Enamel Matrix Derivatives as an Adjunct to Alveolar Ridge Preservation—A Systematic Review
Abstract
1. Background
2. Material & Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Sources & Search Strategy
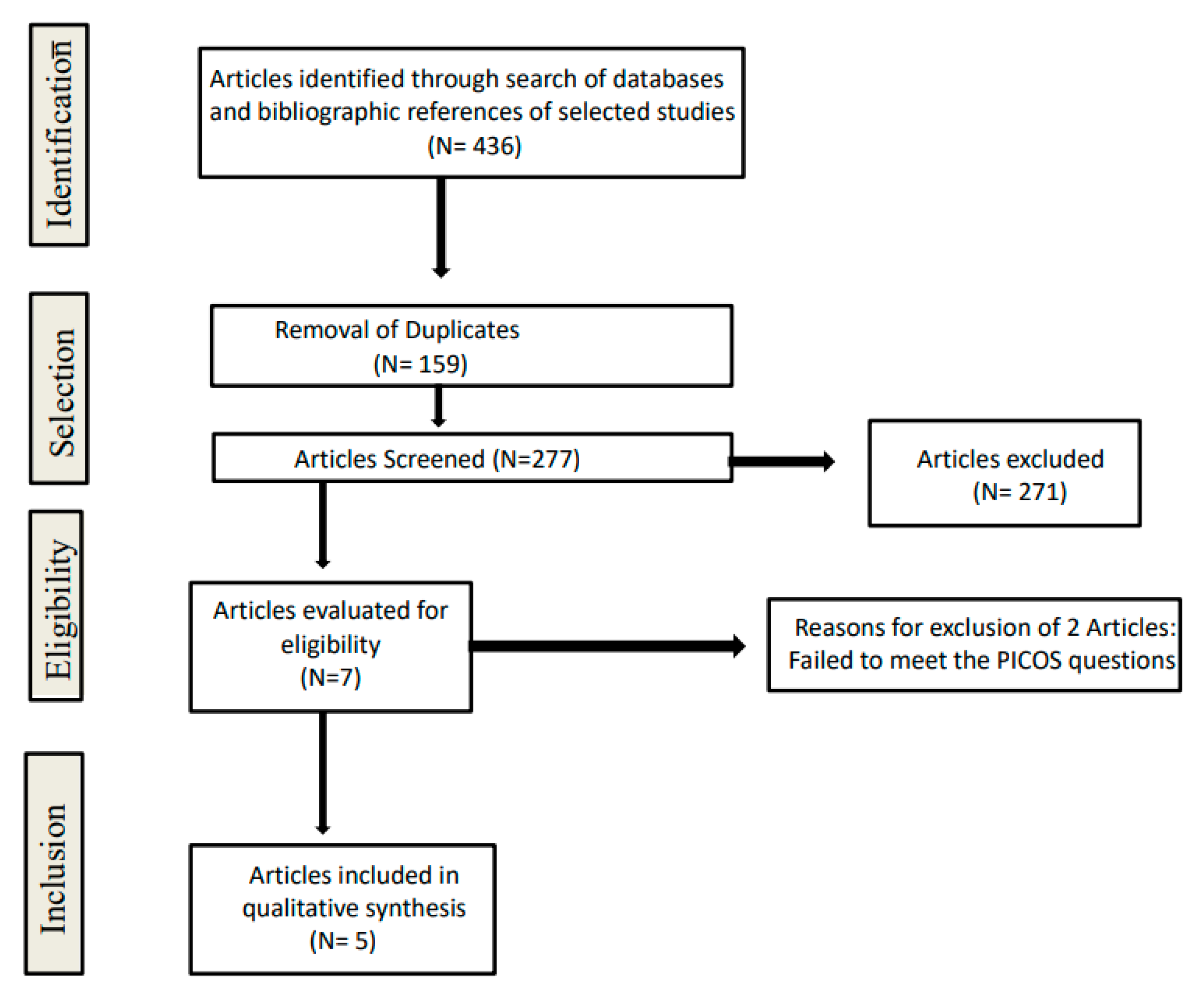
2.4. Study Selection & Data Extraction
2.5. Risk of Bias Assessment
3. Results
3.1. Risk of Bias Assessment
3.2. Study Characteristics
3.3. Intervention Characteristics
3.4. Radiographic Results
3.5. Histologic and Histomorphometric Results
3.6. Postoperative Discomfort
3.7. Feasibility of Implant Placement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EMDs | Enamel matrix derivatives |
| ARP | Alveolar ridge preservation |
| RCTs | Randomized controlled clinical trials |
| BMPs | Bone morphogenetic proteins |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| DBBMC | Deproteinized bovine bone mineral with 10% collagen |
| NB | New bone |
| RG | Residual graft |
| STMs | Soft tissue and marrow spaces |
| CHX | Chlorhexidine |
| CBCT | Cone beam-computed tomography |
| rhBMP-2 | Recombinant human bone morphogenetic protein-2 |
| PRF | Platelet-rich fibrin |
| L-PRF | Leucocyte and platelet-rich fibrin |
| A-PRF+ | Advanced platelet-rich fibrin+ |
References
- Esposito, M.; Grusovin, M.G.; Chew, Y.S.; Coulthard, P.; Worthington, H.V. Interventions for replacing missing teeth: 1-versus 2-stage implant placement. Cochrane Database Syst. Rev. 2009, 3, CD006698. [Google Scholar] [CrossRef] [PubMed]
- Sargolzaie, N.; Moeintaghavi, A.; Shojaie, H. Comparing the Quality of Life of Patients Requesting Dental Implants Before and After Implant. Open Dent. J. 2017, 11, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, M.F.; Kierce, J. Anatomic Basis of Dental Implant Complications. In Complex Dental Implant Complications; Springer: Cham, Switzerland, 2020; pp. 47–72. [Google Scholar]
- Fiorillo, L.; Cervino, G.; Galindo-Moreno, P.; Herford, A.S.; Spagnuolo, G.; Cicciù, M. Growth Factors in Oral Tissue Engineering: New Perspectives and Current Therapeutic Options. BioMed. Res. Int. 2021, 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chappuis, V.; Araújo, M.G.; Buser, D. Clinical relevance of dimensional bone and soft tissue alterations post-extraction in esthetic sites. Periodontology 2016, 73, 73–83. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Cortellini, P.; Graziani, F.; Cairo, F.; Lang, N.P.; Abundo, R.; Conforti, G.P.; Marquardt, S.; Rasperini, G.; Silvestri, M.; et al. Immediate versus delayed implant placement after anterior single tooth extraction: The timing randomized controlled clinical trial. J. Clin. Periodontol. 2017, 44, 215–224. [Google Scholar] [CrossRef]
- Tolstunov, L. Surgical Algorithm for Alveolar Bone Augmentation in Implant Dentistry. Oral Maxillofac. Surg. Clin. North Am. 2019, 31, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Couso-Queiruga, E.; Stuhr, S.; Tattan, M.; Chambrone, L.; Avila-Ortiz, G. Post-extraction dimensional changes: A systematic review and meta-analysis. J. Clin. Periodontol. 2021, 48, 127–145. [Google Scholar] [CrossRef]
- Avila-Ortiz, G.; Chambrone, L.; Vignoletti, F. Effect of alveolar ridge preservation interventions following tooth extraction: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 195–223. [Google Scholar] [CrossRef]
- Lai, P.-C.; Greenwell, H. Ridge Preservation Procedures: Review of Current Literature. Curr. Oral Heal. Rep. 2020, 7, 222–233. [Google Scholar] [CrossRef]
- Lee, D.-W.; Pi, S.-H.; Lee, S.-K.; Kim, E.-C. Comparative histomorphometric analysis of extraction sockets healing implanted with bovine xenografts, irradiated cancellous allografts, and solvent-dehydrated allografts in humans. Int. J. Oral Maxillofac. Implant. 2009, 24, 4. [Google Scholar]
- Barallat, L.; Ruíz-Magaz, V.; Levi, P.A., Jr.; Mareque-Bueno, S.; Galindo-Moreno, P.; Nart, J. Histomorphometric results in ridge preservation procedures comparing various graft materials in extraction sockets with nongrafted sockets in humans: A sys-tematic review. Implant. Dent. 2014, 23, 539–554. [Google Scholar] [CrossRef] [PubMed]
- Bassir, S.H.; Alhareky, M.; Wangsrimongkol, B.; Jia, Y.; Karimbux, N. Systematic Review and Meta-Analysis of Hard Tissue Outcomes of Alveolar Ridge Preservation. Int. J. Oral Maxillofac. Implant. 2018, 33, 979–994. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Xu, Q.; Hou, J.; Wu, Y.; Liu, Y.; Li, R.; Pan, Y.; Zhang, D. Effect of platelet-rich fibrin on alveolar ridge preservation: A systematic review. J. Am. Dent. Assoc. 2019, 150, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S.; Malaiappan, S.; Varghese, S.; Jayakumar, N.D.; Prakasam, G. Additive Effect of Plasma Rich in Growth Factors With Guided Tissue Regeneration in Treatment of Intrabony Defects in Patients With Chronic Periodontitis: A Split-Mouth Randomized Controlled Clinical Trial. J. Periodontol. 2017, 88, 839–845. [Google Scholar] [CrossRef]
- Ramenzoni, L.L.; Hirsiger, C.; Weber, F.E.; Attin, T.; Schmidlin, P.R. Similar inductive effects of enamel and dentin matrix derivatives on osteoblast-like cell response over SLA titanium surface. Arch. Oral Biol. 2020, 109, 104552. [Google Scholar] [CrossRef]
- Miron, R.J.; Sculean, A.; Cochran, D.L.; Froum, S.; Zucchelli, G.; Nemcovsky, C.; Donos, N.; Lyngstadaas, S.P.; Deschner, J.; Dard, M.; et al. Twenty years of enamel matrix derivative: The past, the present and the future. J. Clin. Periodontol. 2016, 43, 668–683. [Google Scholar] [CrossRef]
- Bosshardt, D.D. Biological mediators and periodontal regeneration: A review of enamel matrix proteins at the cellular and molecular levels. J. Clin. Periodontol. 2008, 35 (Suppl. 8), 87–105. [Google Scholar] [CrossRef]
- Miron, R.J.; Dard, M.; Weinreb, M. Enamel matrix derivative, inflammation and soft tissue wound healing. J. Periodontal Res. 2014, 50, 555–569. [Google Scholar] [CrossRef]
- Rojas, M.A.; Marini, L.; Pilloni, A.; Sahrmann, P. Early wound healing outcomes after regenerative periodontal surgery with enamel matrix derivatives or guided tissue regeneration: A systematic review. BMC Oral Heal. 2019, 19, 76. [Google Scholar] [CrossRef]
- Stavropoulos, A.; Bertl, K.; Spineli, L.M.; Sculean, A.; Cortellini, P.; Tonetti, M. Medium- and long-term clinical benefits of periodontal regenerative/reconstructive procedures in intrabony defects: Systematic review and network meta-analysis of randomized controlled clinical studies. J. Clin. Periodontol. 2020, 48, 410–430. [Google Scholar] [CrossRef]
- Zhang, Y.; Jing, D.; Buser, D.; Sculean, A.; Chandad, F.; Miron, R.J. Bone grafting material in combination with Osteogain for bone repair: A rat histomorphometric study. Clin. Oral Investig. 2015, 20, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Konishi, I.; Nishiguchi, M.; Hoshino, T.; Fujiwara, T. Amelogenin binds to both heparan sulfate and bone morphogenetic protein 2 and pharmacologically suppresses the effect of noggin. Bone 2008, 43, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Stout, B.M.; Alent, B.J.; Pedalino, P.; Holbrook, R.; Gluhak-Heinrich, J.; Cui, Y.; Harris, M.A.; Gemperli, A.C.; Cochran, D.L.; Deas, D.E.; et al. Enamel matrix derivative: Protein components and osteoinductive properties. J. Periodontol. 2014, 85, e9–e17. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Fujioka-Kobayashi, M.; Zhang, Y.; Caballé-Serrano, J.; Shirakata, Y.; Bosshardt, D.D.; Buser, D.; Sculean, A. Osteogain improves osteoblast adhesion, proliferation and differentiation on a bovine-derived natural bone mineral. Clin. Oral Implant. Res. 2016, 28, 327–333. [Google Scholar] [CrossRef]
- Tarsilla, M. Cochrane Handbook for Systematic Reviews of Interventions. J. Multidiscip. Eval. 2010, 6, 142–148. [Google Scholar] [CrossRef]
- Moher, D.; Altman, D.G.; Liberati, A.; Tetzlaff, J. PRISMA statement. Epidemiology 2011, 22, 128. [Google Scholar] [CrossRef]
- Schmidlin, P.R.; Khademi, A.; Fakheran, O. Association between periodontal disease and non-apnea sleep disorder: A systematic review. Clin. Oral Investig. 2020, 24, 3335–3345. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.; Chandler, J.; Welch, V.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- Miller, R.J. The Use of Enamel Matrix Derivative in Two-Stage Guided Bone Regeneration Procedures. Clin. Adv. Periodontics 2015, 5, 184–191. [Google Scholar] [CrossRef]
- Alkan, E.; Parlar, A.; Yildirim, B.; Sengüven, B. Histological comparison of healing following tooth extraction with ridge preserva-tion using enamel matrix derivatives versus Bio-Oss Collagen: A pilot study. Int. J. Oral Maxillofac. Surg. 2013, 42, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Nevins, M.L.; Camelo, M.; Schupbach, P.; Nevins, M.; Kim, S.-W.; Kim, D.M. Human buccal plate extraction socket regeneration with recombinant human platelet-derived growth factor BB or enamel matrix derivative. Int. J. Periodontics Restor. Dent. 2011, 31, 5. [Google Scholar]
- Lee, J.H.; Kim, D.H.; Jeong, S.N. Comparative assessment of anterior maxillary alveolar ridge preservation with and without ad-junctive use of enamel matrix derivative: A randomized clinical trial. Clin. Oral Implant. Res. 2020, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeong, S.N. Effect of enamel matrix derivative on alveolar ridge preservation in the posterior maxilla: A randomized controlled clinical trial. Clin. Implant. Dent. Relat. Res. 2020, 22, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Mercado, F.; Vaquette, C.; Hamlet, S.; Ivanovski, S. Enamel matrix derivative promotes new bone formation in xenograft assisted maxillary anterior ridge preservation—A randomized controlled clinical trial. Clin. Oral Implant. Res. 2021, 32, 732–744. [Google Scholar] [CrossRef]
- Bontá, H.; Galli, F.; Gualtieri, A.; Renou, S.; Caride, F. The Effect of an Alloplastic Bone Substitute and Enamel Matrix Derivative on the Preservation of Single Anterior Extraction Sockets: A Histologic Study in Humans. Int. J. Periodontics Restor. Dent. 2022, 42, 361–368. [Google Scholar] [CrossRef]
- Pranskunas, M.; Galindo-Moreno, P.; Padial-Molina, M. Extraction Socket Preservation Using Growth Factors and Stem Cells: A Systematic Review. J. Oral Maxillofac. Res. 2019, 10, e7. [Google Scholar] [CrossRef]
- Jung, R.E.; Ioannidis, A.; Hämmerle, C.H.F.; Thoma, D.S. Alveolar ridge preservation in the esthetic zone. Periodontology 2018, 77, 165–175. [Google Scholar] [CrossRef]
- Coomes, A.M.; Mealey, B.L.; Huynh-Ba, G.; Barboza-Arguello, C.; Moore, W.S.; Cochran, D.L. Buccal bone formation after flapless extraction: A randomized, controlled clinical trial comparing recombinant human bone morphogenetic protein 2/absorbable collagen carrier and collagen sponge alone. J. Periodontol. 2014, 85, 525–535. [Google Scholar] [CrossRef]
- Castro, A.B.; Van Dessel, J.; Temmerman, A.; Jacobs, R.; Quirynen, M. Effect of different platelet-rich fibrin matrices for ridge preservation in multiple tooth extractions: A split-mouth randomized controlled clinical trial. J. Clin. Periodontol. 2021, 48, 984–995. [Google Scholar] [CrossRef]
- Avila-Ortiz, G.; Ambruster, J.; Barootchi, S.; Chambrone, L.; Chen, C.; Dixon, D.R.; Geisinger, M.L.; Giannobile, W.V.; Goss, K.; Gunsolley, J.C.; et al. American Academy of Periodontology best evidence consensus statement on the use of biologics in clinical practice. J. Periodontol. 2022, 93, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Suárez-López del Amo, F.; Monje, A. Efficacy of biologics for alveolar ridge preservation/reconstruction and implant site de-velopment: An American Academy of Periodontology best evidence systematic review. J. Periodontol. 2022, 93, 1827–1847. [Google Scholar] [CrossRef]
- Rathe, F.; Junker, R.; Chesnutt, B.M.; Jansen, J.A. The Effect of Enamel Matrix Derivative (Emdogain®) on Bone Formation: A Systematic Review. Tissue Eng. Part B Rev. 2009, 15, 215–224. [Google Scholar] [CrossRef]
- Lyngstadaas, S.P.; Lundberg, E.; Ekdahl, H.; Andersson, C.; Gestrelius, S. Autocrine growth factors in human periodontal ligament cells cultured on enamel matrix derivative. J. Clin. Periodontol. 2001, 28, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.-R.; Emara, R.; Taher, M.M.; Al-Allaf, F.A.; Almalki, M.; Almasri, M.A.; Siddiqui, S.S. Effects of mineral trioxide aggregate, calcium hydroxide, biodentine and Emdogain on osteogenesis, Odontogenesis, angiogenesis and cell viability of dental pulp stem cells. BMC Oral Heal. 2019, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, K.M.F.; Dörfer, C.; Ungefroren, H.; Kassem, N.; Wiltfang, J.; Paris, S. Effect of Emdogain enamel matrix derivative and BMP-2 on the gene expression and mineralized nodule formation of alveolar bone proper-derived stem/progenitor cells. J. Cranio-Maxillofac. Surg. 2014, 42, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Weishaupt, P.; Bernimoulin, J.-P.; Trackman, P.; Hägewald, S. Stimulation of osteoblasts with Emdogain increases the expression of specific mineralization markers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 106, 304–308. [Google Scholar] [CrossRef]
- Carinci, F.; Piattelli, A.; Guida, L.; Perrotti, V.; Laino, G.; Oliva, A.; Annunziata, M.; Palmieri, A.; Pezzetti, F. Effects of Emdogain on osteoblast gene expression. Oral Dis. 2006, 12, 329–342. [Google Scholar] [CrossRef]
- Fujishiro, N.; Anan, H.; Hamachi, T.; Maeda, K. The role of macrophages in the periodontal regeneration using Emdogain® gel. J. Periodontal Res. 2007, 43, 143–155. [Google Scholar] [CrossRef]
- Galli, C.; Macaluso, G.M.; Guizzardi, S.; Vescovini, R.; Passeri, M.; Passeri, G. Osteoprotegerin and Receptor Activator of Nuclear Factor-Kappa B Ligand Modulation by Enamel Matrix Derivative in Human Alveolar Osteoblasts. J. Periodontol. 2006, 77, 1223–1228. [Google Scholar] [CrossRef]
- Grandin, H.M.; Gemperli, A.C.; Dard, M. Enamel matrix derivative: A review of cellular effects in vitro and a model of molecular arrangement and functioning. Tissue Eng. Part B 2012, 18, 181–202. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Jiang, J.; Safavi, K.E.; Spångberg, L.S.; Zhu, Q. Emdogain promotes osteoblast proliferation and differentiation and stimulates osteoprotegerin expression. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 97, 239–245. [Google Scholar] [CrossRef]
- Van den Dolder, J.; Vloon, A.; Jansen, J. The effect of Emdogain® on the growth and differentiation of rat bone marrow cells. J. Periodontal Res. 2006, 41, 471–476. [Google Scholar] [CrossRef]
- Aspriello, S.D.; Zizzi, A.; Spazzafumo, L.; Rubini, C.; Lorenzi, T.; Marzioni, D.; Bullon, P.; Piemontese, M. Effects of Enamel Matrix Derivative on Vascular Endothelial Growth Factor Expression and Microvessel Density in Gingival Tissues of Periodontal Pocket: A Comparative Study. J. Periodontol. 2011, 82, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Messer, J.; Castillo, E.; Abraham, A.; Jiron, J.; Israel, R.; Yarrow, J.; Thomas, S.; Reynolds, M.; Wnek, R.; Jorgensen, M.; et al. Anti-vascular endothelial growth factor antibody monotherapy causes destructive advanced periodontitis in rice rats (Oryzomys palustris). Bone 2020, 130, 115141. [Google Scholar] [CrossRef]
- Schlueter, S.R.; Carnes, D.L., Jr.; Cochran, D.L. In vitro effects of enamel matrix derivative on microvascular cells. J. Periodontol. 2007, 78, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Furtado Guimarães, G.; Cavalcanti de Araújo, V.; Nery, J.C.; Peruzzo, D.C.; Borges Soares, A. Microvessel Density Evaluation of the Effect of Enamel Matrix Derivative on Soft Tissue After Implant Placement: A Preliminary Study. International Journal of Periodontics & Restorative Dentistry. 2015, 35, 5. [Google Scholar]
- Van der Pauw, M.T.; Van den Bos, T.; Everts, V.; Beertsen, W. Enamel matrix-derived protein stimulates attachment of periodontal ligament fibroblasts and enhances alkaline phosphatase activity and transforming growth factor β1 release of periodontal ligament and gingival fibroblasts. J. Periodontol. 2000, 71, 31–43. [Google Scholar] [CrossRef]
- Kalpidis, C.D.; Ruben, M.P. Treatment of Intrabony Periodontal Defects With Enamel Matrix Derivative: A Literature Review. J. Periodontol. 2002, 73, 1360–1376. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, Y.-S.; Kim, Y.-T.; Kim, D.-H.; Jeong, S.-N. Assessment of early discomfort and wound healing outcomes after periodontal surgery with and without enamel matrix derivative: An observational retrospective case-control study. Clin. Oral Investig. 2019, 24, 229–237. [Google Scholar] [CrossRef]
| First Author Year of Publication Study Design Country | Measurement Methods | Patients (Teeth) Characteristics | Confounding Factors 1. Smoking 2. Periodontitis | Defect Characteristics 1. Socket Location 2. Defect Morphology | Surgical Management 1. Type of Flap 2. Soft Tissue Management 3. Post Operative Antimicrobials | Follow up 1. Healing Period 2. Number of Drop outs 3. Adverse Events | Test and Control Groups |
|---|---|---|---|---|---|---|---|
| Nevins 2011 [33] Single center RCT USA | Histological analysis (light microscopy and B-SEM) Histomorphometric analysis | 15 patients/ 16 teeth Age: 18–70 | 1. No 2. N/R | 1. N/R 2. N/R | 1. Full-thickness with vertical incisions 2. N/R 3. Amoxicillin 1.5 g/day for 5 days, 0.12% CHX for 2 weeks | 1. 5 months 2. 0 3. Uneventful healing | T1: DBBMC alone T2: DBBMC + rhPDGF-BB T3: DBBMC + EMD T4: Bone ceramic + EMD |
| Lee 2019 [34] Single center RCT Republic of Korea | Radiographic analysis (CBCT) Clinical assessment Score of discomfort | 32 participants/32 teeth 8 men (56.3%) 14 women (43.8%) Mean age: 55.1 Y Age range: 31–71 Y | 1. Current smokers (>10 cigarettes/day) considered as exclusion criteria 2. Stable periodontal status (bleeding on probing < 20% and plaque index < 20%) | 1. Maxillary central incisors (N = 16) Maxillary lateral incisors (N = 14) 2. ≤50% buccal bone loss | 1. No flap/No incision 2. N/R 3. Amoxicillin 1.5 g/day for 5 days, 0.12% CHX mouthwash tid for 2 weeks | 1. 5 months 2. 2/32 (6.3%) Test (N = 1) Control (N = 1) 3. Bleeding: (T = 2, C = 2) Persistent swelling: (T = 2, C = 4) Ulceration: (T = 0, C = 1) | T: DBBMC + EMD + 2 layer RCM C: DBBMC + 2 layer RCM |
| Lee 2020 [35] Single center RCT Republic of Korea | Radiographic analysis (CBCT) Clinical assessment Score of discomfort | 36 participants/36 teeth 18 men (64.3%) 10 women (35.7%) Mean age: 52.9 Y Age range: 22–74 Y | 1. Current smokers (>10 cigarettes/day) considered as exclusion criteria 2. Stable periodontal status (bleeding on probing < 25% and plaque index < 25%) | 1. Maxillary first molars (n = 21) maxillary second molars (n = 7) 2. ≤50% buccal bone loss | 1. No flap/No incision 2. N/R 3. Amoxicillin 1.5 g/day for 5 days, 0.12% CHX mouthwash tid for 2 weeks | 1. 5 months 2. 8/36 (22.2%) T1 (N = 2) T2 (N = 2) C (N = 4) 3. Spontaneous bleeding (p= 0.803): T1, (n = 9) T2 (n = 9) C (n = 9) Persistent swelling (p = 0.661): T1 (n = 9) T2 (n = 9) C (n = 9) Ulceration (p= 0.538): T1 (n = 9) T2 (n = 9) C (n = 9) | T1: A: DBBMC + EMD + 2 layer RCM 2: DBBMC + 2 layer RCM C: Empty |
| Mercado 2021 [36] Single center RCT Australia | Radiographic analysis (CBCT) Histological analysis (light microscopy) Histomorphometric analysis | 42 participants/42 teeth T: Mean age: 53.6 ± 10.7 (Female = 66 %) C: Mean age: 51.4 ± 11.3 y (Female = 71%) | 1. Current smokers considered as exclusion criteria 2. Stable periodontal status (No probing depth >4 mm and bleeding on probing < 20% and plaque index < 20%) | 1. Maxillary anterior tooth 2. Buccal dehiscence ≤ 1 mm present at the time of extraction, no palatal defect | 1. Intrasulcular incision/(no flap) 2. FGG3. (0.12% CHX mouthwash) tid for 1 week, 0.12% CHX gel 2nd and 3rd weeks postoperatively | 1. 4 months 2. 0 3. Uneventful healing | T: DBBMC + EMD C: DBBMC only |
| Bonta [37] 2022 Single center RCT Argentina | Histological analysis (light microscopy) Histomorphometric analysis | 21 participants/21 teeth No other data provided regarding the patient (teeth) characteristics | 1. N/R 2. N/R | 1. Single anterior extraction sockets 2. N/R | 1. Laterally sliding flap 2. N/R 3. N/R | 1. 6 months 2. 0 3. N/R | T1: DBBMC only T2: DBBMC + EMD C: Empty |
| First Author | Histomorphometric Results | Histologic Results | Radiographic Results | Postoperative Discomfort | Implant 1. Feasibility of Implant Placement 2. Necessity of Simultaneous Augmentation |
|---|---|---|---|---|---|
| Nevins 2011 [33] | Percentage of new bone: T1: 28.3 ± 17.2 T2: 39.6 ± 11.3 T3: 23.9 ± 9.3 T4: 21.4 ± 4.2 No statistically significant differences | Residual DBBMC graft particulate surrounded by new and native bone. The results of group C was consistent with that of group A specimens. | N/R | N/R | 1. Placement of implants in all C and T sites with good primary stability 2. N/R |
| Lee 2019 [34] | N/R | N/R | Three CBCT images: at baseline, 3 and 5 months. No significant differences between the test and control groups at 3 and 5 months were found. | The severity of pain and swelling did not differ between the two groups, but the duration of pain and swelling were significantly reduced in the test group. | 1. N/R 2. N/R |
| Lee 2020 [35] | N/R | N/R | Two CBCT images: at baseline and 5 months after ARP. No significant differences between T1 and T2 were found regarding horizontal width or vertical height changes. | There were no significant differences in following parameters among four groups: Severity of pain Severity of swelling Duration of pain Duration of swelling | 1. 22 implants placed after 5 months: T1: (N = 9); T2: (N = 8); C: (N = 5) 2. Ten implants placed with additional bone grafting procedures: OSFE/BAOSFE: T1: (N = 3); T2: (N = 3); C: (N = 3) (SFEL): C: (N = 1) |
| Mercado 2021 [36] | Three area fractions (percentage components) were identified in each sample core: NB: T: 45.1 ± 8.8% C: 16.5 ± 6.9% (p < 0.00001) * RG: T: 20.3 ± 7.2 % C: 36.8 ± 8.8% (p < 0.00001) * STM: T: 34.6 ± 13.8 C: 46.5 ± 10.4 (p < 0.003) * | The three types of tissues filling the socket (NB, RG, and STM) in both groups. | Two CBCT images: at baseline and 4 months after ARP. No statistically significant differences when comparing the mean RW, BH, and PH between T and C. There was a significantly greater percentage reduction in ridge dimensions RW, BH, and PH in the <1 mm BT group when compared to the ≥1 mm BT group. | N/R | 1. All patients received the planned dental implants at least 4 months after the extraction 2. N/R |
| Bonta 2022 [37] | NB: (p < 0.05) * T1: 47.30 T2: 32.27 C: 35.62 RG (%): (p > 0.05) T1: 11.61 T2: 18.12 STM: (p < 0.05) * T1: 57.21 T2: 34.57 C: 64.38 | Presence of healthy lamellar bone in all the groups with osteon formation, and evident lack of inflammatory infiltrate in marrow spaces. Residual DBBMC graft particulate surrounded by new and native bone in T1 and T2 groups. | N/R | N/R | 1. Placement of implants in all C and T sites 2. N/R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fakheran, O.; Fischer, K.R.; Schmidlin, P.R. Enamel Matrix Derivatives as an Adjunct to Alveolar Ridge Preservation—A Systematic Review. Dent. J. 2023, 11, 100. https://doi.org/10.3390/dj11040100
Fakheran O, Fischer KR, Schmidlin PR. Enamel Matrix Derivatives as an Adjunct to Alveolar Ridge Preservation—A Systematic Review. Dentistry Journal. 2023; 11(4):100. https://doi.org/10.3390/dj11040100
Chicago/Turabian StyleFakheran, Omid, Kai R. Fischer, and Patrick R. Schmidlin. 2023. "Enamel Matrix Derivatives as an Adjunct to Alveolar Ridge Preservation—A Systematic Review" Dentistry Journal 11, no. 4: 100. https://doi.org/10.3390/dj11040100
APA StyleFakheran, O., Fischer, K. R., & Schmidlin, P. R. (2023). Enamel Matrix Derivatives as an Adjunct to Alveolar Ridge Preservation—A Systematic Review. Dentistry Journal, 11(4), 100. https://doi.org/10.3390/dj11040100








