A Multicenter Prospective Study on the Use of a Mandibular Advancement Device in the Treatment of Obstructive Sleep Apnea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
- -
- Adult patients (age ≥18 years).
- -
- Patients were diagnosed with OSA.
- -
- Patients accepted the treatment with the intraoral appliance APNIA®.
- -
- Have complete records of the evolution of the AHI.
- -
- Signed the informed consent.
- -
- Pregnancy
- -
- Patients with TMJ disorders.
- -
- Patients with active periodontal disease.
- -
- Patients with less than 10 teeth per arch.
2.2. Study Procedures
2.3. Study Variables
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Sleep Disorders Association. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances. Sleep 1995, 18, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Ramar, K.; Dort, L.C.; Katz, S.G.; Lettieri, C.J.; Harrod, C.G.; Thomas, S.M.; Chervin, R.D. Clinical Practice Guideline for the Treatment of Obstructive Sleep Apnea and Snoring with Oral Appliance Therapy: An Update for 2015. J. Clin. Sleep Med. 2015, 11, 773–827. [Google Scholar] [CrossRef] [PubMed]
- Gunaratnam, K.; Taylor, B.; Curtis, B.; Cistulli, P. Obstructive sleep apnoea and periodontitis: A novel association? Sleep Breath. 2009, 13, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.A.; Okeson, J.; Paesani, D.; Gilmore, R. Effect of sleep position on sleep apnea and parafunctional activity. Chest 1986, 90, 424–429. [Google Scholar] [CrossRef]
- Anitua, E.; Duran-Cantolla, J.; Almeida, G.Z.; Alkhraisat, M.H. Association between obstructive sleep apnea and enamel cracks. Am. J. Dent. 2020, 33, 29–32. [Google Scholar]
- Duran-Cantolla, J.; Alkhraisat, M.H.; Martinez-Null, C.; Aguirre, J.J.; Guinea, E.R.; Anitua, E. Frequency of obstructive sleep apnea syndrome in dental patients with tooth wear. J. Clin. Sleep Med. 2015, 11, 445–450. [Google Scholar] [CrossRef]
- Sutherland, K.; Takaya, H.; Qian, J.; Petocz, P.; Ng, A.T.; Cistulli, P.A. Oral Appliance Treatment Response and Polysomnographic Phenotypes of Obstructive Sleep Apnea. J. Clin. Sleep Med. 2015, 11, 861–868. [Google Scholar] [CrossRef]
- Tegelberg, A.; Walker-Engstrom, M.L.; Vestling, O.; Wilhelmsson, B. Two different degrees of mandibular advancement with a dental appliance in treatment of patients with mild to moderate obstructive sleep apnea. Acta Odontol. Scand. 2003, 61, 356–362. [Google Scholar] [CrossRef]
- Aarab, G.; Lobbezoo, F.; Heymans, M.W.; Hamburger, H.L.; Naeije, M. Long-term follow-up of a randomized controlled trial of oral appliance therapy in obstructive sleep apnea. Respiration 2011, 82, 162–168. [Google Scholar] [CrossRef]
- Gauthier, L.; Laberge, L.; Beaudry, M.; Laforte, M.; Rompre, P.H.; Lavigne, G.J. Mandibular advancement appliances remain effective in lowering respiratory disturbance index for 2.5–4.5 years. Sleep Med. 2011, 12, 844–849. [Google Scholar] [CrossRef]
- Ghazal, A.; Sorichter, S.; Jonas, I.; Rose, E.C. A randomized prospective long-term study of two oral appliances for sleep apnoea treatment. J. Sleep Res. 2009, 18, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Walker-Engstrom, M.L.; Tegelberg, A.; Wilhelmsson, B.; Ringqvist, I. 4-year follow-up of treatment with dental appliance or uvulopalatopharyngoplasty in patients with obstructive sleep apnea: A randomized study. Chest 2002, 121, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Umemoto, G.; Toyoshima, H.; Yamaguchi, Y.; Aoyagi, N.; Yoshimura, C.; Funakoshi, K. Therapeutic Efficacy of Twin-Block and Fixed Oral Appliances in Patients with Obstructive Sleep Apnea Syndrome. J. Prosthodont. 2019, 28, e830–e836. [Google Scholar] [CrossRef]
- Pepin, J.L.; Raymond, N.; Lacaze, O.; Aisenberg, N.; Forcioli, J.; Bonte, E.; Bourdin, A.; Launois, S.; Tamisier, R.; Molinari, N. Heat-moulded versus custom-made mandibular advancement devices for obstructive sleep apnoea: A randomised non-inferiority trial. Thorax 2019, 74, 667–674. [Google Scholar] [CrossRef]
- Sutherland, K.; Deane, S.A.; Chan, A.S.; Schwab, R.J.; Ng, A.T.; Darendeliler, M.A.; Cistulli, P.A. Comparative effects of two oral appliances on upper airway structure in obstructive sleep apnea. Sleep 2011, 34, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Aarab, G.; Lobbezoo, F.; Hamburger, H.L.; Naeije, M. Effects of an oral appliance with different mandibular protrusion positions at a constant vertical dimension on obstructive sleep apnea. Clin. Oral Investig. 2010, 14, 339–345. [Google Scholar] [CrossRef]
- Anitua, E.; Duran-Cantolla, J.; Almeida, G.Z.; Alkhraisat, M.H. Minimizing the mandibular advancement in an oral appliance for the treatment of obstructive sleep apnea. Sleep Med. 2017, 34, 226–231. [Google Scholar] [CrossRef]
- Sheats, R.; Essick, G.; Grosdidier, J.; Katz, S.; Kim, C.; Levine, M.; Patel, I. Identifying the appropriate therapeutic position of an oral appliance. J. Dent. Sleep Med. 2020, 7, 7158. [Google Scholar] [CrossRef]
- de Ruiter, M.H.T.; Aarab, G.; de Vries, N.; Lobbezoo, F.; de Lange, J. A stepwise titration protocol for oral appliance therapy in positional obstructive sleep apnea patients: Proof of concept. Sleep Breath. 2020, 24, 1229–1236. [Google Scholar] [CrossRef]
- Almeida, F.R.; Parker, J.A.; Hodges, J.S.; Lowe, A.A.; Ferguson, K.A. Effect of a titration polysomnogram on treatment success with a mandibular repositioning appliance. J. Clin. Sleep Med. 2009, 5, 198–204. [Google Scholar] [CrossRef]
- Gianoni-Capenakas, S.; Kim, D.; Mayoral, P. Lagravère Vich. Mandibular advancement device effects on the upper airway anatomy and function: An umbrella review. J. Dent. Sleep Med. 2023, 10. [Google Scholar] [CrossRef]
- Hans, M.G.; Nelson, S.; Luks, V.G.; Lorkovich, P.; Baek, S.J. Comparison of two dental devices for treatment of obstructive sleep apnea syndrome (OSAS). Am. J. Orthod. Dentofac. Orthop. 1997, 111, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.D.; Gleadhill, I.C.; Cinnamond, M.J.; Gabbey, J.; Burden, D.J. Mandibular advancement appliances and obstructive sleep apnoea: A randomized clinical trial. Eur. J. Orthod. 2002, 24, 251–262. [Google Scholar] [CrossRef]
- Tallamraju, H.; Newton, J.T.; Fleming, P.S.; Johal, A. Factors influencing adherence to oral appliance therapy in adults with obstructive sleep apnea: A systematic review and meta-analysis. J. Clin. Sleep Med. 2021, 17, 1485–1498. [Google Scholar] [CrossRef]
- Tsolakis, I.A.; Palomo, J.M.; Matthaios, S.; Tsolakis, A.I. Dental and Skeletal Side Effects of Oral Appliances Used for the Treatment of Obstructive Sleep Apnea and Snoring in Adult Patients-A Systematic Review and Meta-Analysis. J. Pers. Med. 2022, 12, 483. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Choi, H.I.; Lee, H.; Ahn, S.J.; Noh, G. Biomechanical effect of mandibular advancement device with different protrusion positions for treatment of obstructive sleep apnoea on tooth and facial bone: A finite element study. J. Oral Rehabil. 2018, 45, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Crivellin, G.; Bruno, G.; De Stefani, A.; Mazzoli, A.; Mandolini, M.; Brunzini, A.; Gracco, A. Strength distribution on TMJ using mandibular advancement device for OSAS treatment: A finite element study. Dent. Cadmos 2018, 86, 757–764. [Google Scholar] [CrossRef]
- Cohen-Levy, J.; Petelle, B.; Pinguet, J.; Limerat, E.; Fleury, B. Forces created by mandibular advancement devices in OSAS patients: A pilot study during sleep. Sleep Breath. 2013, 17, 781–789. [Google Scholar] [CrossRef]
- Mediano, O.; Gonzalez Mangado, N.; Montserrat, J.M.; Alonso-Alvarez, M.L.; Almendros, I.; Alonso-Fernandez, A.; Barbe, F.; Borsini, E.; Caballero-Eraso, C.; Cano-Pumarega, I.; et al. International Consensus Document on Obstructive Sleep Apnea. Arch. Bronconeumol. 2022, 58, 52–68. [Google Scholar] [CrossRef]
- Collop, N.A.; Tracy, S.L.; Kapur, V.; Mehra, R.; Kuhlmann, D.; Fleishman, S.A.; Ojile, J.M. Obstructive sleep apnea devices for out-of-center (OOC) testing: Technology evaluation. J. Clin. Sleep Med. 2011, 7, 531–548. [Google Scholar] [CrossRef]
- Ferber, R.; Millman, R.; Coppola, M.; Fleetham, J.; Murray, C.F.; Iber, C.; McCall, W.V.; Nino-Murcia, G.; Pressman, M.; Sanders, M. Portable recording in the assessment of obstructive sleep apnea. ASDA standards of practice. Sleep 1994, 17, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, M.L.; Bortolotti, F.; Raffaelli, E.; D’Anto, V.; Michelotti, A.; Alessandri Bonetti, G. The effectiveness of different mandibular advancement amounts in OSA patients: A systematic review and meta-regression analysis. Sleep Breath. 2016, 20, 911–919. [Google Scholar] [CrossRef]
- Dieltjens, M.; Vanderveken, O.M.; Heyning, P.H.; Braem, M.J. Current opinions and clinical practice in the titration of oral appliances in the treatment of sleep-disordered breathing. Sleep Med. Rev. 2012, 16, 177–185. [Google Scholar] [CrossRef]
- Campbell, A.J.; Reynolds, G.; Trengrove, H.; Neill, A.M. Mandibular advancement splint titration in obstructive sleep apnoea. Sleep Breath. 2009, 13, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Walker-Engstrom, M.L.; Ringqvist, I.; Vestling, O.; Wilhelmsson, B.; Tegelberg, A. A prospective randomized study comparing two different degrees of mandibular advancement with a dental appliance in treatment of severe obstructive sleep apnea. Sleep Breath. 2003, 7, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Mayoral, P.; Lagravere, M.O.; Miguez-Contreras, M.; Garcia, M. Antero-posterior mandibular position at different vertical levels for mandibular advancing device design. BMC Oral Health 2019, 19, 85. [Google Scholar] [CrossRef]
- Bruno, G.; De Stefani, A.; Conte, E.; Caragiuli, M.; Mandolini, M.; Landi, D.; Gracco, A. A Procedure for Analyzing Mandible Roto-Translation Induced by Mandibular Advancement Devices. Materials 2020, 13, 1826. [Google Scholar] [CrossRef]
- Ilea, A.; Timus, D.; Hopken, J.; Andrei, V.; Babtan, A.M.; Petrescu, N.B.; Campian, R.S.; Bosca, A.B.; Sovrea, A.S.; Negucioiu, M.; et al. Oral appliance therapy in obstructive sleep apnea and snoring—Systematic review and new directions of development. Cranio 2021, 39, 472–483. [Google Scholar] [CrossRef]
- Dontsos, V.K.; Chatzigianni, A.; Papadopoulos, M.A.; Nena, E.; Steiropoulos, P. Upper airway volumetric changes of obstructive sleep apnoea patients treated with oral appliances: A systematic review and meta-analysis. Eur. J. Orthod. 2021, 43, 399–407. [Google Scholar] [CrossRef]
- Rangarajan, H.; Padmanabhan, S.; Ranganathan, S.; Kailasam, V. Impact of oral appliance therapy on quality of life (QoL) in patients with obstructive sleep apnea—A systematic review and meta-analysis. Sleep Breath. 2021, 26, 983–996. [Google Scholar] [CrossRef]
- Trzepizur, W.; Cistulli, P.A.; Glos, M.; Vielle, B.; Sutherland, K.; Wijkstra, P.J.; Hoekema, A.; Gagnadoux, F. Health outcomes of continuous positive airway pressure versus mandibular advancement device for the treatment of severe obstructive sleep apnea: An individual participant data meta-analysis. Sleep 2021, 44, zsab015. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Acosta, L.; Hung, Y.L.; Padilla, M.; Enciso, R. Effects of CPAP and mandibular advancement device treatment in obstructive sleep apnea patients: A systematic review and meta-analysis. Sleep Breath. 2018, 22, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Pattipati, M.; Gudavalli, G.; Zin, M.; Dhulipalla, L.; Kolack, E.; Karki, M.; Devarakonda, P.K.; Yoe, L. Continuous Positive Airway Pressure vs Mandibular Advancement Devices in the Treatment of Obstructive Sleep Apnea: An Updated Systematic Review and Meta-Analysis. Cureus 2022, 14, e21759. [Google Scholar] [CrossRef] [PubMed]
- Mecenas, P.; Miranda, G.H.N.; Fagundes, N.C.F.; Normando, D.; Ribeiro, K.C.F. Effects of oral appliances on serum cytokines in adults with obstructive sleep apnea: A systematic review. Sleep Breath. 2021, 26, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, A.; McGrath, C.; Hagg, U. A systematic review of the efficacy of oral appliance design in the management of obstructive sleep apnoea. Eur. J. Orthod. 2011, 33, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Lasserson, T.J.; Fleetham, J.; Wright, J. Oral appliances for obstructive sleep apnoea. Cochrane Database Syst. Rev. 2006, 2006, CD004435. [Google Scholar] [CrossRef] [PubMed]
- Venema, J.; Rosenmoller, B.; de Vries, N.; de Lange, J.; Aarab, G.; Lobbezoo, F.; Hoekema, A. Mandibular advancement device design: A systematic review on outcomes in obstructive sleep apnea treatment. Sleep Med. Rev. 2021, 60, 101557. [Google Scholar] [CrossRef]
- Bortolotti, F.; Corazza, G.; Bartolucci, M.L.; Incerti Parenti, S.; Paganelli, C.; Alessandri-Bonetti, G. Dropout and adherence of obstructive sleep apnoea patients to mandibular advancement device therapy: A systematic review of randomised controlled trials with meta-analysis and meta-regression. J. Oral Rehabil. 2022, 49, 553–572. [Google Scholar] [CrossRef]
- Clark, G.T.; Sohn, J.W.; Hong, C.N. Treating obstructive sleep apnea and snoring: Assessment of an anterior mandibular positioning device. J. Am. Dent. Assoc. 2000, 131, 765–771. [Google Scholar] [CrossRef]
- Pliska, B.T.; Nam, H.; Chen, H.; Lowe, A.A.; Almeida, F.R. Obstructive sleep apnea and mandibular advancement splints: Occlusal effects and progression of changes associated with a decade of treatment. J. Clin. Sleep Med. 2014, 10, 1285–1291. [Google Scholar] [CrossRef]

| Variable | Value |
|---|---|
| Age (years; mean ± SD 1) | 58 ± 11 |
| Sex (%) | 46 females (45%); 56 males (55%) |
| BMI (kg/m2; mean ± SD) | 26.7 ± 3.5 |
| Smokers (number of patients; %) | 12 (12%) |
| Epworth sleepiness score (mean ± SD) | 7.6 ± 3.5 |
| Snoring (number of patients; %) | 70 (69%) |
| Excessive daytime sleepiness (number of patients; %) | 41 (40%) |
| Variable | Value | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
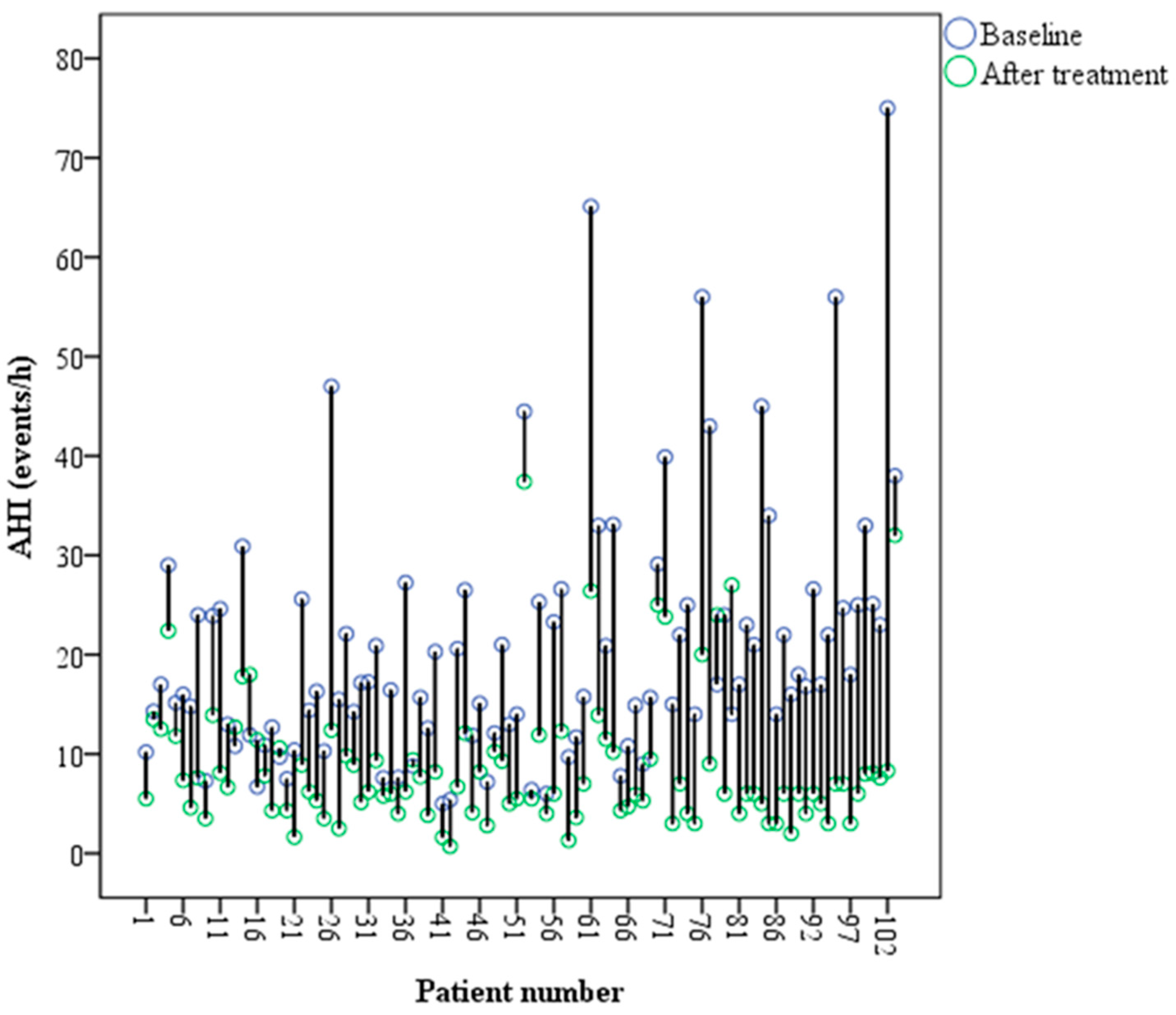
| AHI 1 (events/h) mean ± SD 2 | Before treatment | 20.6 ± 12.7 | p = 0.000 3 | |||||
| After treatment | 8.6 ± 6.7 | |||||||
| Supine AHI (events/h) mean ± SD | Before treatment | 34.8 ± 19.5 | p = 0.000 3 | |||||
| After treatment | 13.8 ± 13.2 | |||||||
| Non-supine AHI (events/h) mean ± SD | Before treatment | 11.7 ± 12.3 | p = 0.000 3 | |||||
| After treatment | 5.7 ± 6.6 | |||||||
| Sleep time in supine position (%) | Before treatment | 41.5 ± 23.8 | p = 0.039 3 | |||||
| After treatment | 37.9 ± 23.6 | |||||||
| Reduction in the AHI (events/h) mean ± SD | Mild OSA 2 | 4.2 ± 5.1 | p = 0.000 4 | |||||
| Moderate OSA | 12.7 ± 5.2 | |||||||
| Severe OSA | 29.3 ± 16.3 | |||||||
| Success (reduction in AHI ≥ 50%) | 69% | |||||||
| Severity OSA (after) | Total | |||||||
| No OSA | Mild | Moderate | Severe | |||||
| Severity of OSA (before) | Mild | Number of patients | 20 | 16 | 2 | 0 | 38 | p = 0.000 5 |
| Percentage | 52.6 | 42.1 | 5.3 | 0.0 | 100 | |||
| Moderate | Number of patients | 9 | 37 | 3 | 0 | 49 | ||
| Percentage | 18.4 | 75.5 | 6.1 | 0.0 | 100 | |||
| Severe | Number of patients | 2 | 7 | 4 | 2 | 15 | ||
| Percentage | 13.3 | 46.7 | 26.7 | 13.3 | 100 | |||
| Total | Number of patients | 31 | 60 | 9 | 2 | 102 | ||
| Percentage | 30.4 | 58.8 | 8.8 | 2.0 | 100 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anitua, E.; Mayoral, P.; Almeida, G.Z.; Durán-Cantolla, J.; Alkhraisat, M.H. A Multicenter Prospective Study on the Use of a Mandibular Advancement Device in the Treatment of Obstructive Sleep Apnea. Dent. J. 2023, 11, 247. https://doi.org/10.3390/dj11110247
Anitua E, Mayoral P, Almeida GZ, Durán-Cantolla J, Alkhraisat MH. A Multicenter Prospective Study on the Use of a Mandibular Advancement Device in the Treatment of Obstructive Sleep Apnea. Dentistry Journal. 2023; 11(11):247. https://doi.org/10.3390/dj11110247
Chicago/Turabian StyleAnitua, Eduardo, Pedro Mayoral, Gabriela Zamora Almeida, Joaquín Durán-Cantolla, and Mohammad Hamdan Alkhraisat. 2023. "A Multicenter Prospective Study on the Use of a Mandibular Advancement Device in the Treatment of Obstructive Sleep Apnea" Dentistry Journal 11, no. 11: 247. https://doi.org/10.3390/dj11110247
APA StyleAnitua, E., Mayoral, P., Almeida, G. Z., Durán-Cantolla, J., & Alkhraisat, M. H. (2023). A Multicenter Prospective Study on the Use of a Mandibular Advancement Device in the Treatment of Obstructive Sleep Apnea. Dentistry Journal, 11(11), 247. https://doi.org/10.3390/dj11110247








