of four modalities of periodontal therapy. Mean probing depth, probing attachment level and recession changes. J. Periodontol. 1988, 59, 783–793. [Google Scholar] [CrossRef]
- Freires, I.A.; Rosalen, P.L. How natural product research has contributed to oral care product development? A critical view. Pharm. Res. 2016, 33, 1311–1317. [Google Scholar] [CrossRef]
- Al-Maweri, S.A.; Nassani, M.Z.; Alaizari, N.; Kalakonda, B.; Al-Shamiri, H.M.; Alhajj, M.N.; Al-Soneidar, W.A.; Alahmary, A.W. Efficacy of aloe vera mouthwash versus chlorhexidine on plaque and gingivitis: A systematic review. Int. J. Dent. Hyg. 2020, 18, 44–51. [Google Scholar] [CrossRef]
- Mullally, B.H.; James, J.A.; Coulter, W.A.; Linden, G.J. The efficacy of a herbal-based toothpaste on the control of plaque and gingivitis. J. Clin. Periodontol. 1995, 22, 686–689. [Google Scholar] [CrossRef]
- Tatikonda, A.; Debnath, S.; Chauhan, V.S.; Chaurasia, V.R.; Taranath, M.; Sharma, A.M. Effects of herbal and non- herbal toothpastes on plaque and gingivitis: A clinical comparative study. J. Int. Soc. Prev. Community Dent. 2014, 4 (Suppl. S2), S126–S129. [Google Scholar] [PubMed]
- Azaripour, A.; Mahmoodi, B.; Habibi, E.; Willershausen, I.; Schmidtmann, I.; Willershausen, B. Effectiveness of a miswak extract-containing toothpaste on gingival inflammation: A randomized clinical trial. Int. J. Dent. Hyg. 2017, 15, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Vajrabhaya, L.; Sappayatosok, K.; Kulthanaamondhita, P.; Korsuwannawong, S.; Sirikururat, P. Desensitizing Efficacy of a Herbal Toothpaste: A Clinical Study. World J. Dent. 2019, 10, 408–412. [Google Scholar] [CrossRef]
| Toothpaste | Active Ingredient | Full Scientific Species |
|---|---|---|
| Test group | Aloe vera | Aloe vera (L.) Burm. f. |
| Clinacanthus nutans | Clinacanthus nutans (Burm.f.) Lindau. | |
| Orange Jessamine leaf | Murraya Exotica L. | |
| Hydrocotyle | Centella asiatica (L.) Urb. | |
| Toothbrush tree | Streblus asper Lour. | |
| Mangosteen peel | Garcinia mangostana Linn. | |
| Active control group | Sodium bicarbonate | - |
| Sodium fluoride | - | |
| Corn mint oil | Mentha Arvensis | |
| Purple coneflower | Echinacea Purpurea | |
| Krameria root extract | Krameria Triandra | |
| Chamomile extract | Chamomilla Recutita | |
| Sage oil | Salvia officinalis | |
| Benchmark group | - | - |
| Number of Samples | Mean Age (years) ± SD |
|---|---|
| Test group (n = 22) | |
| 15 Males | 50.26 ± 7.24 |
| 7 Females | |
| Active control group (n = 13) | |
| 9 Males | 53.70 ± 8.98 |
| 4 Females | |
| Benchmark group (n = 19) | |
| 10 Males | 45.98 ± 6.67 |
| 9 Females | |
| Total n = 54 | 49.98 ± 7.63 |
| Percentage | Time | Test | Benchmark | Active Control | p-Value |
|---|---|---|---|---|---|
| PS | T0 | 44.66 ± 19.84 | 59.18 ± 22.93 | 55.99 ± 25.74 | 0.054 |
| T1 | 21.65 ± 11.27 | 21.55 ± 9.77 | 19.86 ± 7.48 | 0.118 | |
| T2 | 26.20 ± 11.60 | 25.95 ± 14.20 | 35.62 ± 18.03 | 0.169 | |
| BOP | T0 | 47.87 ± 25.40 | 45.81 ± 24.21 | 42.18 ± 25.69 | 0.726 |
| T1 | 17.52 ± 14.66 a,b | 21.03 ± 19.97 a | 13.83 ± 10.61 b | 0.000 | |
| T2 | 17.85 ± 13.47 | 29.13 ± 20.61 | 23.04 ± 18.93 | 0.223 |
| Mean (mm) | Time | Test Mean ± SD (mm) | Benchmark Mean ± SD (mm) | Active Control Mean ± SD (mm) |
|---|---|---|---|---|
| PD | T0 | 3.10 ± 0.44 *,** | 2.95 ± 0.42 #,## | 3.02 ± 0.54p,pp |
| T1 | 2.67 ± 0.48 * | 2.40 ± 0.40 # | 2.44 ± 0.40p | |
| T2 | 2.63 ± 0.41 ** | 2.41 ± 0.36 ## | 2.52 ± 0.31pp | |
| p-value | <0.001 | <0.001 | <0.002 | |
| CAL | T0 | 2.71 ± 1.08 *** | 2.63 ± 0.90 | 2.71 ± 0.70 |
| T1 | 2.30 ± 0.10 | 2.33 ± 0.96 | 2.55 ± 0.70 | |
| T2 | 2.43 ± 0.90 *** | 2.34 ± 0.88 | 2.62 ± 0.72 | |
| p-value | <0.050 | 0.211 | 0.758 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vajrabhaya, L.-o.; Benjasupattananan, S.; Sappayatosok, K.; Dechosilpa, V.; Korsuwannawong, S.; Sirikururat, P. Efficacy of a Herbal Toothpaste During Active Periodontal Treatment: A Clinical Study. Dent. J. 2024, 12, 378. https://doi.org/10.3390/dj12120378
Vajrabhaya L-o, Benjasupattananan S, Sappayatosok K, Dechosilpa V, Korsuwannawong S, Sirikururat P. Efficacy of a Herbal Toothpaste During Active Periodontal Treatment: A Clinical Study. Dentistry Journal. 2024; 12(12):378. https://doi.org/10.3390/dj12120378
Chicago/Turabian StyleVajrabhaya, La-ongthong, Supranee Benjasupattananan, Kraisorn Sappayatosok, Vittawin Dechosilpa, Suwanna Korsuwannawong, and Papatpong Sirikururat. 2024. "Efficacy of a Herbal Toothpaste During Active Periodontal Treatment: A Clinical Study" Dentistry Journal 12, no. 12: 378. https://doi.org/10.3390/dj12120378
APA StyleVajrabhaya, L.-o., Benjasupattananan, S., Sappayatosok, K., Dechosilpa, V., Korsuwannawong, S., & Sirikururat, P. (2024). Efficacy of a Herbal Toothpaste During Active Periodontal Treatment: A Clinical Study. Dentistry Journal, 12(12), 378. https://doi.org/10.3390/dj12120378






