In Vitro Study of the Biological and Physical Properties of Dual-Cure Resin-Modified Calcium Silicate-Based Cement
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Properties
2.1.1. HDPC Culture Preparation
2.1.2. Cell Viability Assay
2.1.3. Odontogenic Differentiation of the hDPCs
2.1.4. Antibacterial Effect
2.2. Physical Properties
2.2.1. Shear Bond Strength Test
2.2.2. Vickers Microhardness Test
2.3. Statistical Analysis
3. Results
3.1. Biological Properties
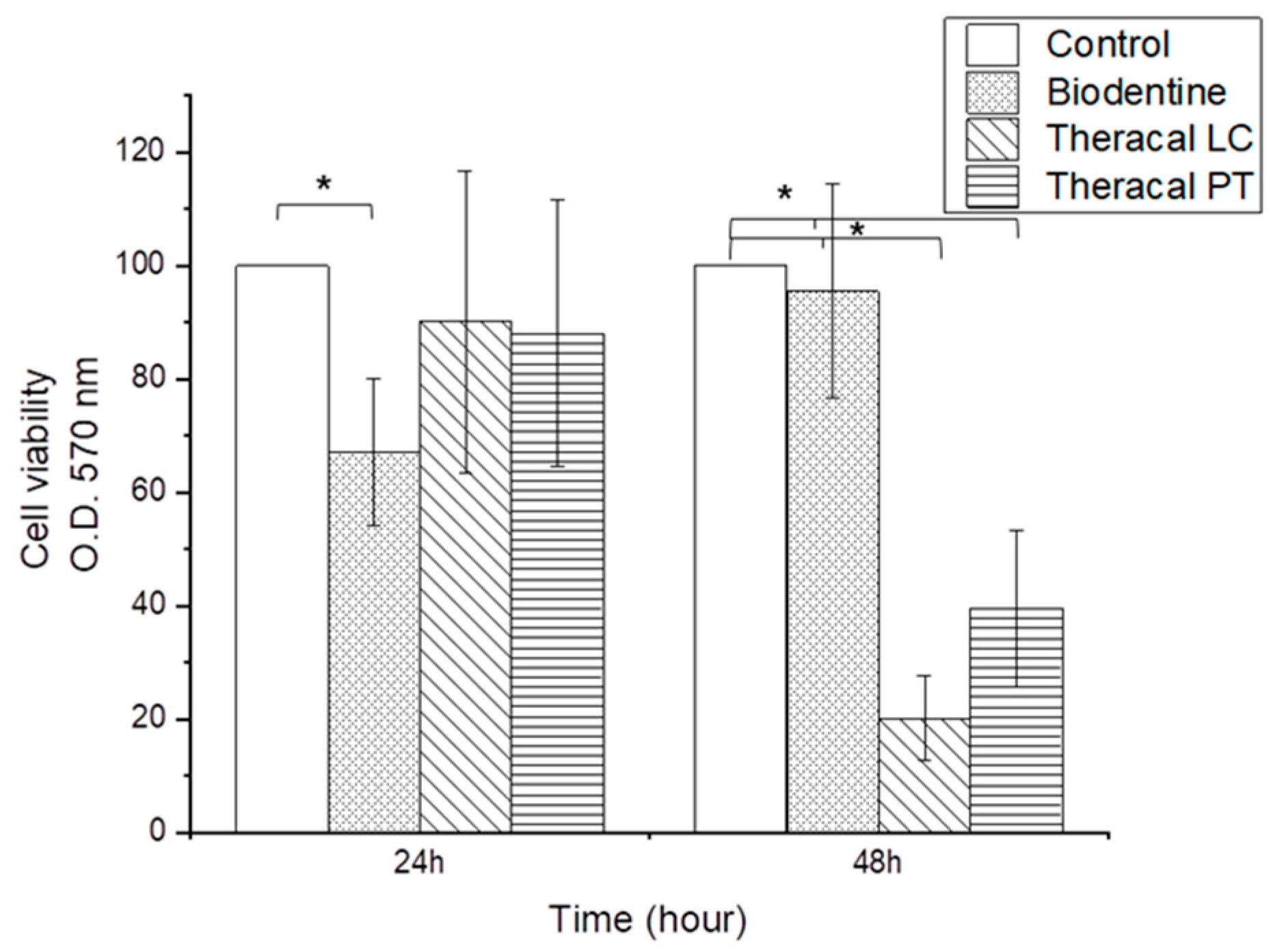
3.1.1. Cell Viability Assay
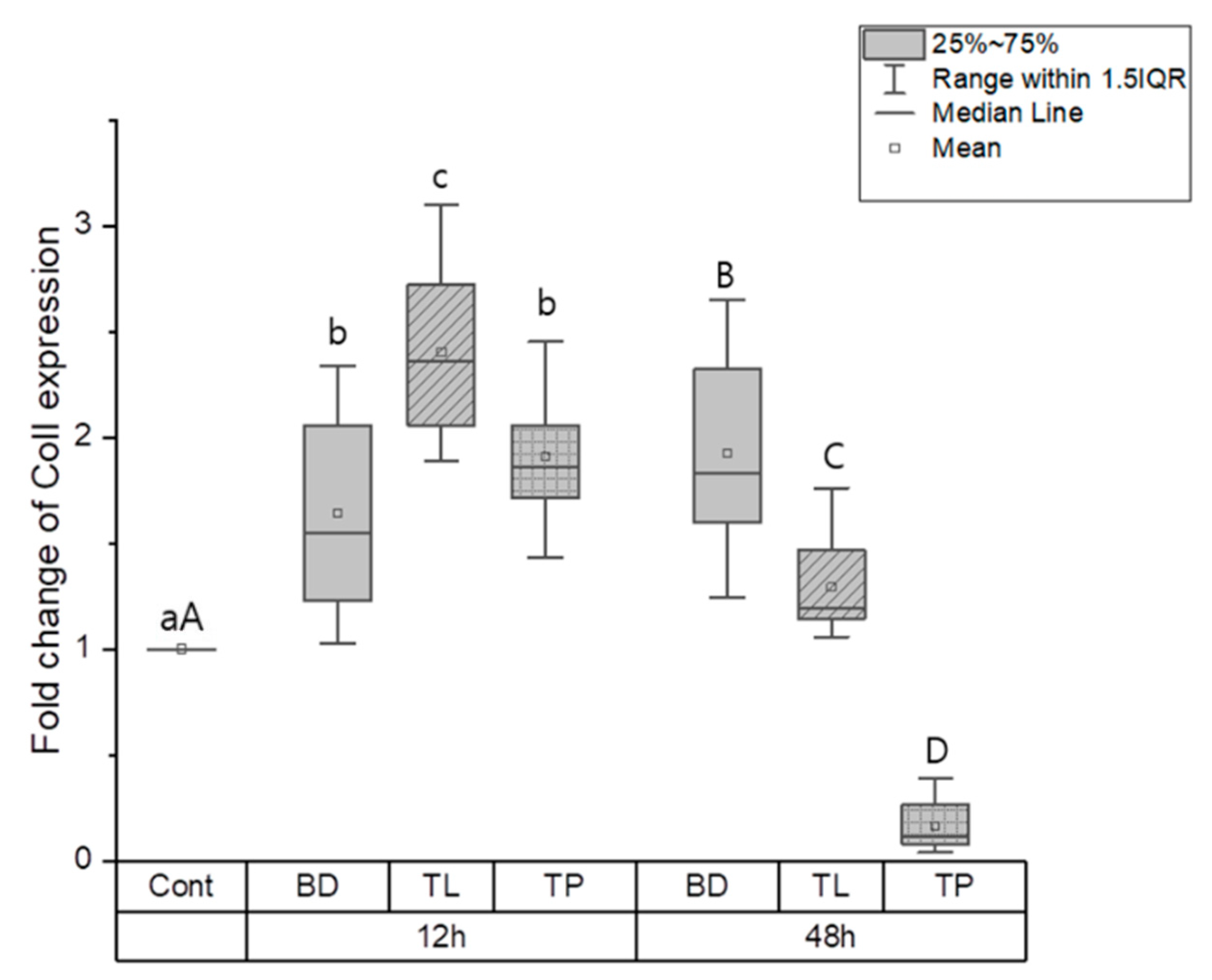
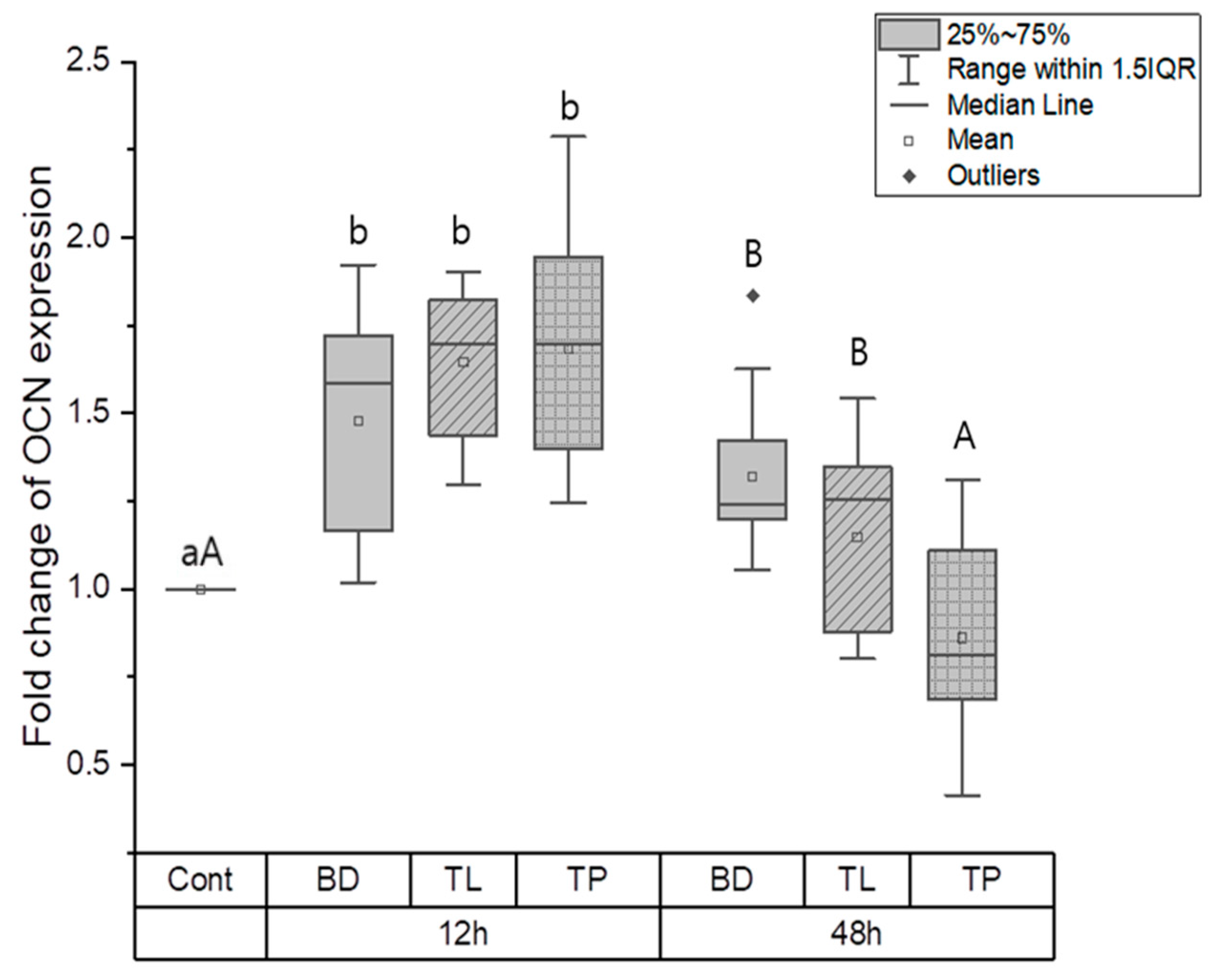
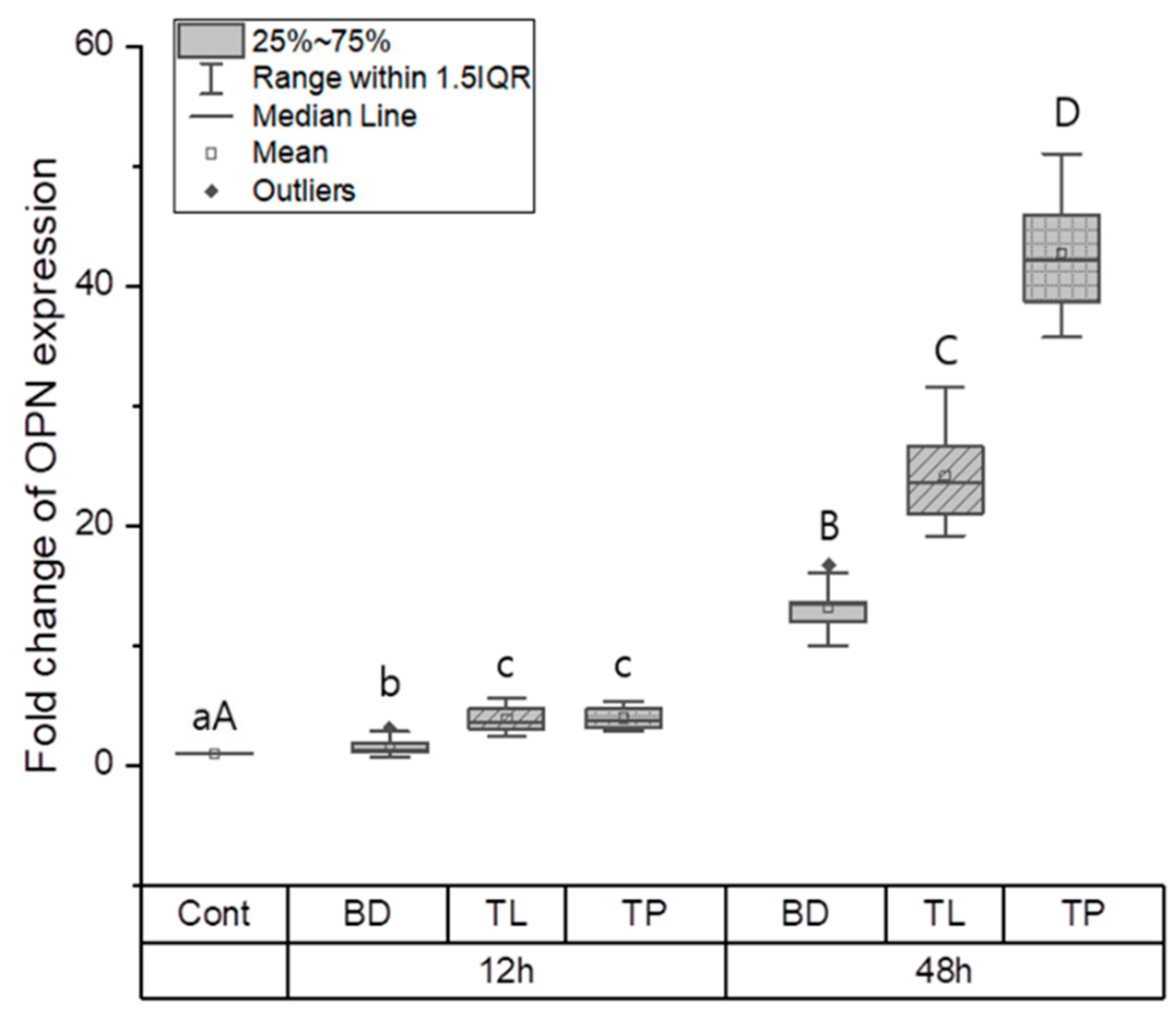
3.1.2. Odontogenic Differentiation
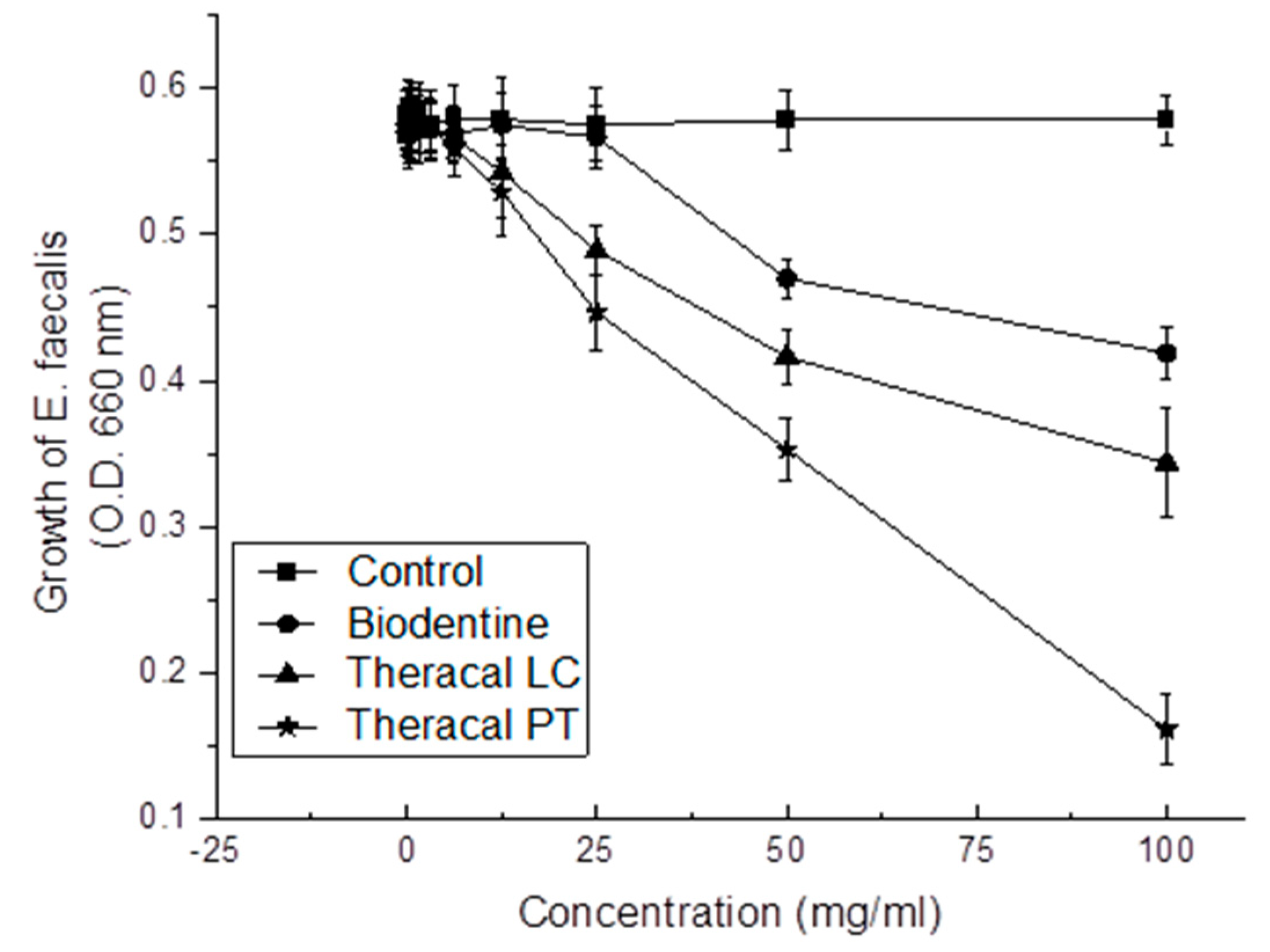
3.1.3. Antibacterial Effect
3.2. Physical Properties
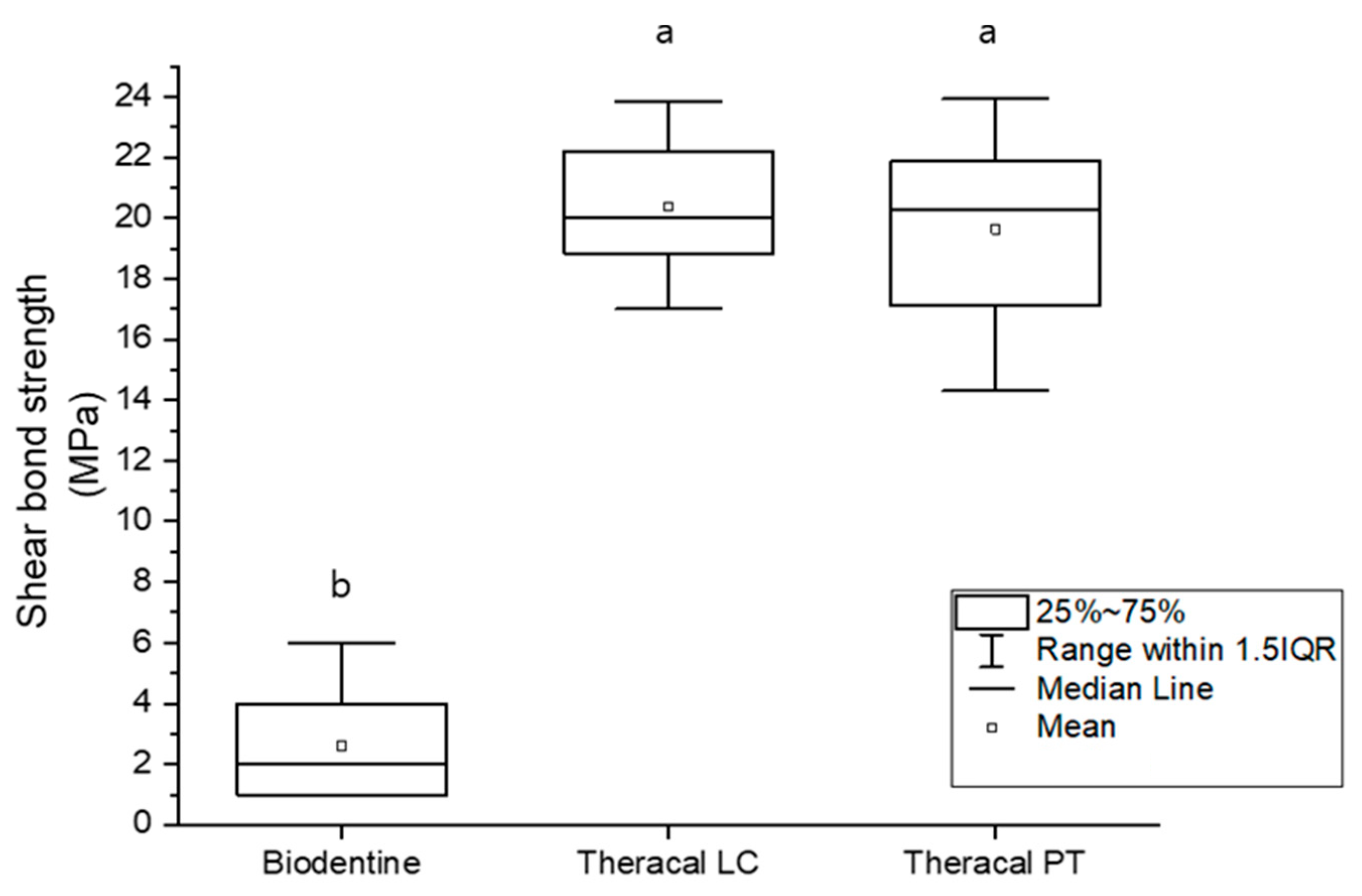
3.2.1. Shear Bond Strength
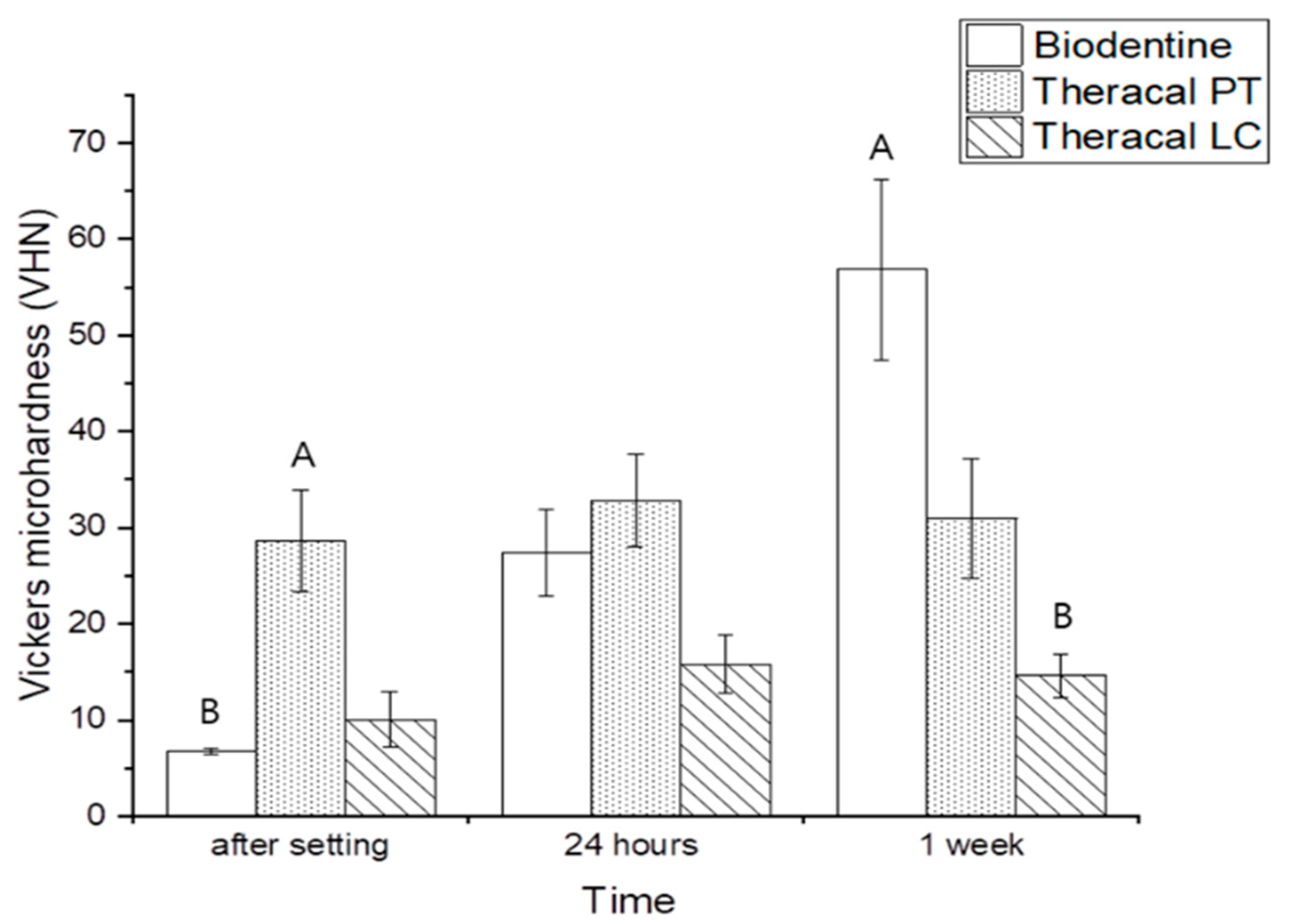
3.2.2. Vickers Microhardness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodriguez-Lozano, F.J.; Lopez-Garcia, S.; Garcia-Bernal, D.; Sanz, J.L.; Lozano, A.; Pecci-Lloret, M.P.; Melo, M.; López-Ginés, C.; Forner, L. Cytocompatibility and bioactive properties of the new dual-curing resin-modified calcium silicate-based material for vital pulp therapy. Clin. Oral Investig. 2021, 25, 5009–5024. [Google Scholar] [CrossRef] [PubMed]
- Collado-González, M.; García-Bernal, D.; Oñate-Sánchez, R.E.; Ortolani-Seltenerich, P.S.; Álvarez-Muro, T.; Lozano, A.; Forner, L.; Llena, C.; Moraleda, J.M.; Rodríguez-Lozano, F.J. Cytotoxicity and bioactivity of various pulpotomy materials on stem cells from human exfoliated primary teeth. Int. Endod. J. 2017, 50, e19–e30. [Google Scholar] [CrossRef] [PubMed]
- Leye, B.F.; Gaye, N.F.; Kane, A.W.; Benoist, H.M.; Farge, P. Evaluation of mineral trioxide aggregate (MTA) versus calcium hydroxide cement (Dycal®) in the formation of a dentine bridge: A randomised controlled trial. Int. Dent. J. 2012, 62, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Poggio, C.; Ceci, M.; Dagna, A.; Beltrami, R.; Colombo, M. In vitro cytotoxicity evaluation of different pulp capping materials: A comparative study. Arh. Za Hig. Rada I Toksikol. 2015, 66, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Alzraikat, H.; Taha, N.A.; Qasrawi, D.; Burrow, M.F. Shear bond strength of a novel light cured calcium silicate based-cement to resin composite using different adhesive systems. Dent. Mater. J. 2016, 35, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Suh, B.I. Cytotoxicity and biocompatibility of resin-free and resin-modified direct pulp capping materials: A state-of-the-art review. Dent. Mater. J. 2017, 36, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fridland, M.; Rosado, R. MTA solubility: A long term study. J. Endod. 2005, 31, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Alzraikat, H.; Taha, N.A.; Salameh, A. A comparison of Physical and Mechanical Properties of Biodentine and Mineral Trioxide Aggregate. J. Res. Med. Dent. Sci. 2016, 4, 121–126. [Google Scholar] [CrossRef]
- Harms, C.S.; Schäfer, E.; Dammaschke, T. Clinical evaluation of direct pulp capping using a calcium silicate cement—Treatment outcomes over an average period of 2.3 years. Clin. Oral Investig. 2019, 23, 3491–3499. [Google Scholar] [CrossRef] [PubMed]
- Dawood, A.E.; Parashos, P.; Wong, R.H.K.; Reynolds, E.C.; Manton, D.J. Calcium silicate-based cements: Composition, properties, and clinical applications. J. Investig. Clin. Dent. 2017, 8, e12195. [Google Scholar] [CrossRef]
- Deepa, V.L.; Dhamaraju, B.; Bollus, I.P.; Balaji, T.S. Shear bond strength evaluation of resin composite bonded to three different liners: TheraCal LC, Biodentine, and resin-modified glass ionomer cement using universal adhesive: An in vitro study. J. Conserv. Dent. 2016, 19, 166. [Google Scholar] [CrossRef] [PubMed]
- Jeanneau, C.; Laurent, P.; Rombouts, C.; Giraud, T.; About, I. Light-cured tricalcium silicate toxicity to the dental pulp. J. Endod. 2017, 43, 2074–2080. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiar, H.; Nekoofar, M.H.; Aminishakib, P.; Abedi, F.; Moosavi, F.N.; Esnaashari, E.; Azizi, A.; Esmailian, S.; Ellini, M.R.; Mesgarzadeh, V. Human pulp responses to partial pulpotomy treatment with TheraCal as compared with Biodentine and ProRoot MTA: A clinical trial. J. Endod. 2017, 43, 1786–1791. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.L.; Soler-Doria, A.; López-García, S.; García-Bernal, D.; Rodríguez-Lozano, F.J.; Lozano, A.; Llena, C.; Forner, L.; Guerrero-Gironés, J.; Melo, M. Comparative Biological Properties and Mineralization Potential of 3 Endodontic Materials for Vital Pulp Therapy: Theracal PT, Theracal LC, and Biodentine on Human Dental Pulp Stem Cells. J. Endod. 2021, 47, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Wayne, P.A. Clinical and Laboratory Standards Institute: Performance Standards for Antimicrobial Susceptibility Testing: 20th Informational Supplement Document M100-S20; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2016. [Google Scholar]
- Wang, Z.; Ma, J.; Shen, Y.; Haapasalo, M. Acidic pH weakens the microhardness and microstructure of three tricalcium silicate materials. Int. Endod. J. 2015, 48, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Nekoofar, M.H.; Oloomi, K.; Sheykhrezae, M.S.; Tabor, R.; Stone, D.F.; Dummer, P.M.H. An evaluation of the effect of blood and human serum on the surface microhardness and surface microstructure of mineral trioxide aggregate. Int. Endod. J. 2010, 43, 849–858. [Google Scholar] [CrossRef]
- Lang, O.; Kohidai, L.; Kohidai, Z.; Dobo-Nagy, C.; Csomo, K.B.; Lajko, M.; Mozes, M.; Keki, S.; Deak, G.; Tian, K.V.; et al. Cell physiological effects of glass ionomer cements on fibroblast cells. Toxicol. In Vitro 2019, 61, 104627. [Google Scholar] [CrossRef] [PubMed]
- Pedano, M.S.; Li, X.; Li, S.; Sun, Z.; Cokic, S.M.; Putzeys, E.; Yoshihara, K.; Yoshida, Y.; Chen, Z.; Van Landuyt, K. Freshly-mixed and setting calcium-silicate cements stimulate human dental pulp cells. Dent. Mater. 2018, 34, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Pedano, M.S.; Li, X.; Yoshihara, K.; Landuyt, K.V.; Van Meerbeek, B. Cytotoxicity and bioactivity of dental pulp-capping agents towards human tooth-pulp cells: A systematic review of in-vitro studies and meta-analysis of randomized and controlled clinical trials. Materials 2020, 13, 2670. [Google Scholar] [CrossRef]
- Peters, O.A. Research that matters–Biocompatibility and cytotoxicity screening. Int. Endod. J. 2013, 46, 195–197. [Google Scholar] [CrossRef]
- Babich, H.; Sinensky, M.C. Indirect cytotoxicity of dental materials: A study with Transwell inserts and the neutral red uptake assay. Altern. Lab. Anim. 2001, 29, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.; Montesin, F.; Di Silvio, L.; Pitt Ford, T.R. The chemical constitution and biocompatibility of accelerated Portland cement for endodontic use. Int. Endod. J. 2005, 38, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Adıgüzel, M.; Ahmetoğlu, F.; Eldeniz, A.Ü.; Tekin, M.G.; Göğebakan, B. Comparison of cytotoxic effects of calcium silicate-based materials on human pulp fibroblasts Mehmet. J. Dent. Res. Dent. Clin. Dent. Prospect. 2019, 13, 241. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, Y. Depth-dependence of Degree of Conversion and Microhardness for Dual-cure and Light-cure Composites. Oper. Dent. 2020, 45, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Ling, J. Expression of mineralization markers in dental pulp cells. J. Endod. 2007, 33, 703–708. [Google Scholar] [CrossRef]
- Tsukamoto, Y.; Fukutani, S.; Shin-Ike, T.; Kubota, T.; Sato, S.; Suzuki, Y.; Mori, M. Mineralized nodule formation by cultures of human dental pulp-derived fibroblasts. Arch. Oral Biol. 1992, 37, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- About, I.; Bottero, M.-J.; de Denato, P.; Camps, J.; Franquin, J.-C.; Mitsiadis, T.A. Human dentin production In Vitro. Exp. Cell Res. 2000, 258, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Daltoé, M.O.; Paula-Silva, F.W.G.; Faccioli, L.H.; Gatón-Hernández, P.M.; De Rossi, A.; Silva, L.A.B. Expression of mineralization markers during pulp response to biodentine and mineral trioxide aggregate. J. Endod. 2016, 42, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Papagerakis, P.; Berdal, A.; Mesbah, M.; Peuchmaur, M.; Malaval, L.; Nydegger, J.; Simmer, J.; Macdougall, M. Investigation of osteocalcin, osteonectin, and dentin sialophosphoprotein in developing human teeth. Bone 2002, 30, 377–385. [Google Scholar] [CrossRef]
- Güven, E.; Taşlı, P.; Yalvac, M.E.; Sofiev, N.; Kayahan, M.B.; Sahin, F. In vitro comparison of induction capacity and biomineralization ability of mineral trioxide aggregate and a bioceramic root canal sealer. Int. Endod. J. 2013, 46, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Rosa, V.; Zhang, Z.; Grande, R.H.M.; Nör, J.E. Dental pulp tissue engineering in full-length human root canals. J. Dent. Res. 2013, 92, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, E.; Rajasekharan, S.; Chitturi, R.T.; Declercq, H.; Martens, L.; De Coster, P. Gene expression profiling and molecular signaling of various cells in response to tricalcium silicate cements: A systematic review. J. Endod. 2016, 42, 1713–1725. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Park, H.C.; Zhu, T.; Yang, H.-C. Inhibition of odontogenic differentiation of human dental pulp cells by dental resin monomers. Biomater. Res. 2015, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Nakatomi, M.; Ida-Yonemochi, H.; Ohshima, H. Osteopontin is essential for type I collagen secretion in reparative dentin. J. Dent. Res. 2016, 95, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Brunn, J.C.; Jones, J.; George, A.; Ramachandran, A.; Gorski, J.P.; Butler, W.T. A comparative study of sialic acid-rich proteins in rat bone and dentin. Eur. J. Oral Sci. 2001, 109, 133–141. [Google Scholar] [CrossRef]
- Sodek, J.; Ganss, B.; McKee, M.D. Osteopontin. Crit. Rev. Oral Biol. Med. 2000, 11, 279–303. [Google Scholar] [CrossRef]
- Patel, M.; Smith, A.J.; Sloan, A.J.; Smith, G.; Cooper, P.R. Phenotype and behaviour of dental pulp cells during expansion culture. Arch. Oral Biol. 2009, 54, 898–908. [Google Scholar] [CrossRef]
- Schwendicke, F.; Brouwer, F.; Schwendicke, A.; Paris, S. Different materials for direct pulp capping: Systematic review and meta-analysis and trial sequential analysis. Clin. Oral Investig. 2016, 20, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Kakehashi, S.; Stanley, H.R.; Fitzgerald, R.J. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg. Oral Med. Oral Pathol. 1965, 20, 340–349. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M. Mineral trioxide aggregate: A comprehensive literature review—Part I: Chemical, physical, and antibacterial properties. J. Endod. 2010, 36, 16–27. [Google Scholar] [CrossRef]
- Zeid, S.; Alothmani, O.S.; Yousef, M.K. Biodentine and mineral trioxide aggregate: An analysis of solubility, pH changes and leaching elements. Life Sci. 2015, 12, 18–23. [Google Scholar]
- Morita, M.; Kitagawa, H.; Nakayama, K.; Kitagawa, R.; Yamaguchi, S.; Imazato, S. Antibacterial activities and mineral induction abilities of proprietary MTA cements. Dent. Mater. J. 2020, 40, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Arandi, N.Z.; Rabi, T. TheraCal LC: From biochemical and bioactive properties to clinical applications. Int. J. Dent. 2018, 2018, 3483653. [Google Scholar] [CrossRef] [PubMed]
- Elbanna, A.; Atta, D.; Sherief, D.I. In vitro bioactivity of newly introduced dual-cured resin-modified calcium silicate cement. Dent. Res. J. 2022, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.A.; Murray, B.E. Lack of correlation of gelatinase production and biofilm formation in a large collection of Enterococcus faecalis isolates. J. Clin. Microbiol. 2005, 43, 5405–5407. [Google Scholar] [CrossRef] [PubMed]
- Nakajo, K.; Komori, R.; Ishikawa, S.; Ueno, T.; Suzuki, Y.; Iwami, Y.; Takahashi, N. Resistance to acidic and alkaline environments in the endodontic pathogen Enterococcus faecalis. Oral Microbiol. Immunol. 2006, 21, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Koutroulis, A.; Kuehne, S.A.; Cooper, P.R.; Camilleri, J. The role of calcium ion release on biocompatibility and antimicrobial properties of hydraulic cements. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xiao, Y.H.; Xing, X.; Li, F.; Ma, S.; Qi, L.; Chen, J. Antibacterial activity and cytotoxicity of two novel cross-linking antibacterial monomers on oral pathogens. Arch. Oral Biol. 2011, 56, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; Willems, G.; Celis, J.-P.; Roos, J.R.; Braem, M.; Lambrechts, P.; Vanherle, G. Assessment by nano-indentation of the hardness and elasticity of the resin-dentin bonding area. J. Dent. Res. 1993, 72, 1434–1442. [Google Scholar] [CrossRef]
- Kaup, M.; Schäfer, E.; Dammaschke, T. An In Vitro study of different material properties of Biodentine compared to ProRoot MTA. Head Face Med. 2015, 11, 16. [Google Scholar] [CrossRef]
- Fuentes, V.; Toledano, M.; Osorio, R.; Carvalho, R.M. Microhardness of superficial and deep sound human dentin. J. Biomed. Mater. Res A 2003, 66, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Forss, H.; Seppä, L.; Lappalaimen, R. In Vitro abrasion resistance and hardness of glass-ionomer cements. Dent. Mater. 1991, 7, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Tunç, E.Ş.; Sönmez, I.S.; Eğilmez, T. The evaluation of bond strength of a composite and a compomer to white mineral trioxide aggregate with two different bonding systems. J. Endod. 2008, 34, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.L.; De Gee, A.J.; Feilzer, A. The competition between the composite-dentin bond strength and the polymerization contraction stress. J. Dent. Res. 1984, 63, 1396–1399. [Google Scholar] [CrossRef] [PubMed]
- Al-Sarheed, M.A. Evaluation of shear bond strength and SEM observation of all-in-one self-etching primer used for bonding of fissure sealants. J. Contemp. Dent. Pract. 2006, 7, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Karadas, M.; Cantekin, K.; Gumus, H.; Ateş, S.M.; Duymuş, Z.Y. Evaluation of the bond strength of different adhesive agents to a resin-modified calcium silicate material (TheraCal LC). Scanning 2016, 38, 403–411. [Google Scholar] [CrossRef]
- Hashem, D.F.; Foxton, R.; Manoharan, A.; Watson, T.F.; Banerjee, A. The physical characteristics of resin composite–calcium silicate interface as part of a layered/laminate adhesive restoration. Dent. Mater. 2014, 30, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Odabaş, M.E.; Bani, M.; Tirali, R.E. Shear bond strengths of different adhesive systems to biodentine. Sci. World J. 2013, 2013, 626103. [Google Scholar] [CrossRef]
| Material Type | Product Name | Compositions | Manufacturer |
|---|---|---|---|
| Tricalcium silicate cement | BiodentineTM | Powder: tricalcium silicate, calcium carbonate, and zirconium dioxide Liquid: water, and calcium chloride | Septodont, St. Maur-des-Fossés, France |
| Light cure resin-modified calcium silicate cement | Theracal LC® | Portland cement type III (20–60%), poly(ethylene glycol) dimethacrylate (10–50%), bis-GMA (5–20%), and barium zirconate (1–10%) | Bisco, Inc., Schamburg, IL, USA |
| Dual cure resin-modified calcium silicate cement | Theracal PT® | Base: SG-Mix cement (50–75%), polyethylene glycol dimethacrylate (10–30%), bis-GMA (5–10%), and barium zirconate (1–5%) Catalyst: barium zirconate (1–5%), ytterbium fluoride (1–5%), and initiator (<1%). | Bisco, Inc., Schamburg, IL, USA |
| Primer | Sequence | |
|---|---|---|
| OCN | F | 5′-CGG TGC AGA GTC CAG CAA AG-3′ |
| R | 5′-TAC AGG TAG CGC CTG GGT CT-3′ | |
| OPN | F | 5′-ACA CAT ATG ATG GCC GAG GTG A-3′ |
| R | 5′-GTG AGG TGA TGT CCT CGT CTG TAG-3′ | |
| ColI | F | 5′-CTG CTG GAC GTC CTG GTG AA-3′ |
| R | 5′-ACG CTG TCC AGC AAT ACC TTG A-3′ | |
| GAPDH | F | 5′-GTG GTG GAC CTG ACC TGC-3′ |
| R | 5′-TGA GCT TGA CAA AGT GGT CG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Lee, S.-H.; Shin, D.-H. In Vitro Study of the Biological and Physical Properties of Dual-Cure Resin-Modified Calcium Silicate-Based Cement. Dent. J. 2023, 11, 120. https://doi.org/10.3390/dj11050120
Kim M, Lee S-H, Shin D-H. In Vitro Study of the Biological and Physical Properties of Dual-Cure Resin-Modified Calcium Silicate-Based Cement. Dentistry Journal. 2023; 11(5):120. https://doi.org/10.3390/dj11050120
Chicago/Turabian StyleKim, Minjung, Sung-Hoon Lee, and Dong-Hoon Shin. 2023. "In Vitro Study of the Biological and Physical Properties of Dual-Cure Resin-Modified Calcium Silicate-Based Cement" Dentistry Journal 11, no. 5: 120. https://doi.org/10.3390/dj11050120
APA StyleKim, M., Lee, S.-H., & Shin, D.-H. (2023). In Vitro Study of the Biological and Physical Properties of Dual-Cure Resin-Modified Calcium Silicate-Based Cement. Dentistry Journal, 11(5), 120. https://doi.org/10.3390/dj11050120





