β-Tricalcium Phosphate as Alveolar Bone Grafting in Cleft Lip/Palate: A Systematic Review
Abstract
:1. Introduction
2. Material and Methods
2.1. Focused Question
2.2. Search Strategies
2.3. Eligibility Criteria
2.4. Study Selection and Data Extraction
2.5. Quality Assessment of Studies
2.6. Risk-of-Bias Assessment
2.7. Statistical Methods
3. Results
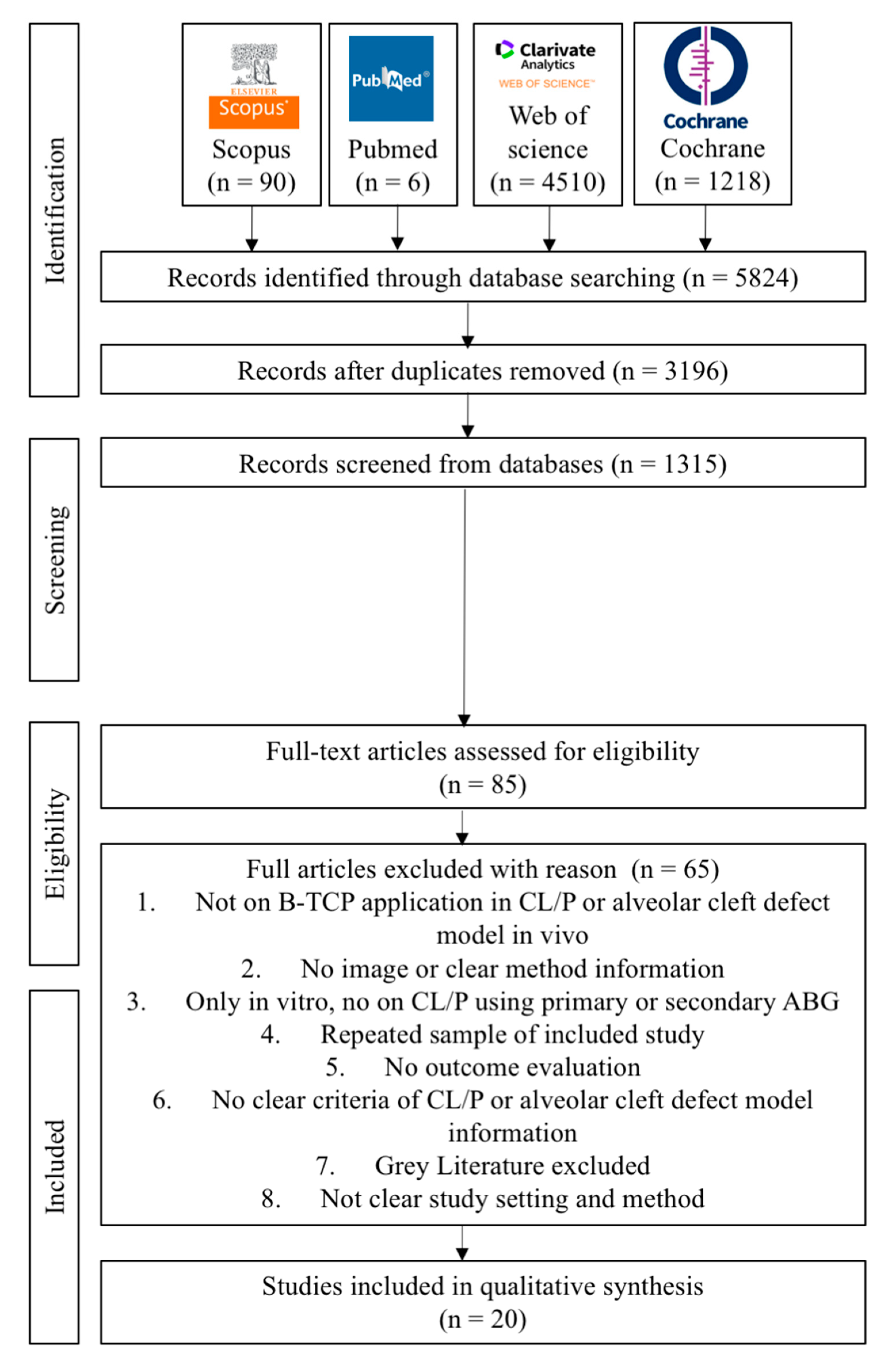
3.1. Study Selection, Data Extraction, and Quality Assessment
3.2. Assessment of the Risk of Bias and Quality
3.3. Qualitative Analysis
4. Discussion
5. Conclusions
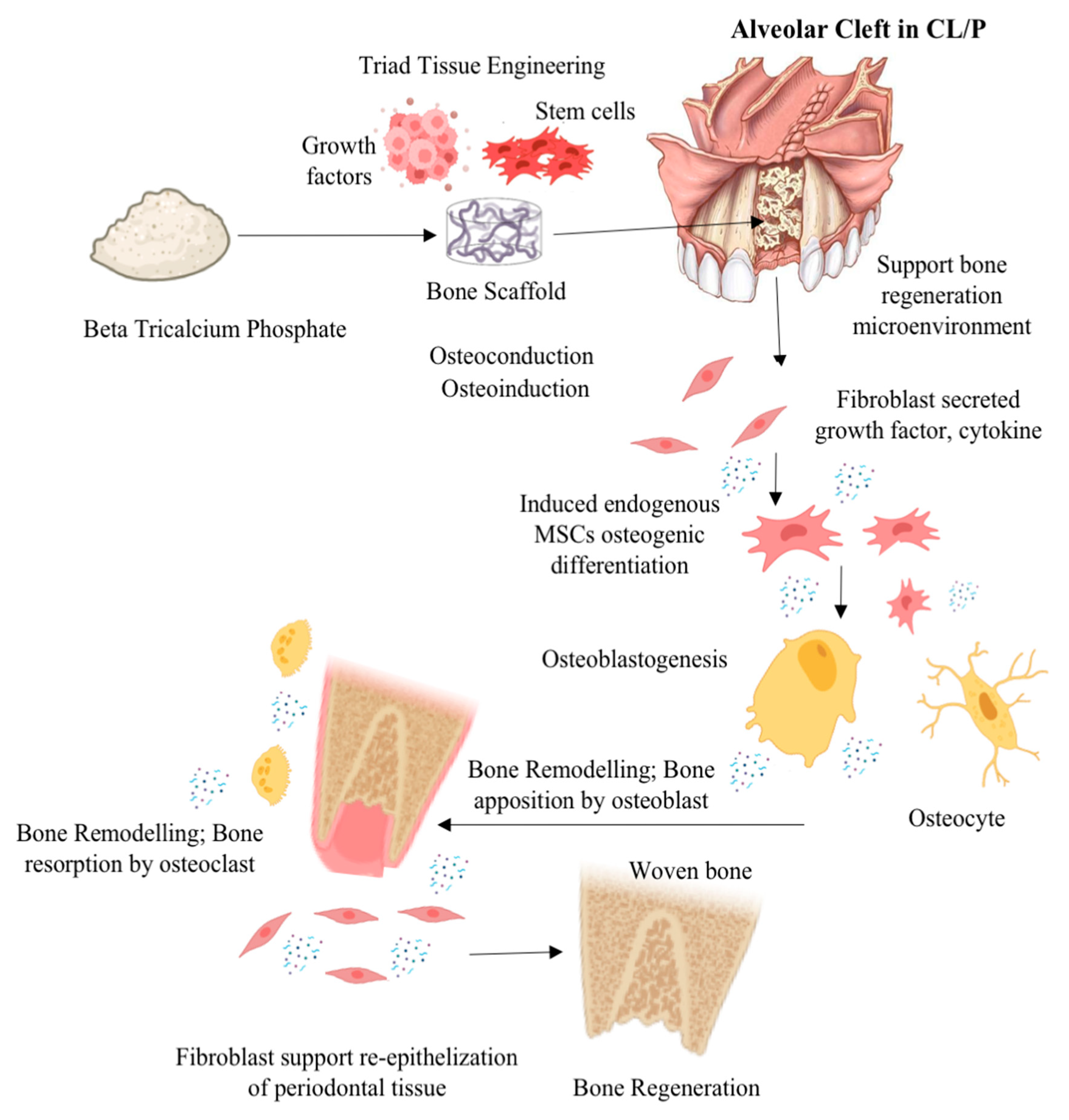
- β-TCP as an ABG material is biocompatible, more visible and practical, offers a less invasive procedure, and does not interfere with orthodontic treatment.
- β-TCP as a synthetic ABG material can be the alternative to autologous bone grafts with several terms and conditions, such as if autografts are hard to come by, there is donor site morbidity, and the size of the defect restricts the size of the autograft.
- The enhancement of osteoinductive and osteoconductive abilities for improvement of β-TCP efficacy for ABG in CL/P or alveolar bone cleft defects can be achieved via a tissue engineering approach combining β-TCP with growth factor, mesenchymal stem cells, or other graft materials and the modification of β-TCP physical properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iskandar, R.P.D.; Proboningrat, A.; Fadholly, A.; Narmada, I.B.; Nidom, C.A.; Sudjarwo, S.A. The Densitometric Analysis of Protein Pattern in Cleft Lip and Palate Patients. J. Int. Soc. Prev. Community Dent. 2019, 9, 240–244. [Google Scholar] [CrossRef]
- Shkoukani, M.A.; Lawrence, L.A.; Liebertz, D.J.; Svider, P.F. Cleft palate: A clinical review. Birth Defects Res. C Embryo Today 2014, 102, 333–342. [Google Scholar] [CrossRef]
- Mossey, P.A.; Little, J.; Munger, R.G.; Dixon, M.J.; Shaw, W.C. Cleft lip and palate. Lancet 2009, 374, 1773–1785. [Google Scholar] [CrossRef]
- Dixon, M.J.; Marazita, M.L.; Beaty, T.H.; Murray, J.C. Cleft lip and palate: Understanding genetic and environmental influences. Nat. Rev. Genet. 2011, 12, 167–178. [Google Scholar] [CrossRef]
- Massenburg, B.B.; Hopper, R.A.; Crowe, C.S.; Morrison, S.D.; Alonso, N.; Calis, M.; Donkor, P.; Kreshanti, P.; Yuan, J. Global Burden of Disease 2017 Orofacial Clefting Collaborators. Global Burden of Orofacial Clefts and the World Surgical Workforce. Plast. Reconstr. Surg. 2021, 148, 568e–580e. [Google Scholar] [CrossRef]
- Tanaka, S.A.; Mahabir, R.C.; Jupiter, D.C.; Menezes, J.M. Updating the epidemiology of cleft lip with or without cleft palate. Plast. Reconstr. Surg. 2012, 129, 511e–518e. [Google Scholar] [CrossRef]
- Panamonta, V.; Pradubwong, S.; Panamonta, M.; Chowchuen, B. Global Birth Prevalence of Orofacial Clefts: A Systematic Review. J. Med. Assoc. Thail. 2015, 98 (Suppl. S7), S11–S21. [Google Scholar]
- Ariawan, D.; Vitria, E.E.; Sulistyani, L.D.; Anindya, C.S.; Adrin, N.S.R.; Aini, N.; Hak, M.S. Prevalence of Simonart’s band in cleft children at a cleft center in Indonesia: A nine-year retrospective study. Dent. Med. Probl. 2022, 59, 509–515. [Google Scholar] [CrossRef]
- Wehby, G.L.; Cassell, C.H. The impact of orofacial clefts on quality of life and healthcare use and costs. Oral Dis. 2010, 16, 3–10. [Google Scholar] [CrossRef]
- Menezes, C.; de Arruda, J.A.; Silva, L.V.; Monteiro, J.L.; Caribé, P.; Álvares, P.; Almeida, M.C.; Coelli, J.C.; Goldemberg, F.; Silveira, M.; et al. Nonsyndromic cleft lip and/or palate: A multicenter study of the dental anomalies involved. J. Clin. Exp. Dent. 2018, 10, e746–e750. [Google Scholar] [CrossRef]
- Wei, S.; Wang, Y.; Ma, X.; Pan, Y.; Zhang, P.; Jiang, H.; Du, Y.; Wan, L. Comparison of Anatomical Features of Alveolar Cleft in Unilateral Cleft Lip and Palate Patients of Different Ages. J. Craniofac. Surg. 2020, 31, 1629–1632. [Google Scholar] [CrossRef] [PubMed]
- Semb, G. Alveolar bone grafting. Front. Oral Biol. 2012, 16, 124–136. [Google Scholar] [PubMed]
- Ebraheim, N.A.; Elgafy, H.; Xu, R. Bone-graft harvesting from iliac and fibular donor sites: Techniques and complications. J. Am. Acad. Orthop. Surg. 2001, 9, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Jupiter, D.C. Bone graft substitute: Allograft and xenograft. Clin. Podiatr. Med. Surg. 2015, 32, 21–34. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46 (Suppl. S21), 92–102. [Google Scholar] [CrossRef]
- Yu, H.; Özcan, M.; Yoshida, K.; Cheng, H.; Sawase, T. Bonding to industrial indirect composite blocks: A systematic review and meta-analysis. Dent. Mater. 2020, 36, 119–134. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Y.L.; Hong, G.; Yu, H. Effects of low-temperature degradation on the surface roughness of yttria-stabilized tetragonal zirconia polycrystal ceramics: A systematic review and meta-analysis. J. Prosthet. Dent. 2021, 125, 222–230. [Google Scholar] [CrossRef]
- Yang, H.; Yang, S.; Attin, T.; Yu, H. Effect of Acidic Solutions on the Surface Roughness and Microhardness of Indirect Restorative Materials: A Systematic Review and Meta-analysis. Int. J. Prosthodont. 2023, 36, 81–90. [Google Scholar] [CrossRef]
- Yu, H.; Chen, Y.H.; Cheng, H.; Sawase, T. Finish-line designs for ceramic crowns: A systematic review and meta-analysis. J. Prosthet. Dent. 2019, 122, 22–30.e5. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Hong, D.-W.; Zheng, M.; Yu, H. Is the bond strength of zirconia-reinforced lithium silicate lower than that of lithium disilicate?—A systematic review and meta-analysis. J. Prosthodont. Res. 2022, 66, 530–537. [Google Scholar] [CrossRef]
- Möhlhenrich, S.C.; Kniha, K.; Magnuska, Z.; Hermanns-Sachweh, B.; Gremse, F.; Hölzle, F.; Danesh, G.; Modabber, A. Evaluation of different grafting materials for alveolar cleft repair in the context of orthodontic tooth movement in rats. Sci. Rep. 2021, 11, 13586. [Google Scholar] [CrossRef] [PubMed]
- Putri, I.L.; Fatchiyah Pramono, C.; Bachtiar, I.; Latief, F.D.E.; Utomo, B.; Rachman, A.; Soesilawati, P.; Hakim, L.; Rantam, F.A.; Perdanakusuma, D.S. Alveolar Repair Using Cancellous Bone and Beta Tricalcium Phosphate Seeded with Adipose-Derived Stem Cell. Cleft Palate Craniofac. J. 2022. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Andersson, L.; Tolba, R.; Al-Asfour, A.; Bartella, A.K.; Gremse, F.; Rosenhain, S.; Hölzle, F.; Kessler, P.; Lethaus, B. Bone regeneration using composite non-demineralized xenogenic dentin with beta-tricalcium phosphate in experimental alveolar cleft repair in a rabbit model. J. Transl. Med. 2017, 15, 263. [Google Scholar] [CrossRef] [PubMed]
- Shahnaseri, S.; Sheikhi, M.; Hashemibeni, B.; Mousavi, S.A.; Soltani, P. Comparison of autogenous bone graft and tissue-engineered bone graft in alveolar cleft defects in canine animal models using digital radiography. Indian J. Dent. Res. 2020, 31, 118–123. [Google Scholar] [CrossRef]
- Pourebrahim, N.; Hashemibeni, B.; Shahnaseri, S.; Torabinia, N.; Mousavi, B.; Adibi, S.; Heidari, F.; Alavi, M.J. A comparison of tissue-engineered bone from adipose-derived stem cell with autogenous bone repair in maxillary alveolar cleft model in dogs. Int. J. Oral Maxillofac. Surg. 2013, 42, 562–568. [Google Scholar] [CrossRef]
- Huang, J.; Tian, B.; Chu, F.; Yang, C.; Zhao, J.; Jiang, X.; Qian, Y. Rapid maxillary expansion in alveolar cleft repaired with a tissue-engineered bone in a canine model. J. Mech. Behav. Biomed. Mater. 2015, 48, 86–99. [Google Scholar] [CrossRef]
- Ito, M.; Toriumi, T.; Imura, H.; Akiyama, Y.; Arai, Y.; Natsume, N.; Honda, M. Rat Palatine Fissure: A Suitable Experimental Model for Evaluating Bone Regeneration. Tissue Eng. Part C Methods 2019, 25, 513–522. [Google Scholar] [CrossRef]
- Möhlhenrich, S.C.; Kniha, K.; Magnuska, Z.; Chhatwani, S.; Hermanns-Sachweh, B.; Gremse, F.; Hölzle, F.; Danesh, G.; Modabber, A. Development of root resorption during orthodontic tooth movement after cleft repair using different grafting materials in rats. Clin. Oral Investig. 2022, 26, 5809–5821. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Kyomen, S.; Tanne, K. Biologic responses of autogenous bone and beta-tricalcium phosphate ceramics transplanted into bone defects to orthodontic forces. Cleft Palate Craniofac. J. 1996, 33, 277–283. [Google Scholar] [CrossRef]
- de Ruiter, A.; Meijer, G.; Dormaar, T.; Janssen, N.; van der Bilt, A.; Slootweg, P.; de Bruijn, J.; van Rijn, L.; Koole, R. β-TCP versus autologous bone for repair of alveolar clefts in a goat model. Cleft Palate Craniofac. J. 2011, 48, 654–662. [Google Scholar]
- Zhang, D.; Chu, F.; Yang, Y.; Xia, L.; Zeng, D.; Uludağ, H.; Zhang, X.; Qian, Y.; Jiang, X. Orthodontic tooth movement in alveolar cleft repaired with a tissue engineering bone: An experimental study in dogs. Tissue Eng. Part. A 2011, 17, 1313–1325. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.G.; de Ruiter, A.P.; van Hout, W.M.M.T.; van Miegem, V.; Gawlitta, D.; Groot, F.B.; Meijer, G.J.; Rosenberg, A.J.W.P.; Koole, R. Microstructured β-Tricalcium Phosphate Putty Versus Autologous Bone for Repair of Alveolar Clefts in a Goat Model. Cleft Palate Craniofac. J. 2017, 54, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Ekin, O.; Calis, M.; Aliyev, A.; Yar, A.S.; Korkusuz, P.; Bilgic, E.; Aydin, H.M.; Celik, H.H.; Ozgur, F.; Vargel, I. Poly(L-Lactide)/Poly(ε-Caprolactone) and Collagen/β-Tricalcium Phosphate Scaffolds for the Treatment of Critical-Sized Rat Alveolar Defects: A Microtomographic, Molecular-Biological, and Histological Study. Cleft Palate Craniofac. J. 2016, 53, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Tokugawa, Y.; Kubota, M.; Nishimura, M.; Haruyama, N.; Igarashi, K. Bone regeneration of canine artificial alveolar clefts using bone-marrow-derived mesenchymal stromal cells and β-tricalcium phosphate: A preliminary study. Orthod. Wave 2012, 71, 51–58. [Google Scholar] [CrossRef]
- Trujillo, R.L.; Kadioglu, O.; Currier, G.F.; Smith, K.S.; Yetkiner, E. Volumetric Cleft Changes in Treatment with Bone Morphogenic Protein/β-Tricalcium Phosphate Versus Grafts From the Iliac Crest or Symphysis. J. Oral Maxillofac. Surg. 2018, 76, 1991–1997. [Google Scholar] [CrossRef]
- Janssen, N.G.; Schreurs, R.; de Ruiter, A.P.; Sylvester-Jensen, H.C.; Blindheim, G.; Meijer, G.J.; Koole, R.; Vindenes, H. Microstructured beta-tricalcium phosphate for alveolar cleft repair: A two-centre study. Int. J. Oral Maxillofac. Surg. 2019, 48, 708–711. [Google Scholar] [CrossRef]
- Du, F.; Wu, H.; Li, H.; Cai, L.; Wang, Q.; Liu, X.; Xiao, R.; Yin, N.; Cao, Y. Bone Marrow Mononuclear Cells Combined with Beta-Tricalcium Phosphate Granules for Alveolar Cleft Repair: A 12-Month Clinical Study. Sci. Rep. 2017, 7, 13773. [Google Scholar] [CrossRef]
- Miyagawa, K.; Tanaka, S.; Hiroishi, S.; Matsushita, Y.; Murakami, S.; Kogo, M. Comparative evaluation of bone microstructure in alveolar cleft repair by cone beam CT: Influence of different autologous donor sites and additional application of β-tricalcium phosphate. Clin. Oral Investig. 2020, 24, 2789–2797. [Google Scholar] [CrossRef]
- de Ruiter, A.; Janssen, N.; van Es, R.; Frank, M.; Meijer, G.; Koole, R.; Rosenberg, T. Micro-structured Beta-Tricalcium Phosphate for Repair of the Alveolar Cleft in Cleft Lip and Palate Patients: A Pilot Study. Cleft Palate Craniofac. J. 2015, 52, 336–340. [Google Scholar] [CrossRef]
- Weijs, W.L.; Siebers, T.J.; Kuijpers-Jagtman, A.M.; Bergé, S.J.; Meijer, G.J.; Borstlap, W.A. Early secondary closure of alveolar clefts with mandibular symphyseal bone grafts and beta-tri calcium phosphate (beta-TCP). Int. J. Oral Maxillofac. Surg. 2010, 39, 424–429. [Google Scholar] [CrossRef]
- Ducheyne, P.; Radin, S.; King, L. The effect of calcium phosphate ceramic composition and structure on in vitro behavior. I. Dissolution. J. Biomed. Mater. Res. 1993, 27, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.; Liss, P.; Jacquemaire, B.; Lecestre, P.; Frayssinet, P. Biphasic synthetic bone substitute use in orthopaedic and trauma surgery: Clinical, radiological and histological results. J. Mater. Sci. Mater. Med. 1999, 10, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Oberoi, S.; Chigurupati, R.; Gill, P.; Hoffman, W.Y.; Vargervik, K. Volumetric assessment of secondary alveolar bone grafting using cone beam computed tomography. Cleft Palate Craniofac. J. 2009, 46, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, M.; Wojtaszek-Słomińska, A.; Racka-Pilszak, B. A novel method for alveolar bone grafting assessment in cleft lip and palate patients: Cone-beam computed tomography evaluation. Clin. Oral Investig. 2021, 25, 1967–1975. [Google Scholar] [CrossRef]
- Kamperos, G.; Theologie-Lygidakis, N.; Tsiklakis, K.; Iatrou, I. A novel success scale for evaluating alveolar cleft repair using cone-beam computed tomography. J. Craniomaxillofac. Surg. 2020, 48, 391–398. [Google Scholar] [CrossRef]
- European Commission. Radiation Protection No 172: Cone Beam CT for Dental and Maxillofacial Radiology—Evidence Based Guidelines; European Commission: Luxembourg, 2012. [Google Scholar]
- Francisco, I.; Paula, A.B.; Oliveiros, B.; Fernandes, M.H.; Carrilho, E.; Marto, C.M.; Vale, F. Regenerative Strategies in Cleft Palate: An Umbrella Review. Bioengineering 2021, 8, 76. [Google Scholar] [CrossRef]
| Database | Search Strategy |
|---|---|
| Scopus | β-tricalcium AND phosphate OR β-TCP or bone graft OR bone grafting OR alveolar AND bone AND graft AND alveolar AND bone AND cleft OR cleft AND palate AND (LIMIT-TO (PUBSTAGE, “final”)) AND (LIMIT-TO (OA, “all”)) AND (LIMIT-TO (DOCTYPE, “ar”)) AND (LIMIT-TO (LANGUAGE, “English”)) AND (LIMIT-TO (SRCTYPE, “j”)) |
| PubMed | β-tricalcium phosphate OR β-TCP OR bone graft OR bone grafting OR biomaterial OR alveolar bone graft AND alveolar bone cleft AND cleft palate Filters applied: Free full text, Clinical Trial, Randomized Controlled Trial, English, Exclude preprints, MEDLINE. |
| Web of Science | ((((((ALL = (β-tricalcium phosphate)) OR TS = (β-TCP)) OR TS = (bone graft)) OR TS = (bone grafting)) OR TS = (alveolar bone graft)) AND TS = (alveolar bone cleft)) OR TS = (cleft palate) Refined By: Open Access. Click to remove this refine from your search. Document Types: Article. Click to remove this refine from your search. Open Access: All Open Access. Click to remove this refine from your search. Languages: English. |
| Cochrane Library | β-tricalcium phosphate OR β-TCP OR bone graft OR bone grafting OR alveolar bone graft AND alveolar bone cleft OR cleft palate in Title Abstract Keyword—in Trials, Clinical Answers (Word variations have been searched) |
| Authors, Year, Country | Study Design | Sample/ Subject Criteria (n) | Type of Cleft | Type of Tissue Engineering | Examinations and Variables | Outcome | Conclusion |
|---|---|---|---|---|---|---|---|
| Möhlhenrich et al., 2021, German [21] | True experimental, post-test-only control group design | Twenty-one 8-week-old male Wistar-HAN rats; average weight of 465 ± 34 g. | 1.7 mm alveolar clefts at the left side of the upper jaws with 0.14 N continuous orthodontic tooth movement application | Autologous rat hip, xenograft human bone substitute material and synthetic graft β-TCP and HA. | µCT: BMD, BV/TV. Histomorphometric analysis: lamellar bone and woven bone | Autologous ABG better than synthetic ABG in new bone formation, is less resorbed, and is better integrated into the cleft defect. | β-TCP and HA combined with growth factors and cells might be used as an alternative to autologous ABG. |
| Putri et al., 2022, Indonesia [22] | True experimental, post-test-only control group design | Thirty-six male Wistar rats (Rattus novergicus) | 5 × 5 mm alveolar defect of the upper jaws | Autologous rat alveolar bone graft (ABG), human cancellous freeze-dried graft (HCG)–human adipose stem cell (hADSC), β-TCP–hADSC | Immunohistochemical analysis: runt-related transcription factor 2 (RUNX2), alkaline phosphatase (ALP), osterix (OSX), and bone morphogenetic protein 2 (BMP2) µCT: BV/TV (mm3), BF (%), and trabecular thickness of bone (TT, mm). | RUNX2, OSX, ALP, and BMP2 expression was enhanced in HCG-hADSC compared to β-TCP-hADSC and ABG. | Exogenous hADSC improved the ability of HCG and β-TCP to enhance osteogenesis, osteoconduction, and osteoinduction. |
| Kamal et al., 2017 German [23] | True experimental, post-test-only control group design | Sixteen male New Zealand rabbits. | Unilateral alveolar cleft defects | β-TCP, composite xenogenic dentin with β-TCP. | µCT: Defect size (mm3) Hounsfield unit (HU) % BV/TV, BMD. histomorphometric analysis: % bone formation % residual graft. | Dentin/β-TCP group showed significantly larger bone volume fraction (%) and residual graft (%) compared to β-TCP group. | Alveolar cleft defects repaired with dentin/β-TCP resulted in a larger graft residual volume and bone volume fraction. |
| Shahnaseri et al., 2020; Iran [24] | True experimental, post-test-only control group design | Four male canines | Unilateral alveolar bone cleft (15 mm) | Autologous AMSCs osteogenic differentiated seeded in HA/β-TCP scaffold, autologous tibia bone graft | Densitometer software with digital radiography: bone density histomorphometric: Bone regeneration (%) | Bone density and bone regeneration in autologous tibia bone grafts and autologous AMSCs with osteogenic differentiation planted in HA/β-TCP scaffolds did not differ in a statistically meaningful way. | Autologous AMSCs that are osteogenically differentiated and seeded in HA/β-TCP scaffolds can be used to reconstruct bone defects in patients who are unable to receive autogenous bone grafting when the size of the defect restricts the size of the autograft. |
| Pourebrahim et al., 2013; Iran [25] | True experimental, post-test-only control group design | Four male mongrel dogs | 15 mm alveolar cleft in the crest to nasal floor via removal of two of the three incisors bilaterally | Autogenous tibial graft, HA/β-TCP loaded canine AMSCs | Histomorphometric: bone regeneration (%) and collagen regeneration (%) | At 15 and 60 days, the autograft sides had more bone growth than the stem cell sides, at 45% and 96%, compared to 5% and 70%, respectively, with significant differences between groups. | Tissue-engineered HA/β-TCP-loaded cAMSCs might be a viable alternative, particularly if autografts are hard to come by or there is donor site morbidity. |
| Huang et al., 2015; China [26] | True experimental, post-test-only control group design | Fourteen 24-week-old male beagles | Unilateral alveolar bone defect with 15 mm size | Autogenous iliac crest bone graft; tissue-engineered bone (TEB) BMSCs/β-TCP with rapid maxillary expansion (RME) | Occlusal radiograph: height of the bone graft; Histomorphometric: bone formation (%) | In comparison to untreated dogs or dogs just receiving autogenous iliac bone after 8 weeks of therapy with TEB BMSCs-β-TCP, and RME, the dogs’ new bone production and mineralization were dramatically accelerated. | BMSCs-β-TCP also have the capacity to replace autogenous bone, and their combination with RME may be another option for treating alveolar clefts. |
| Ito et al., 2019; Japan [27] | True experimental, post-test-only control group design | Twenty male Sprague Dawley rats | Alveolar bone cleft in the palatine | Autogenous bone graft, β-TCP. | µCT: BV and BMD Histology analysis: Osteoblast, osteoclast, alkaline phosphatase, tartate-resistant acid phosphatase | Autologous bone grafts had a considerably larger bone volume and BMD than β-TCP. | β-TCP resulted in lower bone volume and BMD than autologous bone transplants. |
| Möhlhenrich et al., 2022 [28] | True experimental, post-test-only control group design | Twenty-one male Wistar rats (R. novergius) | Alveolar bone cleft | Autografts, human xenografts and synthetic bone substitute β-TCP/HA | μCT and histopathological investigation: tooth movement, and root resorption. | The differences in root resorption and tooth movement between the bone graft replacements and autologous bone were not statistically significant at any time. | Autografts, human xenografts, and synthetic bone substitutes used for cleft repair all appear to have a comparable influence on later orthodontic tooth movement and root resorption. |
| Hossain et al., 1996; Bangladesh [29] | True experimental, post-test-only control group design | Nine male beagles dog | Alveolar bone cleft | Autogenous particulate marrow and cancellous bone (PMCB), β-TCP and combination with experimental tooth movement | Radiograph analysis: bone deposition Histopathological investigation: bone regeneration | β-TCP showed a more pronounced biodegradative reaction to orthodontic force in connection with the production of new cementum. Root resorption was considerably lower in the β-TCP region than in the PMCB zone. | β-TCP is a more biocompatible option for autogenous bone transplantation into alveolar bone cleft defects that support orthodontic tooth movement. |
| de Ruiter et al., 2011; Netherland [30] | True experimental, post-test-only control group design | Ten adult Dutch milk goats (Capra hircus) | Alveolar bone cleft | β-TCP, autologous iliac crest bone graft | Histologic assessment: new bone formation and bone graft resorption. Radiographic measurement: orthodontic tooth movement. | An average tooth movement of 43.2% was measured in clefts restored with iliac bone and 41% in clefts rebuilt with β-TCP. | The bone substitute β-TCP is at least as successful as autologous iliac crest bone in the healing of alveolar clefts in goats, according to surgical, orthodontic, histologic, and radiologic viewpoints. |
| Zhang et al., 2011; China [31] | True experimental, post-test-only control group design | Six canines | Alveolar bone cleft | Porous β-TCP combined with osteogenically induced BMSCs and autologous iliac bone with experimental tooth movement | Occlusal radiographic: repaired alveolar cleft, residual alveolar height (%). Immunofluorescence: rate of bone formation and mineralization. Histological examination: area of the residual scaffold in the grafted region (%) and area of bone formation in the grafted region (%) | When compared to β-TCP alone, which was absorbed severely, BMSC-porous b-TCP significantly encouraged new bone formation and mineralization and attained a satisfactory height of the repaired alveolar cleft. | For patients with alveolar clefts and resultant orthodontic tooth movement, porous β-TCP in combination with osteogenically produced BMSCs may be a practical therapeutic technique as a replacement for bone transplants. |
| Janssen et al., 2017, Netherland [32] | True experimental, post-test-only control group design | Ten Dutch milk goats | Alveolar bone cleft defect | Microstructured beta-tricalcium phosphate (β-TCP) putty with autologous iliac bone | Histomorphometric and μCT: bone quality and BV/TV | There was no statistically significant difference between cleft sites and bone area percentages in β-TCP-CMCG and autologous bone grafts. | β-TCP-CMCG putty provides superior surgical handling in the correction of alveolar cleft deformities. |
| Ekin et al., 2015; Turkey [33] | True experimental, post-test-only control group design | Fifty-six Sprague Dawley rats | Critical-sized alveolar bone cleft defect | autograft, col/β-TCP scaffolds, and PLLA/PCL scaffolds | μCT: mineralized matrix formation, new bone formation, BV HPA: defect healing and new bone formation. RT-qPCR: Runx2, OSC, SPARC, BSP, ALP, and OSX | The autograft group had the greatest new bone volume rate at 1 month and 4 months. | The synthetic tissue scaffolds reported herein have significant potential as an alternative treatment option when cost, donor region morbidity, and hospitalization time are considered. |
| Tokugawa et al., 2012; Japan [34] | True experimental, post-test-only control group design | Ten female beagles dogs | Alveolar bone cleft defect | Canine BMSCs cultured on β-TCP, β-TCP. | μCT: BMD (mg/cm3) Bone mineral content (mg/mm); histopathological investigation: bone regeneration. | The regenerated bone in the MSCs/β-TCP group exhibited a bone mineral density that was midway between that of normal bone and that of β-TCP only. | cBMSCs-β-TCP-based bone regeneration offers a less invasive alternative to standard cancellous iliac bone autografts for alveolar bone replacement. |
| Authors, Year, Country | Study Design | Sample/ Subject Criteria (n) | Type of Cleft | Type of Tissue Engineering | Examinations and Variables | Outcome | Conclusion |
|---|---|---|---|---|---|---|---|
| Trujillo et al., 2018, USA [35] | Retrospective cohort study | Twenty-five patient CL/P: 15 and 10 females | Unilateral or bilateral clefts. | Iliac crest bone autograft Mandibular symphyseal bone graft, rhBMP-2/β-TCP bone substitute. | CBCT: Preoperative defect volume (cm3), postoperative residual defect volume (cm3), bone formation (%) | The bone formation was shown in the iliac crest group, which was followed by the rhBMP-2/ACS/β-TCP group and the mandibular symphysis group but was not significantly different between groups. | In alveolar cleft patients, rhBMP2 administered in a β-TCP scaffold may be an effective substitute for autogenous iliac crest. |
| Janssen et al., 2019, Netherland [36] | Case–control randomized clinical study, | Total of 20 CL/P patients: 14 males and 6 females | Unilateral CL/P | Microporous β-TCP | CBCT: Residual calcified tissue, spontaneous eruption of canine/lateral incisor, continuous alveolar process, residual oronasal fistula | No significant granule loss, surgical infection, or wound dehiscence occurred. The operating patients, who had an average residual calcified tissue volume of 65% one year after surgery, had no orinasal fistulas left. | In the clinical setting, SABG using microporous β-TCP is safe to utilize. |
| Du et al., 2017; China [37] | Clinical study | Ten CL/P patients (5 males and 5 females) | Unilateral alveolar cleft defects | Autologous iliac crest bone graft (ICBG), bone marrow mononuclear cells (BMMNCs) combined with β-TCP granules | CBCT and computer-aided engineering technology: bone volume (mm3), bone formation ratio (%), Bone volume (mm3) bone Formation ratio (%). bone union; Chelsea scale; duration of hospital stay (days). | Average defect volume, bone formation ratio (%), bone volume (mm3), and bone formation ratio (%) were not significantly different between groups. | Alveolar cleft repair with autologous BMMNCs and β-TCP granules was radiographically similar to using ICBG. |
| Miyagawa et al., 2020; Japan [38] | Clinical study | Thirty-one CL/P patients | Non-syndromic unilateral cleft lip and alveolus (UCLA) and cleft lip palate (UCLP) | Iliac crest bone, Mandibular symphysis, Mandibular symphysis combined with β-TCP granules. | CBCT: BV/TV, trabecular thickness (Tb.Th), trabecular number (Tb.N), trabecular separation (Tb.Sp), trabecular bone pattern factor (TBPf), structure model index (SMI), and fractal dimension (FD). | TBPs revealed variations between the IC and MS groups, leading to higher bone volume density values and a lower TBPf value in the IC group when compared to the MS group. | The use of β-TCP granules could produce similar results in the microstructure of the bone bridge. |
| de Ruiter et al., 2015; Netherland [39] | Prospective clinical study | Seven CL/P patients (5 males and 2 females) | Unilateral alveolar cleft | Micro-structured β-TCP | CBCT: cleft volume pre-operation; graft volume post-operation; bone volume 6 months post-operation | The bone volume thus gained was satisfactory six months following the surgical grafting of micro-structured β-TCP into the alveolar cleft: comparing the average bone volume to the initial cleft volume, 73% to 6%. | The therapeutic application of microstructured β-TCP bone replacement in alveolar cleft repair. |
| Weijs et al., 2010; Netherland [40] | Clinical study | Forty-seven CL/P patients (24 males and 23 females) | Unilateral alveolar cleft | Autogenous mandibular symphyseal bone only, mandibular symphyseal bone wrapped in /β-TCP granules | Occlusal radiograph: alveolar height and eruption disturbance. | There was no discernible difference in alveolar height or eruption disruption between the β-TCP granule group and the mandibular symphysis bone alone. | Autogenous mandibular symphyseal bone grafts enhanced with β-TCP granules can be utilized effectively in circumstances when the alveolar cleft is too big to be grafted with mandibular symphyseal bone alone. |
| Authors, Year, Country | CL/P or Alveolar Cleft Defect | β-TCP Utilization | Sample Preparation | Randomization | Blind Examiner | Test Method Clearly Reported | Complete Results | Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Möhlhenrich et al., 2021, German [21] | Y | Y | Y | Y | Y | Y | Y | Low |
| Putri et al., 2022, Indonesia [22] | Y | Y | Y | Y | Y | Y | Y | Low |
| Kamal et al., 2017 German [23] | Y | Y | Y | Y | Y | Y | Y | Low |
| Shahnaseri et al., 2020; Iran [24] | Y | Y | Y | N | N | N | N | High |
| Pourebrahim et al., 2013; Iran [25] | Y | Y | Y | Y | Y | Y | Y | Low |
| Huang et al., 2015; China [26] | Y | Y | Y | Y | Y | Y | Y | Low |
| Ito et al., 2019; Japan [27] | Y | Y | Y | Y | Y | Y | Y | Low |
| Möhlhenrich et al., 2022 [28] | Y | Y | Y | Y | Y | Y | Y | Low |
| Hossain et al., 1996; Bangladesh [29] | Y | Y | Y | Y | N | N | Y | Moderate |
| de Ruiter et al., 2011; Netherland [30] | Y | Y | Y | Y | Y | Y | Y | Low |
| Zhang et al., 2011; China [31] | Y | Y | Y | Y | Y | Y | Y | Low |
| Janssen et al., 2017, Netherland [32] | Y | Y | Y | Y | Y | Y | Y | Low |
| Ekin et al., 2015; Turkey [33] | Y | Y | Y | Y | Y | Y | Y | Low |
| Tokugawa et al., 2012; Japan [34] | Y | Y | Y | Y | Y | Y | Y | Low |
| Trujillo et al., 2018, USA [35] | Y | Y | Y | N | N | Y | Y | Moderate |
| Janssen et al., 2019, Netherland [36] | Y | Y | Y | N | N | Y | Y | Moderate |
| Du et al., 2017; China [37] | Y | Y | Y | Y | Y | Y | Y | Low |
| Miyagawa et al., 2020; Japan [38] | Y | Y | Y | N | N | Y | Y | Moderate |
| de Ruiter et al., 2015; Netherland [39] | Y | Y | Y | N | N | Y | Y | Moderate |
| Weijs et al., 2010; Netherland [40] | Y | Y | Y | N | N | N | Y | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nugraha, A.P.; Yang, H.; Chen, J.; Yang, K.; Kraisintu, P.; Zaww, K.; Ma, A.; Wang, R.; Alhadi, N.E.A.M.; Vanegas Sáenz, J.R.; et al. β-Tricalcium Phosphate as Alveolar Bone Grafting in Cleft Lip/Palate: A Systematic Review. Dent. J. 2023, 11, 234. https://doi.org/10.3390/dj11100234
Nugraha AP, Yang H, Chen J, Yang K, Kraisintu P, Zaww K, Ma A, Wang R, Alhadi NEAM, Vanegas Sáenz JR, et al. β-Tricalcium Phosphate as Alveolar Bone Grafting in Cleft Lip/Palate: A Systematic Review. Dentistry Journal. 2023; 11(10):234. https://doi.org/10.3390/dj11100234
Chicago/Turabian StyleNugraha, Alexander Patera, Hui Yang, Junduo Chen, Kunhua Yang, Ploypim Kraisintu, Kyaw Zaww, Aobo Ma, Ruixian Wang, Nada Emad Alshafei Mohamed Alhadi, Juan Ramón Vanegas Sáenz, and et al. 2023. "β-Tricalcium Phosphate as Alveolar Bone Grafting in Cleft Lip/Palate: A Systematic Review" Dentistry Journal 11, no. 10: 234. https://doi.org/10.3390/dj11100234
APA StyleNugraha, A. P., Yang, H., Chen, J., Yang, K., Kraisintu, P., Zaww, K., Ma, A., Wang, R., Alhadi, N. E. A. M., Vanegas Sáenz, J. R., & Hong, G. (2023). β-Tricalcium Phosphate as Alveolar Bone Grafting in Cleft Lip/Palate: A Systematic Review. Dentistry Journal, 11(10), 234. https://doi.org/10.3390/dj11100234








