Upper Airway Dimensions among Different Skeletal Malocclusions: A Retrospective Observational Study by Cephalometric Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, Participants, Variables
- -
- ANB = 2° ± 2° → Class I,
- -
- ANB > 4° → Class II,
- -
- ANB < 0° → Class III.
- -
- SN-GoGn = 32° ± 5 → normodivergence,
- -
- SN-GoGn < 27° → hypodivergence,
- -
- SN-GoGn > 37° → hyperdivergence.
- -
- SNA > 84° and SNB > 82°.
- -
- SNA < 80° and SNB > 78°.
2.2. Data Measurement and Bias
2.3. Study Size
2.4. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Freitas, M.R.; Alcazar, N.M.; Janson, G.; de Freitas, K.M.; Henriques, J.F. Upper and lower pharyngeal airways in subjects with Class I and Class II malocclusions and different growth patterns. Am. J. Orthod. Dentofac. Orthop. 2006, 130, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.N.; Lacerda, R.H.; Silva, A.W.; Ramos, T.B. Assessment of upper airways measurements in patients with mandibular skeletal Class II malocclusion. Dent. Press J. Orthod. 2015, 20, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolska-Zarzycka, M.; Dunin-Wilczyńska, I.; Mitura, I.; Dąbała, M. Evaluation of upper airways depth among patients with skeletal Class I and III. Folia Morphol. 2013, 72, 155–160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Keim, R.G.; Vogels Iii, D.S.; Vogels, P.B. 2020 JCO Study of Orthodontic Diagnosis and Treatment Procedures Part 1: Results and Trends. J. Clin. Orthod. 2020, 54, 581–610. [Google Scholar] [PubMed]
- Zimmerman, J.N.; Vora, S.R.; Pliska, B.T. Reliability of upper airway assessment using CBCT. Eur. J. Orthod. 2019, 41, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Eslami, E.; Katz, E.S.; Baghdady, M.; Abramovitch, K.; Masoud, M.I. Are three-dimensional airway evaluations obtained through computed and cone-beam computed tomography scans predictable from lateral cephalograms? A systematic review of evidence. Angle Orthod. 2017, 87, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Moss-Salentijn, L.; Melvin, L. Moss and the functional matrix. J. Dent. Res. 1997, 76, 1814–1817. [Google Scholar] [CrossRef]
- Di Carlo, G.; Polimeni, A.; Melsen, B.; Cattaneo, P.M. The relationship between upper airways and craniofacial morphology studied in 3D. A CBCT study. Orthod. Craniofac. Res. 2015, 18, 1–11. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, X.; Huang, Y.; Mu, L.; Yang, L.; Zhao, M.; Xie, F.; Zhang, C.; Xu, J.; Lu, J.; et al. Craniofacial and Upper Airway Development in Patients with Treacher Collins Syndrome. J. Craniofac. Surg. 2021, 32, 2305–2309. [Google Scholar] [CrossRef]
- Chen, L.J.; Zhao, M.C.; Pan, X.F.; Wei, Y.Q.; Wang, D.Y. X-cephalometric study of different parts of the upper airway space and changes in hyoid position following mandibular fractures. West Indian Med. J. 2013, 62, 642–648. [Google Scholar]
- Ryu, H.H.; Kim, C.H.; Cheon, S.M.; Bae, W.Y.; Kim, S.H.; Koo, S.K.; Kim, M.S.; Kim, B.J. The usefulness of cephalometric measurement as a diagnostic tool for obstructive sleep apnea syndrome: A retrospective study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 119, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Ertugrul, B.Y. Evaluation of effects of removable functional orthodontic apparatus on the upper airway size by cephalometric films. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, e121–e125. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Savoldi, F.; Chan, E.Y.L.; Tse, C.S.K.; Lau, M.T.W.; Wey, M.C.; Hägg, U.; Yang, Y. Changes in the upper airway, hyoid bone and craniofacial morphology between patients treated with headgear activator and Herbst appliance: A retrospective study on lateral cephalometry. Orthod. Craniofac. Res. 2021, 24, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Chen, H.H.; Hsu, L.F.; Wang, S.H.; Chen, Y.J.; Lai, E.H.; Chang, J.Z.; Yao, C.J. Airway increase after open bite closure with temporary anchorage devices for intrusion of the upper posteriors: Evidence from 2D cephalometric measurements and 3D magnetic resonance imaging. J. Oral Rehabil. 2018, 45, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.N.; Yoon, H.J.; Park, J.H.; Park, Y.G.; Kim, S.J. Effect of extraction treatment on upper airway dimensions in patients with bimaxillary skeletal protrusion relative to their vertical skeletal pattern. Korean J. Orthod. 2021, 51, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Gonçales, E.S.; Rocha, J.F.; Gonçales, A.G.; Yaedú, R.Y.; Sant’Ana, E. Computerized cephalometric study of the pharyngeal airway space in patients submitted to orthognathic surgery. J. Maxillofac. Oral Surg. 2014, 13, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Engboonmeskul, T.; Leepong, N.; Chalidapongse, P. Effect of surgical mandibular setback on the occurrence of obstructive sleep apnea. J. Oral Biol. Craniofac. Res. 2020, 10, 597–602. [Google Scholar] [CrossRef]
- Bucci, R.; Montanaro, D.; Rongo, R.; Valletta, R.; Michelotti, A.; D’Antò, V. Effects of maxillary expansion on the upper airways: Evidence from systematic reviews and meta-analyses. J. Oral Rehabil. 2019, 46, 377–387. [Google Scholar] [CrossRef]
- Richard, A.R. The relation of maxillary structures to cranium in malocclusion and in normal occlusion. Angle Orthod. 1952, 22, 142–145. [Google Scholar]
- Steiner, C.C. Cephalometrics for you and me. Am. J. Orthod. 1953, 39, 729–755. [Google Scholar] [CrossRef]
- Sprenger, R.; Martins, L.A.C.; Dos Santos, J.C.B.; de Menezes, C.C.; Venezian, G.C.; Degan, V.V. A retrospective cephalometric study on upper airway spaces in different facial types. Prog. Orthod. 2017, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Solow, B.; Kreiborg, S. Soft-tissue stretching: A possible control factor in craniofacial morphogenesis. Scand. J. Dent. Res. 1977, 85, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Harvold, E.P.; Tomer, B.S.; Vargervik, K.; Chierici, G. Primate experiments on oral respiration. Am. J. Orthod. 1981, 79, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Warren, D.; Spalding, P. Dentofacial morphology and breathing: A century of controversy. In Current Controversies in Orthodontics; Melsen, B., Ed.; Quintessence Publishing: Chicago, IL, USA, 1991; pp. 45–76. [Google Scholar]
- Abu Allhaija, E.S.; Al-Khateeb, S.N. Uvulo-glosso-pharyngeal dimensions in different anteroposterior skeletal patterns. Angle Orthod. 2005, 75, 1012–1018. [Google Scholar] [PubMed]
- Shastri, D.; Tandon, P.; Nagar, A.; Singh, A. Cephalometric norms for the upper airway in a healthy North Indian population. Contemp. Clin. Dent. 2015, 6, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tang, H.; Liu, X.; Luo, Q.; Jiang, Z.; Martin, D.; Guo, J. Comparison of dimensions and volume of upper airway before and after mini-implant assisted rapid maxillary expansion. Angle Orthod. 2020, 90, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Koenig, L.J.; Pruszynski, J.E.; Bradley, T.G.; Bosio, J.A.; Liu, D. Dimensional changes of upper airway after rapid maxillary expansion: A prospective cone-beam computed tomography study. Am. J. Orthod. Dentofac. Orthop. 2013, 143, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Kavand, G.; Lagravère, M.; Kula, K.; Stewart, K.; Ghoneima, A. Retrospective CBCT analysis of airway volume changes after bone-borne vs tooth-borne rapid maxillary expansion. Angle Orthod. 2019, 89, 566–574. [Google Scholar] [CrossRef]
- Lanteri, V.; Farronato, M.; Ugolini, A.; Cossellu, G.; Gaffuri, F.; Parisi, F.M.R.; Cavagnetto, D.; Abate, A.; Maspero, C. Volumetric Changes in the Upper Airways after Rapid and Slow Maxillary Expansion in Growing Patients: A Case-Control Study. Materials 2020, 13, 2239. [Google Scholar] [CrossRef]
- Feng, X.; Lie, S.A.; Hellén-Halme, K.; Shi, X.Q. Effect of Rapid Maxillary Expansion on Upper Airway Morphology: A Retrospective Comparison of Normal Patients versus Patients with Enlarged Adenoid Tissue. J. Clin. Pediatr. Dent. 2021, 45, 208–215. [Google Scholar] [CrossRef]
- Galeotti, A.; Festa, P.; Viarani, V.; Pavone, M.; Sitzia, E.; Piga, S.; Cutrera, R.; De Vincentiis, G.C.; D’Antò, V. Correlation between cephalometric variables and obstructive sleep apnoea severity in children. Eur. J. Paediatr. Dent. 2019, 20, 43–47. [Google Scholar] [PubMed]
- Cornelis, M.A.; Cattaneo, P.M. Voies aérifères supérieures: Analyse tridimensionnelle et effets du traitement par appareils fonctionnels [Upper airways: Tridimensional analysis and effect of treatment by functional appliances]. Orthod. Fr. 2019, 90, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Caiado, G.M.; Evangelista, K.; Freire, M.D.C.M.; Almeida, F.T.; Pacheco-Pereira, C.; Flores-Mir, C.; Cevidanes, L.H.S.; Ruelas, A.C.O.; Vasconcelos, K.F.; Preda, F.; et al. Orthodontists’ criteria for prescribing cone-beam computed tomography-a multi-country survey. Clin. Oral Investig. 2022, 26, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
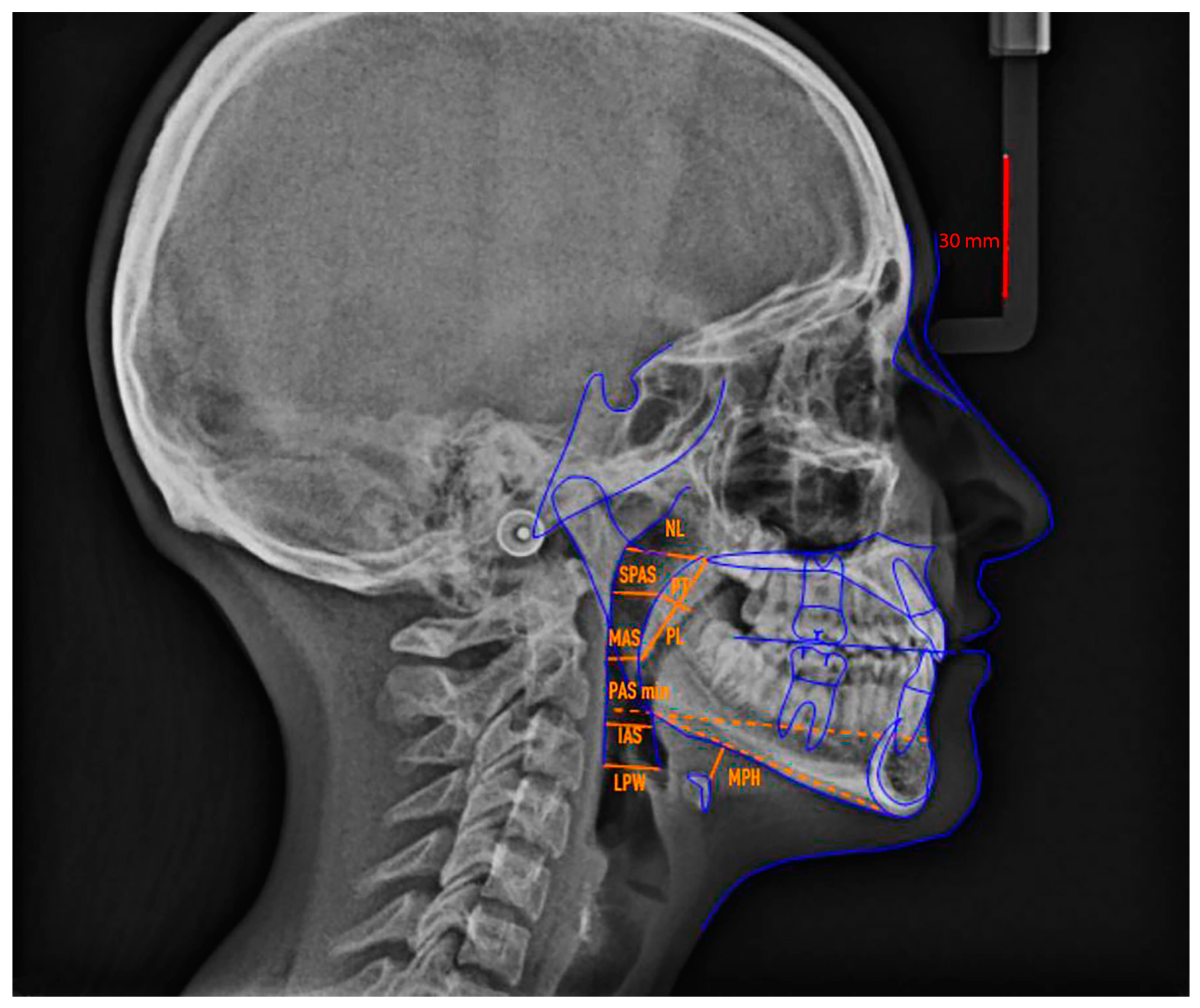
| Points and Measurements (Abbreviation) | Definition |
|---|---|
| Nasion (N) | Most anterior point on the frontonasal suture in the midsagittal plane |
| Sella (S) | Centre of the pituitary fossa of the sphenoid bone |
| Point A (A) | Deepest point of the curve of the anterior border of the maxilla |
| Point B (B) | Most posterior point in the concavity along the anterior border of the symphysis |
| Gonion (Go) | Point along the angle of the mandible, midway between the lower border of the mandible and the posterior border of the ascending ramus |
| Gnathion (Gn) | Most anterior and inferior points of the chin between the pogonion and menton |
| Basion (Ba) | Most anterior point of the foramen magnum |
| Anterior nasal spine (ANS) | Anterior tip of the sharp bony process of the maxilla at the lower margin of the anterior nasal aperture |
| Posterior nasal spine (PNS) | Posterior limit of the palatine bone |
| SNA | Angle between the sella, nasion and A point |
| SNB | Angle between the sella, nasion and B point |
| ANB | Angle between point A, the nasion and point B |
| SN | Plane between the sella and nasion |
| Sna-Snp | Bispinal plane |
| GoGn | Mandibular plane |
| Points and Measurements (Abbreviations) | Definition |
|---|---|
| Soft palate | |
| MPP | Middle point of the posterior wall of the soft palate |
| MPA | Middle point of the anterior wall of the soft palate |
| PT | Soft palate thickness |
| PL | Soft palate length |
| Nasopharynx | |
| Ad1 | Point at the intersection between the posterior pharyngeal wall and PNS-Ba line |
| NL (nasopharynx length) | Distance between Ad1 and PNS |
| PD (nasopharynx depth) | Line parallel to the bispinal plane and connecting PNS and the posterior margin of pharynx |
| Oropharynx | |
| SPAS (point) | Projection of the MPP point on the pharyngeal wall |
| SPAS | Superior Pharyngeal Airway Space: from half of the posterior border of the soft palate (MMP) perpendicular to the closest point on the posterior wall of the pharynx |
| U1 | Terminal part of the uvula |
| U2 | Projection of the U1 point on the pharyngeal posterior wall |
| MAS | Mean Airway Space: U1—U2 |
| T1 | Intersection of the tongue’s base and the line of point B and Go |
| T2 | T1 projection on the posterior wall of the pharynx along the parallel line Go-B |
| PAS min | Pharyngeal Airway Space minimum: T1—T2 distance along the Go-B parallel line |
| Ph1 | Intersection of the tongue’s base and vallecula of epiglottis |
| Ph2 | Ph1 projection on the posterior wall of the pharynx |
| IAS | Inferior Airway Space: from the superior margin of the epiglottis (Ph1) to the closest perpendicular point on the posterior wall of the pharynx |
| Hypopharynx | |
| Va1 | Base of the epiglottic vallecula |
| Va2 | Va1 projection on the posterior wall of pharynx |
| LPW | Lateral Pharyngeal Wall: distance between the epiglottic vallecula (Va1) and the posterior wall of pharynx perpendicular to Va1 |
| Hyoid bone | |
| H1 | The most cranial point of the body of the hyoid bone |
| H2 | Projection of the H1 point on the perpendicular line of the inferior margin of the jaw (Go—Gn) |
| MPH | Mandibular Plane—Hyoid bone: distance from the most anterior and superior point of the hyoid bone (H1) perpendicular to the mandibular plane |
| Angle Class I | Angle Class II | Angle Class III | ||||
|---|---|---|---|---|---|---|
| n | Age | n | Age | n | Age | |
| Female | 27 | 27.86 ± 8.29 | 31 | 27.05 ± 7.59 | 13 | 28.36 ± 9.47 |
| Male | 13 | 27.68 ± 8.03 | 9 | 27.29 ± 8.09 | 27 | 28.38 ± 9.18 |
| Class I | Class II | Class III | Biretruded (n = 24) | Normoposition (n = 70) | Biprotruded (n = 26) | Hypodivergent (n = 24) | Normodivergent (n = 70) | Hyperdivergent (n = 26) | |
|---|---|---|---|---|---|---|---|---|---|
| NL | 19.88 ± 3.30 A | 21.07 ± 3.49 A | 17.26 ± 3.37 B | 18.19 ± 4.17 A | 18.62 ± 3.86 A | 27.3 ± 37.29 A | 19.82 ± 3.23 A | 22.36 ± 23.92 A | 18.55 ± 3.66 A |
| PD | 21.06 ± 3.22 A | 21.97 ± 3.45 A | 18.41 ± 3.45 B | 18.86 ± 3.95 A | 19.73 ± 3.59 A | 21.08 ± 3.1 A | 20.96 ± 3.16 A | 20.55 ± 3.77 A | 19.72 ± 3.86 A |
| SPAS | 11.95 ± 2.36 A | 12.57 ± 2.96 A | 11.25 ± 3.07 A | 11.26 ± 1.8 A | 11.78 ± 2.94 A | 12.27 ± 2.58 A | 11.60 ± 2.49 A | 11.88 ± 2.73 A | 12.04 ± 2.89 A |
| MAS | 8.99 ± 2.37 A | 10.19 ± 2.98 A | 10.08 ± 3.06 A | 8.9 ± 2.07 A | 9.64 ± 2.86 A | 10.04 ± 2.72 A | 10.13 ± 2.4 A | 9.51 ± 2.70 A | 9.65 ± 2.67 A |
| PAS min | 11.11 ± 2.85 A | 11.41 ± 3.40 A | 11.19 ± 3.52 A | 9.64 ± 2.24 A | 11.07 ± 3.25 A,B | 11.97 ± 3.2 B | 11.90 ± 2.76 A | 10.81 ± 3.19 A | 11.33 ± 3.12 A |
| IAS | 10.73 ± 2.51 A | 10.97 ± 3.39 A | 10.30 ± 3.33 A | 9.31 ± 2.72 A | 10.53 ± 3.17 A | 11.32 ± 2.62 A | 11.41 ± 2.7 A | 10.44 ± 3.12 A | 10.02 ± 2.51 A |
| LPW | 14.02 ± 3.24 A | 13.89 ± 3.02 A | 12.58 ± 3.75 A | 12.5 ± 3.44 A | 13.20 ± 3.65 A | 13.98 ± 3.19 A | 14.43 ± 2.81 A | 13.23 ± 3.50 A | 12.89 ± 3.16 A |
| PL | 33.29 ± 4.33 A | 30.93 ± 5.43 A | 30.85 ± 5.80 A | 29.4 ± 4.74 A | 32.22 ± 6.08 A | 32.21 ± 4.6 A | 32.17 ± 4.76 A | 32.23 ± 5.66 A | 30.20 ± 4.15 A |
| PT | 9.38 ± 1.64 A | 9.37 ± 2.61 A | 9.71 ± 2.51 A | 8.54 ± 1.83 A | 9.37 ± 2.25 A,B | 10.19 ± 1.74 B | 10.26 ± 1.74 A | 9.71 ± 2.51 A | 9.21 ± 1.99 A |
| MPH | 12.81 ± 4.72 A | 13.18 ± 5.30 A | 14.64 ± 4.17 A | 12.09 ± 4.94 A | 14.20 ± 4.00 A | 13.71 ± 4.15 A | 13.98 ± 3.84 A | 13.65 ± 4.77 A | 12.66 ± 5.40 A |
| SNA | SNB | ANB | SN^GoGn | ANS-PNS | PL | PT | MPH | Age | Sex | |
|---|---|---|---|---|---|---|---|---|---|---|
| Nasopharynx | ||||||||||
| NL | 0.043 (0.023) | 0.0441 (0.021) | ||||||||
| PD | 0.0758 (0.002) | 0.1351 (0.001) | ||||||||
| Oropharynx | ||||||||||
| SPAS | 0.0509 (0.013) | |||||||||
| MAS | 0.0528 (0.012) | 0.0519 (0.012) | ||||||||
| IAS | 0.063 (0.012) | |||||||||
| PAS min | 0.0342 (0.043) | 0.038 (0.033) | ||||||||
| Hypopharynx | ||||||||||
| LPW | 0.0337 (0.034) | |||||||||
| Soft palate | ||||||||||
| PT | ||||||||||
| PL | 0.1808 (1.29 × 10−6) | |||||||||
| Hyoid bone | ||||||||||
| MPH |
| Class Divergence Sex | NL | PD | SPAS | MAS | PAS Min | IAS | LPW | MPH | PL | PT |
|---|---|---|---|---|---|---|---|---|---|---|
| Class I | / | / | 13.1 ± 2.6 | 9.7 ± 3.1 | / | 12.3 ± 4.4 | / | 18.2 ± 4.4 | 36.8 ± 4 | 7.4 ± 1.3 |
| Class II | / | / | 14 ± 3.8 | 10.1 ± 3.1 | / | 12.9 ± 3.9 | / | 15.8 ± 4.8 | 37 ± 4 | 7.4 ± 1.44 |
| Class III | / | / | 12.8 ± 4.4 | 10.6 ± 4.5 | / | 13.9 ± 4.6 | / | 18.8 ± 5 | 34 ± 9.3 | 7.3 ± 2.1 |
| Ipodivergence | / | / | 12.9 ± 2.74 | / | / | / | / | 18.95 ± 4.37 | 34.39 ± 8.42 | / |
| Normodivergence | / | / | 12.64 ± 2.3 | / | / | / | / | 15.97 ± 4.97 | 31.07 ± 4.16 | / |
| Iperdivergence | / | / | 10.64 ± 1.83 | / | / | / | / | 18.74 ± 5.53 | 33.12 ± 3.96 | / |
| Males | / | 27.9 ± 2.5 | / | 10.9 ± 2.8 | 11.1 ± 3.2 | / | 19.7 ± 2.6 | / | 38.3 ± 1.9 | 11.1 ± 1.4 |
| Female | / | 25.1 ± 1.3 | / | 10.1 ± 2.4 | 10.5 ± 2.8 | / | 16.5 ± 3.1 | / | 35.6 ± 1.7 | 9.5 ± 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sfondrini, M.F.; Gallo, S.; Pascadopoli, M.; Gandini, P.; Roncoroni, C.; Scribante, A. Upper Airway Dimensions among Different Skeletal Malocclusions: A Retrospective Observational Study by Cephalometric Analysis. Dent. J. 2024, 12, 12. https://doi.org/10.3390/dj12010012
Sfondrini MF, Gallo S, Pascadopoli M, Gandini P, Roncoroni C, Scribante A. Upper Airway Dimensions among Different Skeletal Malocclusions: A Retrospective Observational Study by Cephalometric Analysis. Dentistry Journal. 2024; 12(1):12. https://doi.org/10.3390/dj12010012
Chicago/Turabian StyleSfondrini, Maria Francesca, Simone Gallo, Maurizio Pascadopoli, Paola Gandini, Caterina Roncoroni, and Andrea Scribante. 2024. "Upper Airway Dimensions among Different Skeletal Malocclusions: A Retrospective Observational Study by Cephalometric Analysis" Dentistry Journal 12, no. 1: 12. https://doi.org/10.3390/dj12010012
APA StyleSfondrini, M. F., Gallo, S., Pascadopoli, M., Gandini, P., Roncoroni, C., & Scribante, A. (2024). Upper Airway Dimensions among Different Skeletal Malocclusions: A Retrospective Observational Study by Cephalometric Analysis. Dentistry Journal, 12(1), 12. https://doi.org/10.3390/dj12010012









