1. Introduction
Accurate diagnosis of pulp and adjacent periapical tissues is essential for making informed therapeutic decisions [
1]. Currently, this diagnosis relies on evaluating clinical symptoms, conducting oral inspections, reviewing radiographs, and performing pulp ‘vitality’ tests. A correlation between the histopathological status of the pulp and clinical findings, both subjective and objective, can be drawn through a thorough evaluation of the patient’s health history, oral inspection, pulp tests, and radiographic results [
2]. However, diagnosing pulp conditions is challenging because conventional pulp vitality tests rely on nerve stimulation to reproduce the symptoms of various pulpal pathologies, which makes the process dependent on the patient’s subjective response [
3,
4] and the operator’s interpretation [
3]. These tests cannot detect pulpal blood flow [
3,
4], which should be the primary factor in assessing true pulp vitality [
5]. Therefore, decisions regarding pulp therapy should be based on objective assessments of blood flow rather than subjective nerve responses [
4]. Although conventional cold pulp testing (CPT) is the most commonly used method and is considered reliable [
6,
7], it cannot be regarded as the ‘ideal’ pulp test. It is invasive and often causes discomfort or pain, which can lead to false positive or false negative results, especially if neighboring oral structures are affected [
8]. Measuring pulpal blood circulation and oxygen supply would offer an objective way to differentiate between healthy and unhealthy pulp tissue. Laser Doppler flowmetry (LDF) [
4,
9,
10,
11], pulse oximetry (PO), oxygen saturation [
12,
13], and other non-traditional methods [
14] have been investigated to determine for their potential for accurately assessing pulp health status. These light transmission or reflection-based optical methods aim to measure pulpal blood flow and oxygen saturation, providing a quantifiable and non-invasive approach to assessing pulp vitality [
4,
11]. While vital pulp therapy (VPT) has become increasingly important due to advances in materials and research, there remain significant gaps in knowledge and variations in clinical practice, particularly concerning the need for better diagnostics based on stronger evidence [
15] to improve diagnostic outcomes and treatment success. A clinical assessment of the pulp tissue’s cellular structure without opening the tooth is not yet feasible, and despite the advancements of the last century, a reliable method for accurate pulp diagnosis remains unavailable in dentistry [
16].
Thus, this study aimed to investigate the diagnostic accuracy of a pulp vitality scanning device (OPS device) compared with clinically conventional and non-conventional pulp diagnostic tests and to generate further clinical data to improve the algorithm’s classification. The null hypothesis postulated that specificity and sensitivity are equal to or smaller than 90% and 80%, respectively.
2. Materials and Methods
This single-centered prospective study conducted at the Department of Periodontology and Operative Dentistry, Johannes Gutenberg University, Mainz, Germany, is consistent with the EN-ISO standard —Clinical investigation of medical devices for human subjects—Good Clinical Practice in the form at the time of submission to the ethics committee (
https://www.iso.org/standard/71690.html; last access 9 October 2024). It was approved by the independent ethics committee of the State Chamber of Physicians of Rhineland-Palatinate and German Federal Institute of Drugs and Medical Devices, Germany. All procedures were carried out in accordance with the relevant guidelines and regulations. Five certified and calibrated operators (Interdisciplinary Center for Clinical Trials, Mainz, Germany) were responsible for the clinical procedures. Operator calibration consisted of assessment and training regarding clinical conventional diagnostic parameters using the Optical Pulp Scanning (OPS/VDW GmbH, Munich, Germany) device, which was developed specifically for this study. The ethics committee also stipulated that the OPS-derived pulp status results should not influence the clinical treatment decisions. The pulp chambers were clinically inspected visually using 2.5-times magnification.
A total of 421 teeth from 107 emergency dental patients were included in this study. Inclusion criteria were teeth conventionally diagnosed (ConvDia) as having acute or chronic pulpitis, non-vital pulp, or periapical tissue showing a radiological pathological condition, or teeth that were symptomatically endodontically treated (EndTe). These teeth were classified as ‘emergency teeth’ (EmeTe). Additionally, mesially and distally located teeth were included as control teeth when feasible. An access cavity was prepared only for EmeTe requiring endodontic treatment based on ConvDia. Symptomatically treated EndTe were excluded from the EmeTe group. The study aimed to include at least 200 teeth diagnosed as non-vital through ConvDia, with a minimum of 100 being non-EndTe. Exclusion criteria were individuals who were not able to give informed consent, pregnant and nursing patients, those with primary dentition, those with a missing endodontic treatment indication, individuals unable to comply with the study protocol due to a medical, social, or psychological condition, and individuals who were financially or otherwise dependent on the research or sponsoring institution. The distinction between acute and chronic pulpitis was identified by means of pain symptoms. If there was pain, it was classified as acute pulpitis, and no pain was classified chronic pulpitis (often an incidental finding). In cases of acute pulpitis, the tooth could be either vital or non-vital, whereas chronic pulpitis always indicated a non-vital tooth.
The pulp health status was diagnosed using conventional pulp diagnostic (ConvDia) tests, cold pulp testing (CPT), and a “final” diagnosis (FinDia) according to the in-situ pulp chamber content (PCC) observations after endodontic access (
Table 1). CPT (−44 °C; Provotest; Hoechst-Pharma AG, Zurich, Switzerland) was performed with a foam pellet applied to the vestibular or palatal surface of the tooth. Uncertain CPT responses were repeated after 30 s. The FinDia findings either confirmed or revoked the ConvDia and were correspondingly electronically recorded. The electrical test (Vitality Scanner, Sybron Endo, Kerr Corporation, Gilbert, AZ, USA) was used when the cold test produced unclear results. Percussion testing, gingival status, and diagnostic radiographs were re-evaluated by an unbiased observer only when ConvDia or FinDia results did not align with those obtained from the Optical Pulp Scanning (OPS) device. EmeTe, EndTe, and control teeth were screened with the OPS device following CPT and ConvDia, and the results were recorded in a double-blinded manner (patient codes anonymized).
The OPS device’s working principle is based on transmitted light spectroscopy in a wavelength between 450 nm and 750 nm and a spectral response analysis. The OPS handpiece is provided by light-conductive fibers (Ø 0.2 mm) from an LED source to a spectrometer (
Figure 1). The probes were optically shielded with stainless-steel tubes and transparent single-use plastic sleeves to avoid cross-contamination. Through a specific embedded software, developed for this purpose, the OPS automatically adjusted the measuring time, depending on the tooth width and translucency. The spectral data were obtained within 10 to 6000 ms and recorded in an electrically unplugged notebook using a USB connection (
Figure 1).
A specific algorithm was developed to analyze the measured light transmission spectra in a wavelength range between 500 and 650 nm, thus allowing for the determination of the maximum absorption of hemoglobin in its oxygenated (λ = 541 nm and λ = 577 nm) and deoxygenated (λ = 555 nm) states, and other spectral features associated with the pulp status. Spectral features were extracted by differentiation (first and second derivative) and integration in determined wavelength areas. Four features were extracted from the respective spectral curve integration and five features were from the first and second derivatives. From those nine spectral features, eight combinations of two features each were combined in x–y diagrams (one feature on the x- and one on the y-axis, respectively). From a subset of the patient measurements with a known diagnosis, a range between the min and max values on both diagram axes was defined, which was typical for a vital pulp or a non-vital pulp. These value ranges on the y and x axes together defined rectangle “boxes” in the x–y diagram, with one box containing the most probable values for vital pulp and the other box for non-vital pulp, yet without an overlap of both boxes. Other areas in the diagrams were considered “undefined”. This was carried out for all eight combinations selected from the nine spectral features. If the data points were located in all eight diagrams within the vital box, then the probability for this measurement to be vital was considered as 1. If the data points were found within only, e.g., four vital boxes, the probability of being vital was considered as 0.5. Through comparison of the vital and non-vital probabilities, a decision was taken to determine it as either a vital, non-vital, or unclear pulp diagnosis. An unclear pulp diagnosis was defined when the probability of being vital and non-vital was approximately the same or when most data points were located outside the predefined rectangular areas.
The embedded software started a measurement by itself and recorded a successful measurement advised with a signal tone when the specific parameters (signal stability and intensity) were within the predefined range. During the OPS measurement, the operator’s voice including the anonymized patient record, tooth number being examined, and probe site placement was also recorded. The OPS conductive fiberglass probes were placed in the buccal and lingual aspects of the teeth at the gingival margin (G0), under a crown margin where this was existing (
Figure 1), to be able to avoid light conduction interferences during the measurements. A rather short training period was required to understand and master how the probes should effectively be placed for the anterior and posterior teeth. After completing the OPS measurements, a patient’s procedure comfort/discomfort was recorded according to the VAS scale. The spectral intensity signal was split into spectral regions and analyzed (Excel 16.40 Microsoft, Redmond, WA, USA) as follows:
by integrating the intensity within interesting spectral regions (e.g., peak oxygenated and non-oxygenated blood regions) and
by calculating the spectral absorption curves slope and turning points using differentiation within the various spectral regions.
The different integration and differentiation results were then fed into a classifier with predefined boundaries. The pulp/tooth status decision (vital, non-vital, or an EndTe) was derived from weighted values, which were calculated from the raw results inside or outside of areas separated by boundaries.
An access cavity was prepared after OPS measurement completion, and the FinDia was established after removing the pulp chamber roof of the EmeTe and its contents were visually inspected (
Table 1). EndTe underwent ConvDia and OPS measurement and they were protocoled correspondingly, being classified as negative with the CPT. The results obtained with the OPS device were defined as being of a vital, non-vital, not-EndTe, non-vital EndTe, or unknown (pulp health) status. The specificity, sensitivity, and positive and negative predictive values of the OPS device were calculated according to the results obtained with the CPT, ConvDia, and FinDia, which were defined as reference (gold) standards. Three research groups were created for purposes of comparison: ConvDia/OPS (all teeth and EmeTe), FinDia/OPS (EmeTe), and CPT/OPS (all teeth and EmeTe). For a possible generalization of the results, a post-hoc sample size calculation was carried out (
www.openepi.com; last access 7 October 2024) to confirm whether the analyzed sample was statistically significant [
17]. The null hypothesis postulated that specificity and sensitivity are equal to or smaller than 90% and 80%, respectively.
3. Results
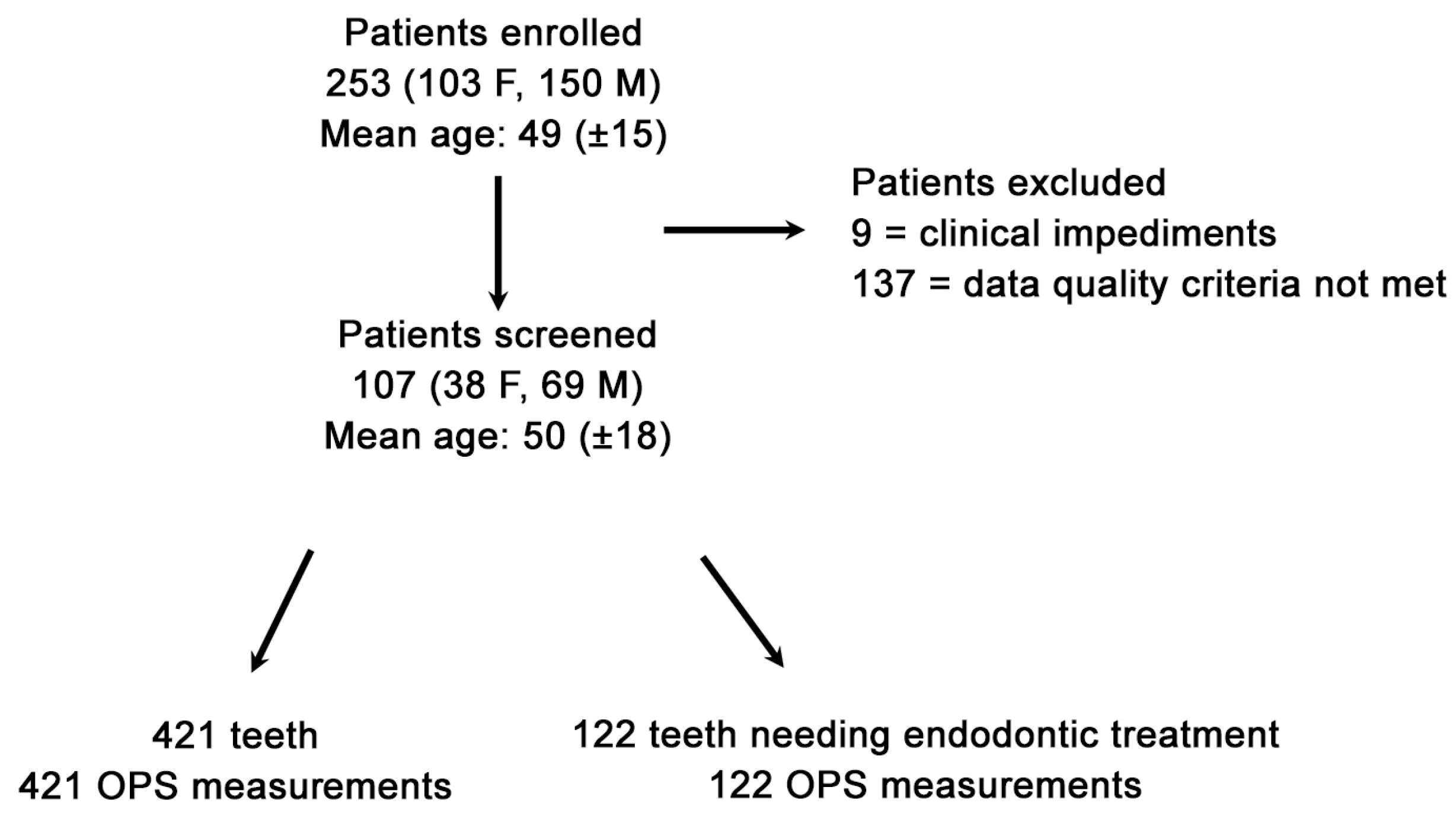
Due to the research’s stringent stipulated inclusion conditions, 146 out of 253 patients seeking dental emergency treatment were excluded, attributable to clinical impediments or Optical Pulp Scanning (OPS) device deficiencies (
Figure 1). The final validated datasets were 107 patients (Power = Φ(13.556) = 1 = 100% power analyses) with 543 OPS measurements made at the gingival line (G0). The specifically developed algorithm allowed the identification of lower-grade quality data that was impacted by any interference source (e.g., neighboring fluorescent light source fingerprints); thus, out of the validated datasets, 107 patients with a total of 421 OPS measurements (122 emergency teeth and 299 control teeth) who met the stringent inclusion criterion (
Figure 2) were analyzed. A total of 139 measurements were not included in the final analysis due to an OPS measurement error or device feedback. A complete patient OPS screening took, at most, five minutes and was performed immediately after the patient had been recruited and ConvDia had been carried out. As expected, after this point, no further dropouts occurred.
Due to the unexpected OPS low specificities and sensitivities of EndTe, and although the results were identifiable as such, they were classified and statistically analyzed as negative teeth in the corresponding group. The sensitivities, specificities, and positive and negative predictive OPS values were calculated assuming that the CPT, ConvDia, or FinDia were 100% correct. A summary of the results of all teeth divided into tooth-type groups according to the compared gold standard is given in
Table 2,
Table 3,
Table 4,
Table 5,
Table 6 and
Table 7. According to the VAS scale (0–10), 92 (86.0%) patients reported no discomfort (scale value = 0) and 6 (5.6%), 8 (7.5%), and 1 (0.9%) patients reported a relatively low discomfort level of 1, 2, and 3, respectively, according to the VAS scale, during the OPS procedure (n = 107). The mean VAS-scale response was 0.23%. The OPS diagnostic/screening procedure proved to be uncomplicated and rapid, and measurements under crown margins were feasible and effortless.