Abstract
This retrospective study addressed the role of oral potentially malignant disorders and the presence of intraepithelial Candida hyphae in the carcinogenesis of the oral tongue squamous cell carcinoma and its association with smoking, alcohol consumption, and oral inflammatory burden. The medical records of 183 subjects diagnosed with oral tongue squamous cell carcinoma at the Helsinki University Hospital were investigated. Preceding oral lichen planus, lichenoid reaction, and leukoplakia diagnosis were recorded. Further, the data on Candida hyphae in histological samples as an indicator of oral candidiasis, oral inflammatory burden, smoking, and alcohol consumption were recorded and analyzed. The histopathological diagnosis of oral lichen planus/lichenoid reaction (p < 0.001) and the presence of Candida hyphae (p = 0.005) were associated significantly with female gender. Oral lichen planus/lichenoid reaction patients were less often smokers than patients without these lesions. Candida hyphae were more often recorded in patients without alcohol use (p = 0.012). Oral lichen planus/lichenoid reaction and Candida hyphae in histological samples were associated with female gender and lower levels of typical risk factors, such as alcohol use and smoking, in oral tongue squamous cell carcinoma patients. Therefore, these patients should be well monitored despite a potential lack of the classical risk factors of oral carcinoma.
1. Introduction
Oral potentially malignant disorders (OPMDs) include leukoplakia, erythroplakia, erythroleukoplakia, proliferative verrucous leukoplakia, oral lichen planus, oral submucous fibrosis, palatal lesions in reverse smokers, smokeless tobacco keratosis, oral lupus erythematosus, actinic keratosis, dyskeratosis congenita, oral lichenoid lesion, oral graft versus host disease, syphilic glossitis, and chronic candidiasis [1,2,3,4]. OPMD conditions have an increased risk of progressing into malignancy, but the risk varies individually [5,6,7].
The composition of oral microbiota may be one factor contributing to the malignant transformation of premalignant lesions to oral squamous cell carcinoma (OSCC) [8,9,10,11]. In particular, changes in the relative abundance of certain oral microbes such as Candida species, Porphyromonas gingivalis, Fusobacterium nucleatum, and Streptococcus species have been shown to associate with OSCC [12].
Mucosal barrier abnormalities and the use of immunosuppressive medication, both typical for OLP, predispose to candidiasis [13,14]. Indeed, oral Candida infections are observed more commonly in patients with oral lichen planus (OLP) than patients without OLP [15,16]. Non-Candida albicans (C. albicans) species, particularly C. glabrata and C. krusei, have been found more often in patients with erosive OLP, while C. albicans has been more frequently observed in patients with non-erosive OLP [15,16,17].
Candida species and their biotypes are believed to have a role in oral carcinogenesis [15,18,19]. Candida isolates from oral tongue squamous cell carcinoma (OTSCC) patients have been shown to produce more virulence factors than isolates from non-OTSCC patients [20]. Candida proteases can activate latent proMMP-8 and promote tissue destruction [21]. The virulence factors of Candida vary according to the oral mucosal lesion type [20]. In erosive and non-erosive OLP patients, Candida phospholipase enzyme activity is higher than in the controls [17] and Candida species and biotypes differ between these OLP lesions as well as between homogeneous and non-homogeneous leukoplakia lesions [19]. Non-homogeneous leukoplakia is believed to develop more often into oral cancer than homogeneous leukoplakia [19,22]. C. albicans has been shown to have different biotypes in non-homogeneous leukoplakia, suggesting that it might play a role in carcinogenesis [19].
A correlation between the prevalence of Candida colonization of oral mucosal lesions and the severity of epithelial dysplasia has been reported [23,24]. It is unclear whether colonization is a consequence of oral epithelial changes or precedes them. The tongue lesions are more often infected with Candida species than lesions in other parts of the oral cavity [23,24]. OSCC patients are more frequently colonized with Candida species than controls [25]. Candida species from OSCC patients have a greater ability to form biofilm than those from non-OSCC patients [26]. In addition, Candida biofilms from OSCC patients produce more carcinogenic acetaldehyde and have higher metabolic activity than biofilms from non-oral cancer [26]. C. parapsilosis and C. albicans genotype B is significantly more common in non-oral cancer patients, whereas C. albicans genotype A is more frequently isolated in oral cancer patients, indicating that genotypic differences in Candida species may have a role in carcinogenesis [25].
C. albicans has been shown to produce carcinogens including nitrosamines, which can activate proto-oncogenes triggering carcinomatous changes [27]. Furthermore, candidal proteases in concert with proteases from oral dysbiotic periodontopathogens can activate host cell-derived latent pro matrix metalloproteases in tissue destruction cascades, which is important in the development and etiopathogenesis of oral malignancies [21,28,29].
Previous studies have shown that C. albicans isolates in potentially carcinogenic oral lesions can produce mutagenic levels of acetaldehydes and tissue destructive proteases [21]. Smoking and alcohol abuse may favor adaptive changes, resulting in the upregulation of Candida acetaldehyde metabolism [30]. Therefore, all Candida species may produce potentially carcinogenic amounts of acetaldehyde, especially in combination with smoking or alcohol consumption [26]. Acetaldehyde production is known to vary among species, and C. albicans, C. tropicalis, and C. parapsilosis produce more acetaldehyde than other species in the same genus [31].
Candida species can produce mutagenic amounts of acetaldehyde and tissue destructive proteases in mucosal lesions [21]. Furthermore, it is recognized that smoking and alcohol use may favor adaptive changes, resulting in re-regulation of Candida acetaldehyde metabolism and accelerated acetaldehyde production [26,30].
Oral polymicrobial interactions may further favor oral colonization and biofilm formation as well as modify inflammatory reactions by the Candida species [32]. With this background, the primary aim of this retrospective observational study was to clarify the role of intraepithelial Candida hyphae finding, indicative of Candida infection, in association with certain OPMD lesions in OTSCC carcinogenesis. The secondary objective was to evaluate the effect of dental health, alcohol consumption, and smoking as contributing factors—both independently and in conjunction with the primary factors. The nature of the study was retrospective, collecting information from the patient files and pathology reports.
2. Materials and Methods
2.1. Study Design
We studied retrospectively the medical records of 183 consecutive patients with OTSCC treated in 2016–2017 at the Department of Oral Maxillofacial Diseases, Helsinki University Hospital, Finland. The patients were identified from the hospital electronic medical records using the International Classification of Diseases, Tenth Revision (ICD-10) codes C01, C02.0, C02.1, C02.11, C02.2, and C02.3. All patients identified with these codes and confirmed to have OTSCC based on the medical records were included in the study. A lack of electronic medical records or electronic histopathological report was used as the exclusion criteria. No informed consent was required due to the retrospective nature of the study.
Information on OTSCC, concurrent systemic autoimmune diseases, immunosuppressive medications, alcohol consumption, smoking, clinical oral health status, and panoramic tomography findings were gathered from the medical records. Any previous diagnosis of oral leukoplakia, OLP, and lichenoid reaction (LR) and the presence of oral Candida hyphae were ascertained from histopathological reports. Histopathological samples were taken from some patients with OPMD years before oral cancer diagnoses and some patients’ first samples from cancer lesions. The follow-up time ranged from 11 years to 0 years.
All oral mucosal tissue samples had been processed with hematoxylin and eosin staining and were investigated further by Periodic acid-Schiff (PAS) staining in order to visualize Candida hyphae. We used the World Health Organization’s diagnostic criteria for the determination of LR and OLP [33,34]. Due to a lack of precise differential diagnostic information on clinical manifestation and the retrospective nature of the study, patients with LR and/or OLP were analyzed as one group. Accordingly, we could not evaluate the presence of possible clinical findings typical for candidiasis. Candida culture results were only available from very few patients. Therefore, we were unable to acquire all the necessary information to meet all the criteria for Candida infection [35]. Instead, the detection of intraepithelial Candida hyphae by PAS staining was used as the criterion of active candidiasis.
Dental health and oral infection burden were evaluated using the following parameters: Periodontal Inflammatory Burden Index (PIBI), describing the inflammatory load caused by periodontitis, Total Dental Index (TDI), describing the inflammatory load caused by oral infections, and Panoramic Tomography Index (PTI), additionally describing the inflammatory load by oral infections and the total number of teeth, reflecting long-term inflammatory load [36,37,38] (Table 1).

Table 1.
Classification of oral inflammatory burden. Oral inflammatory burden can be assessed by recording different indices, such as the PIBI, TDI, and PTI, as shown in the next tables [16,24].
2.2. Statistical Analysis
All statistical analyses were performed with the software package IBM SPSS for Macintosh (version 26.0, IBM Corp., Armonk, NY, USA). Categorical variables were cross-tabulated and analyzed with Pearson’s Chi-square test or Fisher’s exact test if expected values in cell frequencies were <5. For differences in continuous variables, Student’s t-test, Mann–Whitney U test, one-way analysis of variance, and Kruskal–Wallis H test were used. Pairwise comparisons were performed as post hoc analyses for Pearson’s Chi-square test using Z test and Dunn’s (1964) procedure for the Kruskal–Wallis H test, both with a Bonferroni correction for multiple comparisons. p-values < 0.05 were considered statistically significant throughout the study.
3. Results
3.1. Characteristics of OTSCC Patients
None of the patients needed to be excluded based on the exclusion criteria. Findings of, in total, 183 consecutive patients with OTSCC were included in the final study cohort. The age of the patients ranged from 23 to 91 years, and males (n = 102) were more numerous than females (n = 81). Anamnestic information was available for 133 patients about smoking status and 87 patients about alcohol consumption (Table 2).

Table 2.
Characteristics of study participants.
3.2. Differences in Male and Female Gender
Alcohol consumption was more prevalent among males than females (p = 0.028). Additionally, smoking was more common among males; however, the difference was not statistically significant. OLP and/or LR (p < 0.001) and intraepithelial Candida hyphae (p = 0.005) were significantly associated with the female gender. Males had higher PTI values than females (IQR 0–4 vs. 0–2, p = 0.036), but no significant differences existed between the sexes in TDI or PIBI values or number of teeth (Table 3).

Table 3.
Study variables stratified by gender.
3.3. OPMDs Association with Smoking and Alcohol Use
There was a significant association of the studied OPMDs (oral leukoplakia, OLP, and lichenoid reaction) both with smoking (p = 0.040) and alcohol use (p = 0.005). Patients with OLP/LR but not with leukoplakia were less often smokers than patients with neither OLP/LR nor leukoplakia. Patients with OLP/lichenoid reaction but not with leukoplakia and patients with leukoplakia but no OLP/LR were associated with abstinence from alcohol usage (p = 0.005) (Table 4).

Table 4.
Study variables stratified by presence of oral mucosal diseases.
3.4. Oral Candida Hyphae Associated with No Alcohol Use
Patients with histologically verified oral Candida hyphae (Figure 1) were significantly older than patients without (p = 0.003), with a medium effect size (Hedges’ g = 0.544). Oral Candida hyphae were more often observed in patients with no alcohol usage (p = 0.012) (Table 5).
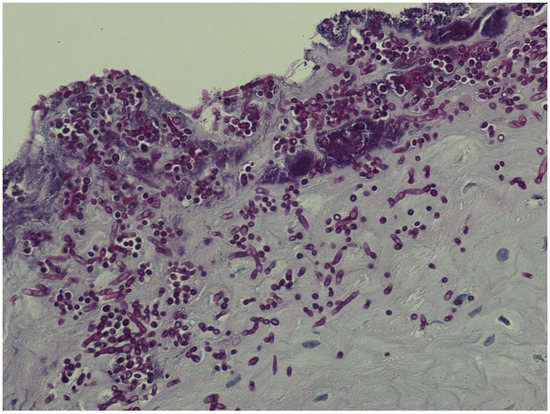
Figure 1.
Plenty of candida hyphae in oral epithelium.

Table 5.
Study variables stratified by presence of Candida by histopathologic examination (any species).
4. Discussion
The purpose of this study was to address the role of OPMDs and intraepithelial Candida hyphae, indicative of Candida infection, in OTSCC development and to evaluate the effect of smoking and oral infection disease burden on this process. To address the oral inflammatory burden of common oral infections, such as dental caries and periodontal disease, the number of teeth, PIBI, TDI, and PTI were utilized as described elsewhere [36,37,38].
In our study, females more often had a history of OLP/LR and oral Candida hyphae, and less often smoking or alcohol consumption. By contrast, leukoplakia was evenly distributed between the genders. The prevalence of autoimmune diseases or immunosuppressive medication did not show a difference between men and women.
Patients with OLP/LR and patients who developed cancer of the tongue had no significant association with underlying risk factors, such as smoking or alcohol use, while in the group of patients with no premalignant mucosal lesions smoking and alcohol use were more common. Presumably, the patients’ gender partly explains the associations between smoking and alcohol consumption, as females, non-smokers, and non-alcohol-using groups had more OLP/LR. The results underline the fact that OLP/LR obviously belong to the OPMD group of mucosal lesions.
The clinical or radiological oral health status indices PIBI and TDI were not associated with OPMDs or the Candida hyphae findings. However, males had higher PTI values than females, which is in accordance with the 2011 national survey on the oral health of Finnish citizens (Health 2011 Survey) [39].
We did not observe a significant association between oral Candida hyphae findings and OPMDs in OTSCC patients [26]. This might reflect the fact that OPMDs and Candida both take part in the malignant process independently.
Intraepithelial Candida hyphae were less common in patients with smoking or alcohol consumption, even though these are generally known to predispose to Candida [40]. The reason for this may be that in our study, oral Candida hyphae associated significantly with the female sex, and women were less often smokers and alcohol users. Therefore, the difference in prevalence of Candida hyphae may also be, at least in part, due to the gender. It might additionally reflect the fact that information about the smoking status or alcohol use may not always be registered properly in medical records. There is a potential risk of underreporting unhealthy habits to healthcare professionals. Moreover, smoking and alcohol consumption can alter the virulence factors of Candida species [30]. Future studies should investigate the impact of smoking and alcohol use on carcinogenesis in patients with OLP/LR and oral candidiasis.
The information on Candida species by culture was available only for very few patients and, therefore, the meaning of specific species for carcinogenesis could not be addressed in our study. Further prospective studies on the association of these factors and OTSCC are warranted. Additionally, the biotype, genotype, and virulence factors of Candida species in different forms of OLP and LR and whether they are relevant in OTSCC carcinogenesis should be studied.
Hyposalivation and poor oral or denture hygiene are well-known predisposing factors for oral candidiasis in addition to mucosal barrier abnormality, systemic immunosuppressive diseases and medications, antibiotics, and heavy smoking [13,14,41]. Due to the retrospective nature of our study, we did not obtain a comprehensive understanding of the frequency of dry mouth or the use of dentures in patients. Related to oral hygiene as a risk factor for carcinogenesis, Candida and polymicrobial oral biofilms have been reported to increase the expression of the cancer-associated inflammatory cytokines IL-6 and IL-8 in normal and cancer cells [32]. Further prospective studies should be conducted to compare the differences in oral and dental health in subjects with OPMD lesions with or without progression to cancer to clarify the role of oral infection burden in inflammatory mediators and related carcinogenesis.
Limitations of this study include the lack of a control group. Therefore, this study does not address the causality of findings to the development of OTSCC. Another limitation is the retrospective nature of the study because patient information in medical records is not always thoroughly and uniformly recorded. In addition, appropriate information for the differential diagnostics between OLP and LR was not available due to the retrospective nature of the research. The strengths of the study are that the patients are consecutive OTSCC patients, and the number of patients is sufficiently large for statistical analyses. Further studies should be performed with a larger OTSCC patient cohort in order to verify if the current findings are generalizable to a larger population. A prospective study would enable the comprehensive collection of Candida samples for species identification to provide more detailed insight into the mechanisms underlying the relation between Candida infection and OTSCC.
To conclude, OLP/LR and intraepithelial Candida hyphae were found to be associated with the female gender and less with typical OTSCC risk factors such as alcohol use and smoking. It is important to recognize that this group of patients is at risk for oral cancer development and to monitor these patients frequently enough to enable the early diagnosis of cancer.
Author Contributions
O.S., J.H., H.V. and H.R. conceptualization; O.S., J.F., J.H., T.S., V.R., T.T., H.V. and H.R. data curation; O.S., J.F. and V.R. formal analysis; H.R. funding acquisition; O.S., J.F., J.H., T.S., V.R., T.T., H.V. and H.R. investigation; O.S., J.F., J.H., T.S., V.R., H.V. and H.R. methodology; H.R. and H.V. project administration; H.R. resources; O.S., J.F. and V.R. software; H.R. and H.V. supervision; O.S., J.F., J.H., T.S., V.R., T.T., H.V. and H.R. validation; O.S., J.F., J.H., T.S., V.R., T.T., H.V. and H.R. visualization; O.S., H.V. and H.R. writing—original draft; O.S., J.F., J.H., T.S., V.R., T.T., H.V. and H.R. writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Helsinki University Central Hospital.
Institutional Review Board Statement
The study protocol was approved by the Helsinki and Uusimaa Hospital District Ethics Committee, Helsinki, Finland (DNRO HUS/996/2018) and conducted in adherence with the Declaration of Helsinki. This is a retrospective study and therefore no informed consent was required.
Informed Consent Statement
This is a retrospective study and therefore no written consent was required.
Data Availability Statement
The data supporting the results reported here are available from the corresponding author upon request.
Acknowledgments
Open access funding provided by University of Helsinki.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef]
- Reibel, J.; Gale, N.; Hille, J.; Hunt, J.L.; Lingen, M.; Muller, S.; Sloan, P.; Tilakaratne, W.M.; Westra, W.H.; Williams, M.D.; et al. Oral potentially malignant disorders and oral epithelial dysplasia. In WHO Classification of Head and Neck Tumours, 4th ed.; El-Naggar, A.K., Chan, J.K.C., Grandis, J.R., Takata, T., Slootweg, P.J., Eds.; IARC: Lyon, France, 2017; pp. 112–115. [Google Scholar]
- Kumari, P.; Debta, P.; Dixit, A. Oral Potentially Malignant Disorders: Etiology, Pathogenesis, and Transformation Into Oral Cancer. Front. Pharmacol. 2022, 13, 825266. [Google Scholar]
- Warnakulasuriya, S.; Johnson, N.W.; van der Waal, I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J. Oral Pathol. Med. 2007, 36, 575–580. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Oral potentially malignant disorders: A comprehensive review on clinical aspects and management. Oral Oncol. 2020, 102, 104550. [Google Scholar] [CrossRef] [PubMed]
- Speight, P.M.; Khurram, S.A.; Kujan, O. Oral potentially malignant disorders: Risk of progression to malignancy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 612–627. [Google Scholar] [CrossRef] [PubMed]
- Iocca, O.; Sollecito, T.P.; Alawi, F.; Weinstein, G.S.; Newman, J.G.; De Virgilio, A.; Di Maio, P.; Spriano, G.; Pardiñas López, S.; Shanti, R.M. Potentially malignant disorders of the oral cavity and oral dysplasia: A systematic review and meta-analysis of malignant transformation rate by subtype. Head Neck 2020, 42, 539–555. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Zheng, H.J.; Zhang, C.P. The Oral Microbiota May Have Influence on Oral Cancer. Front. Cell Infect. Microbiol. 2019, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hu, Y.; Zhou, X.; Liu, S.; Han, Q.; Cheng, L. Role of Oral Bacteria in the Development of Oral Squamous Cell Carcinoma. Cancers 2020, 12, 2797. [Google Scholar] [CrossRef]
- Stasiewicz, M.; Karpiński, T.M. The oral microbiota and its role in carcinogenesis. Semin. Cancer Biol. 2022, 86, 633–642. [Google Scholar]
- La Rosa, G.R.M.; Gattuso, G.; Pedullà, E.; Rapisarda, E.; Nicolosi, D.; Salmeri, M. Association of oral dysbiosis with oral cancer development. Oncol. Lett. 2020, 19, 3045–3058. [Google Scholar] [CrossRef]
- Vyhnalova, T.; Danek, Z.; Gachova, D.; Linhartova, P.B. The Role of the Oral Microbiota in the Etiopathogenesis of Oral Squamous Cell Carcinoma. Microorganisms 2021, 9, 1549. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.Y. Oral Candidosis: Pathophysiology and Best Practice for Diagnosis, Classification, and Successful Management. J. Fungi 2021, 7, 555. [Google Scholar] [CrossRef]
- Jainkittivong, A.; Kuvatanasuchati, J.; Pipattanagovit, P.; Sinheng, W. Candida in oral lichen planus patients undergoing topical steroid therapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 104, 61–66. [Google Scholar] [CrossRef]
- Arora, S.; Verma, M.; Gupta, S.R.; Urs, A.B.; Dhakad, M.S.; Kaur, R. Phenotypic variability and therapeutic implications of Candida species in patients with oral lichen planus. Biotech. Histochem. 2016, 91, 237–241. [Google Scholar] [CrossRef]
- Lundström, I.M.; Anneroth, G.B.; Holmberg, K. Candida in patients with oral lichen planus. Int. J. Oral Surg. 1984, 13, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Hou, X.; Wang, Z.; Jiang, L.; Xiong, C.; Zhou, M.; Chen, Q. Carriage rate and virulence attributes of oral Candida albicans isolates from patients with oral lichen planus: A study in an ethnic Chinese cohort. Mycoses 2009, 52, 161–165. [Google Scholar] [CrossRef]
- Bombeccari, G.P.; Giannì, A.B.; Spadari, F. Oral Candida colonization and oral lichen planus. Oral Dis. 2017, 23, 1009–1010. [Google Scholar] [CrossRef]
- Krogh, P.; Holmstrup, P.; Thorn, J.J.; Vedtofte, P.; Pindborg, J.J. Yeast species and biotypes associated with oral leukoplakia and lichen planus. Oral Surg. Oral Med. Oral Pathol. 1987, 63, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Castillo, G.D.V.; de Blanc, S.L.; Sotomayor, C.E.; Azcurra, A.I. Study of virulence factor of Candida species in oral lesions and its association with potentially malignant and malignant lesions. Arch. Oral Biol. 2018, 91, 35–41. [Google Scholar] [CrossRef]
- Pärnänen, P.; Sorsa, T.; Tervahartiala, T.; Nikula-Ijäs, P. Isolation, characterization and regulation of moonlighting proteases from Candida glabrata cell wall. Microb. Pathog. 2020, 149, 104547. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Ariyawardana, A. Malignant transformation of oral leukoplakia: A systematic review of observational studies. J. Oral Pathol. Med. 2016, 45, 155–166. [Google Scholar] [CrossRef]
- Barrett, A.W.; Kingsmill, V.J.; Speight, P.M. The frequency of fungal infection in biopsies of oral mucosal lesions. Oral Dis. 1998, 4, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Hebbar, P.B.; Pai, A.; Sujatha, D. Mycological and histological associations of Candida in oral mucosal lesions. J. Oral Sci. 2013, 55, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Alnuaimi, A.D.; Wiesenfeld, D.; O’Brien-Simpson, N.M.; Reynolds, E.C.; McCullough, M.J. Oral Candida colonization in oral cancer patients and its relationship with traditional risk factors of oral cancer: A matched case-control study. Oral Oncol. 2015, 51, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Alnuaimi, A.D.; Ramdzan, A.N.; Wiesenfeld, D.; O’Brien-Simpson, N.M.; Kolev, S.D.; Reynolds, E.C.; McCullough, M.J. Candida virulence and ethanol-derived acetaldehyde production in oral cancer and non-cancer subjects. Oral Dis. 2016, 22, 805–814. [Google Scholar] [CrossRef]
- Liao, M.; Cheng, L.; Zhou, X.D.; Ren, B. Research progress of Candida albicans on malignant transformation of oral mucosal diseases. Hua Xi Kou Qiang Yi Xue Za Zhi Huaxi Kouqiang Yixue Zazhi West China J. Stomatol. 2020, 38, 431–437. [Google Scholar]
- Nieminen, M.T.; Listyarifah, D.; Hagström, J.; Haglund, C.; Grenier, D.; Nordström, D.; Uitto, V.J.; Hernandez, M.; Yucel-Lindberg, T.; Tervahartiala, T.; et al. Treponema denticola chymotrypsin-like proteinase may contribute to orodigestive carcinogenesis through immunomodulation. Br. J. Cancer 2018, 118, 428–434. [Google Scholar] [CrossRef]
- Sorsa, T.; Ingman, T.; Suomalainen, K.; Haapasalo, M.; Konttinen, Y.T.; Lindy, O.; Saari, H.; Uitto, V.J. Identification of proteases from periodontopathogenic bacteria as activators of latent human neutrophil and fibroblast-type interstitial collagenases. Infect. Immun. 1992, 60, 4491–4495. [Google Scholar] [CrossRef]
- Gainza-Cirauqui, M.L.; Nieminen, M.T.; Novak Frazer, L.; Aguirre-Urizar, J.M.; Moragues, M.D.; Rautemaa, R. Production of carcinogenic acetaldehyde by Candida albicans from patients with potentially malignant oral mucosal disorders. J. Oral Pathol. Med. 2013, 42, 243–249. [Google Scholar] [CrossRef]
- Nieminen, M.T.; Uittamo, J.; Salaspuro, M.; Rautemaa, R. Acetaldehyde production from ethanol and glucose by non-Candida albicans yeasts in vitro. Oral Oncol. 2009, 45, e245–e248. [Google Scholar] [CrossRef]
- Arzmi, M.H.; Cirillo, N.; Lenzo, J.C.; Catmull, D.V.; O’Brien-Simpson, N.; Reynolds, E.C.; Dashper, S.; McCullough, M. Monospecies and polymicrobial biofilms differentially regulate the phenotype of genotype-specific oral cancer cells. Carcinogenesis 2019, 40, 184–193. [Google Scholar] [CrossRef] [PubMed]
- van der Meij, E.H.; Mast, H.; van der Waal, I. The possible premalignant character of oral lichen planus and oral lichenoid lesions: A prospective five-year follow-up study of 192 patients. Oral Oncol. 2007, 43, 742–748. [Google Scholar] [CrossRef] [PubMed]
- van der Meij, E.H.; van der Waal, I. Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J. Oral Pathol. Med. 2003, 32, 507–512. [Google Scholar] [CrossRef]
- Coronado-Castellote, L.; Jiménez-Soriano, Y. Clinical and microbiological diagnosis of oral candidiasis. J. Clin. Exp. Dent. 2013, 5, e279–e286. [Google Scholar] [CrossRef] [PubMed]
- Mattila, K.J.; Nieminen, M.S.; Valtonen, V.V.; Rasi, V.P.; Kesäniemi, Y.A.; Syrjälä, S.L.; Jungell, P.S.; Isoluoma, M.; Hietaniemi, K.; Jokinen, M.J. Association between dental health and acute myocardial infarction. BMJ 1989, 298, 779–781. [Google Scholar] [CrossRef]
- Ruokonen, H.; Nylund, K.; Furuholm, J.; Meurman, J.H.; Sorsa, T.; Kotaniemi, K.; Ortiz, F.; Heikkinen, A.M. Oral Health and Mortality in Patients with Chronic Kidney Disease. J. Periodontol. 2017, 88, 26–33. [Google Scholar] [CrossRef]
- Lahdentausta, L.S.J.; Paju, S.; Mäntylä, P.; Buhlin, K.; Tervahartiala, T.; Pietiäinen, M.; Alfthan, H.; Nieminen, M.S.; Sinisalo, J.; Sorsa, T.; et al. Saliva and serum biomarkers in periodontitis and coronary artery disease. J. Clin. Periodontol. 2018, 45, 1045–1055. [Google Scholar] [CrossRef]
- Suominen, M.; Vehkalahti, M.; Knuuttila, M. Suunterveys. In Terveys, Toimintakyky ja Hyvinvointi Suomessa 2011; Koskinen, S., Lundqvist, A., Ristiluoma, N., Eds.; Terveyden Ja Hyvinvoinnin Laitos, Report 68_2012; 2012; p. 102-7. ISBN 978-952-245-769-1. Available online: http://www.julkari.fi/handle/10024/90832 (accessed on 11 March 2023).
- Soysa, N.S.; Ellepola, A.N.B. The impact of cigarette/tobacco smoking on oral candidosis: An overview. Oral. Dis. 2005, 11, 268–273. [Google Scholar] [CrossRef]
- Muzurovic, S.; Babajic, E.; Masic, T.; Smajic, R.; Selmanagic, A. The relationship between oral hygiene and oral colonisation with Candida species. Med. Arch. Sarajevo Bosnia Herzeg. 2012, 66, 415–417. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).