In-Vitro Phenotypic Response of Human Osteoblasts to Different Degrees of Titanium Surface Roughness
Abstract
:1. Introduction
- There is no difference in the proliferation of the HOB cells in contact with Ti discs of various degrees of surface roughness;
- There is no difference in the cytotoxicity of the HOB cells in contact with Ti discs of various degrees of surface roughness and that;
- There is no effect on either the proliferation or cytotoxicity of the HOB cells with different exposure times for each surface roughness degree.
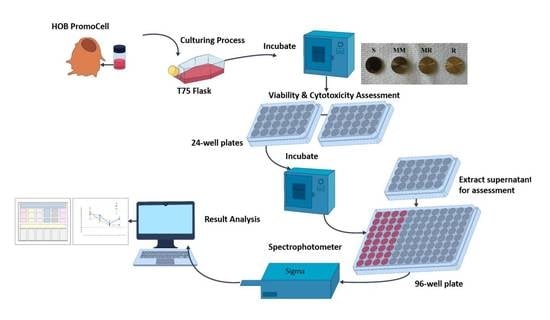
2. Materials and Methods
2.1. Specimens’ Preparation
2.2. Surface Roughness Measurements
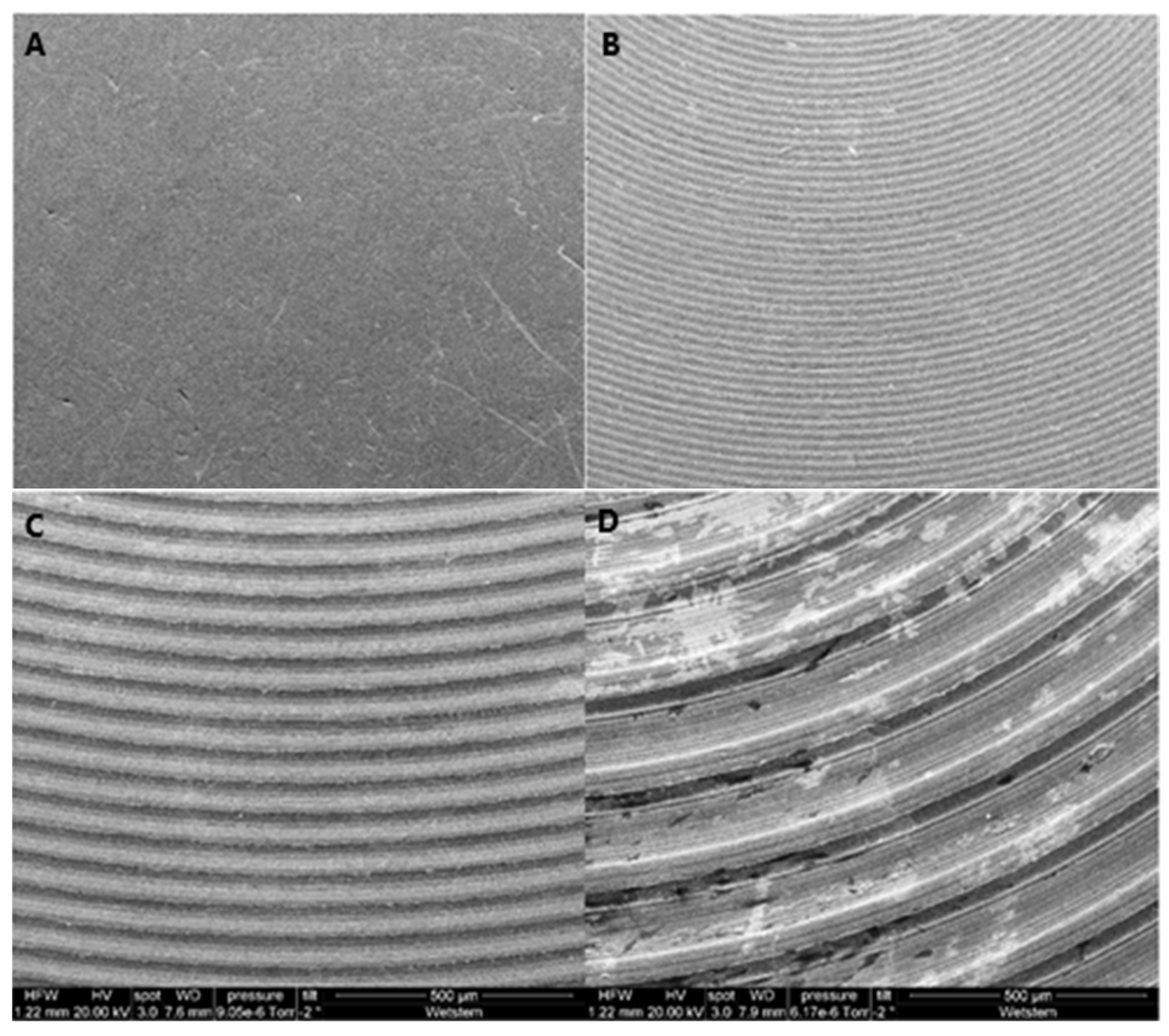
2.3. Surface Morphology Analysis
2.4. HOB Cell Culture Preparation
2.5. Cell Viability
2.6. Cytotoxicity
2.7. Statistical Analysis
3. Results
3.1. Surface Roughness
3.2. Surface Morphology Analysis
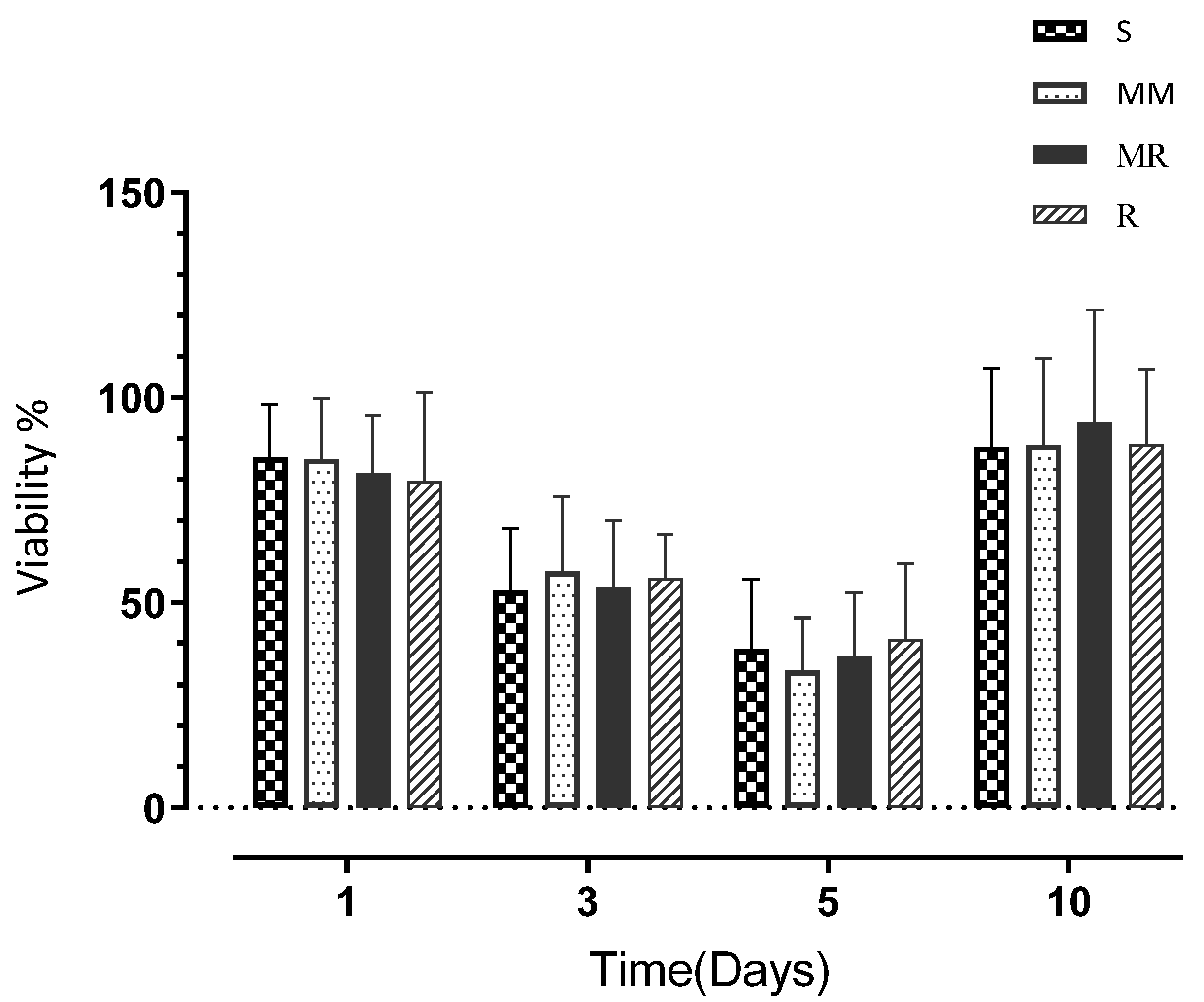
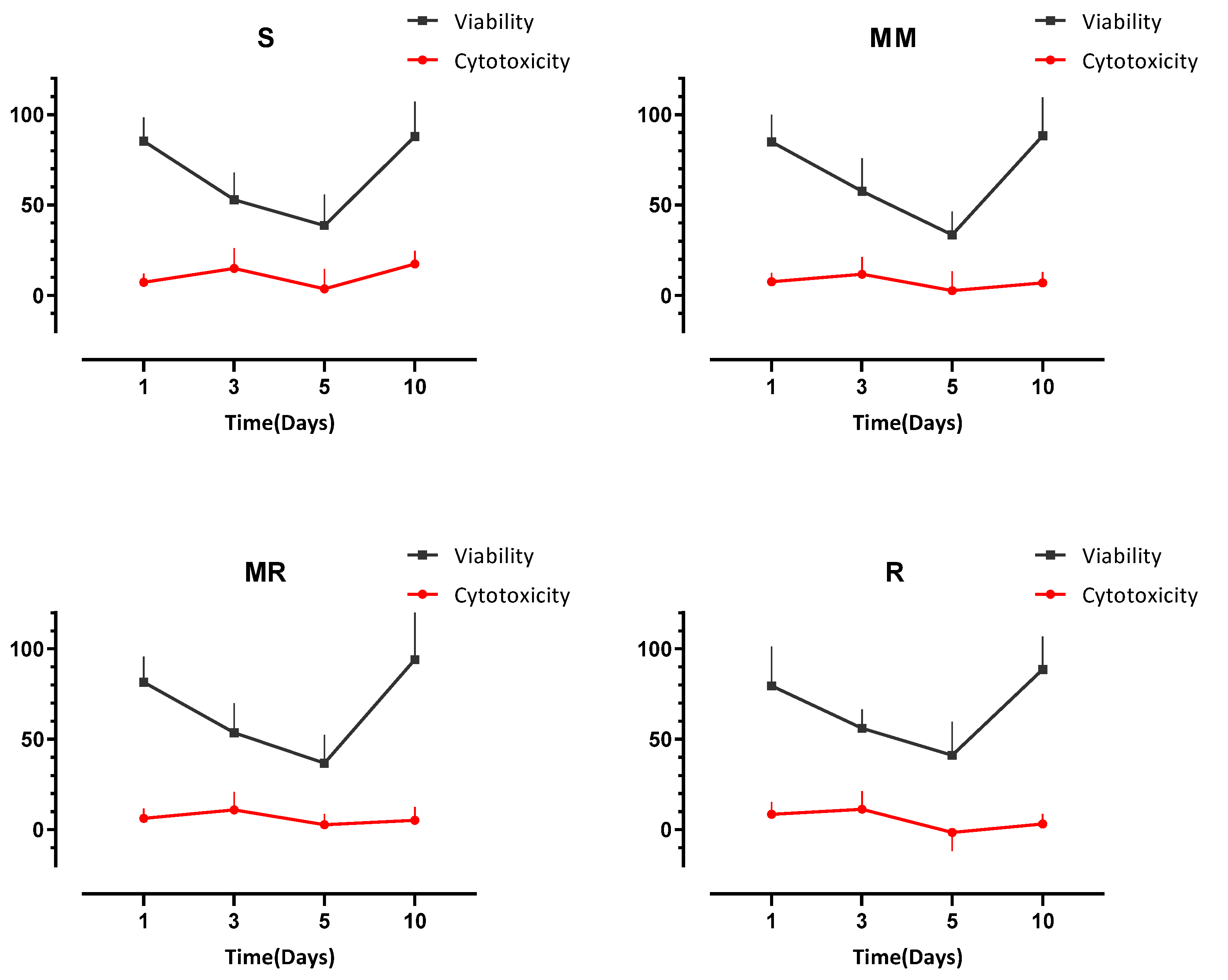
3.3. Cell Viability
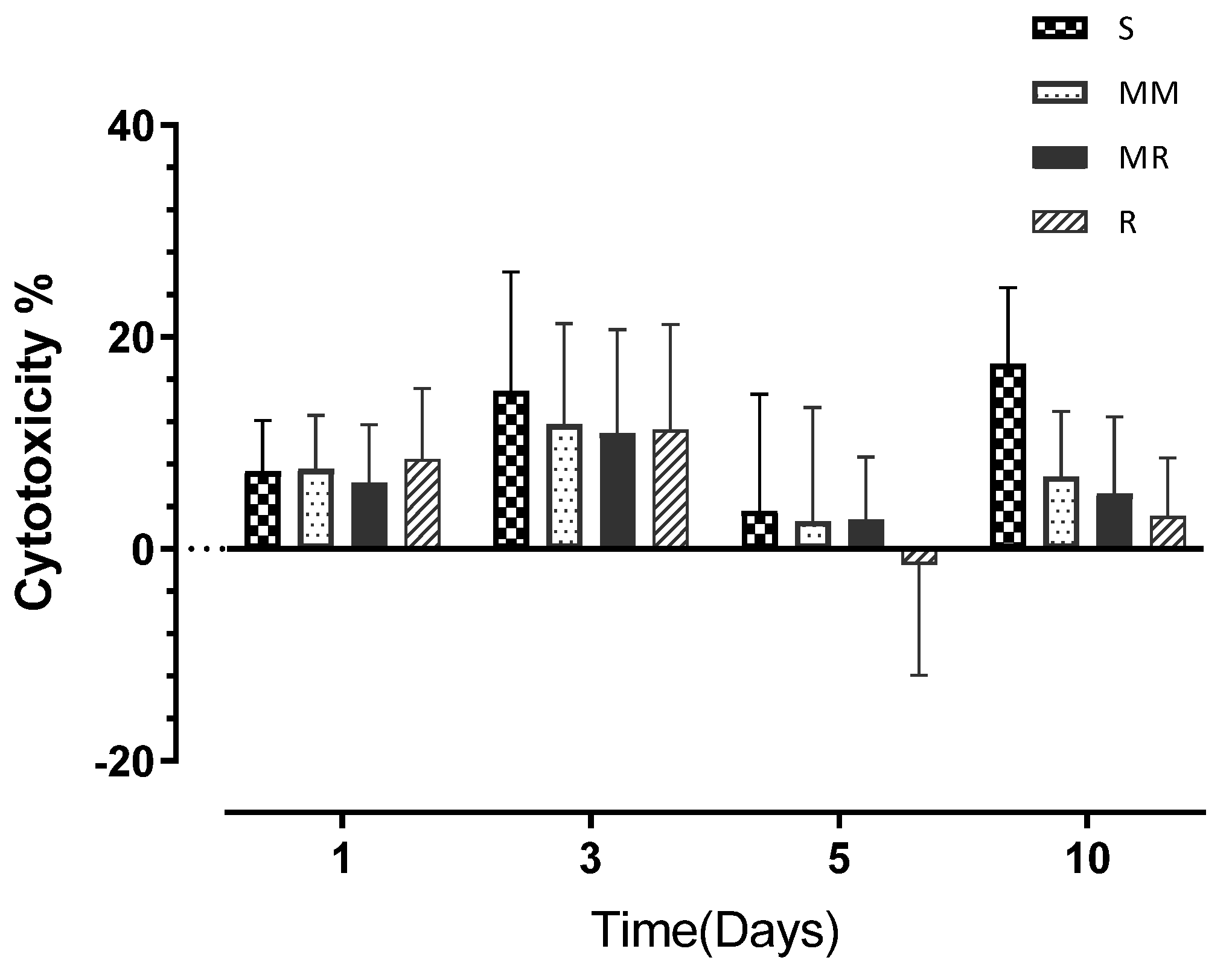
3.4. Cell Cytotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adell, R.; Hansson, B.O.; Branemark, P.I.; Breine, U. Intra-osseous anchorage of dental prostheses. II. Review of clinical approaches. Scand. J. Plast. Reconstr. Surg. 1970, 4, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Wennerberg, A. The impact of oral implants—Past and future, 1966–2042. J. Can. Dent. Assoc. 2005, 71, 327. [Google Scholar] [PubMed]
- Branemark, P.I.; Adell, R.; Breine, U.; Hansson, B.O.; Lindstrom, J.; Ohlsson, A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Branemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindstrom, J.; Hallen, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surg. Suppl. 1977, 16, 1–132. [Google Scholar] [PubMed]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Huttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Thomsen, P.; Larsson, C.; Ericson, L.E.; Sennerby, L.; Lausmaa, J.; Kasemo, B. Structure of the interface between rabbit cortical bone and implants of gold, zirconium and titanium. J. Mater. Sci. Mater. Med. 1997, 8, 653–665. [Google Scholar] [CrossRef]
- Brunette, D.M.; Tengvall, P.; Textor, M.; Thomsen, P. Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Sola-Ruiz, M.F.; Perez-Martinez, C.; Labaig-Rueda, C.; Carda, C.; Martin De Llano, J.J. Behavior of Human Osteoblast Cells Cultured on Titanium Discs in Relation to Surface Roughness and Presence of Melatonin. Int. J. Mol. Sci. 2017, 18, 823. [Google Scholar] [CrossRef] [Green Version]
- Albrektsson, T.; Branemark, P.I.; Hansson, H.A.; Lindstrom, J. Osseointegrated titanium implants: Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [Green Version]
- Ananth, H.; Kundapur, V.; Mohammed, H.S.; Anand, M.; Amarnath, G.S.; Mankar, S. A Review on Biomaterials in Dental Implantology. Int. J. Biomed. Sci. 2015, 11, 113–120. [Google Scholar]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New Developments of Ti-Based Alloys for Biomedical Applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef] [Green Version]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases 2015, 3, 52–57. [Google Scholar] [CrossRef]
- Civantos, A.; Martinez-Campos, E.; Ramos, V.; Elvira, C.; Gallardo, A.; Abarrategi, A. Titanium Coatings and Surface Modifications: Toward Clinically Useful Bioactive Implants. ACS Biomater. Sci. Eng. 2017, 3, 1245–1261. [Google Scholar] [CrossRef]
- Bostman, O.; Hirvensalo, E.; Makinen, J.; Rokkanen, P. Foreign-body reactions to fracture fixation implants of biodegradable synthetic polymers. J. Bone Jt. Surg. Br. Vol. 1990, 72, 592–596. [Google Scholar] [CrossRef]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [Green Version]
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Asri, R.I.M.; Harun, W.S.W.; Samykano, M.; Lah, N.A.C.; Ghani, S.A.C.; Tarlochan, F.; Raza, M.R. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. C 2017, 77, 1261–1274. [Google Scholar] [CrossRef] [Green Version]
- Neoh, K.G.; Hu, X.; Zheng, D.; Kang, E.T. Balancing osteoblast functions and bacterial adhesion on functionalized titanium surfaces. Biomaterials 2012, 33, 2813–2822. [Google Scholar] [CrossRef]
- Boyan, B.D.; Lossdorfer, S.; Wang, L.; Zhao, G.; Lohmann, C.H.; Cochran, D.L.; Schwartz, Z. Osteoblasts generate an osteogenic microenvironment when grown on surfaces with rough microtopographies. Eur. Cells Mater. 2003, 6, 22–27. [Google Scholar] [CrossRef]
- Zhao, G.; Zinger, O.; Schwartz, Z.; Wieland, M.; Landolt, D.; Boyan, B.D. Osteoblast-like cells are sensitive to submicron-scale surface structure. Clin. Oral Implant. Res. 2006, 17, 258–264. [Google Scholar] [CrossRef]
- Schwartz, Z.; Raz, P.; Zhao, G.; Barak, Y.; Tauber, M.; Yao, H.; Boyan, B.D. Effect of micrometer-scale roughness of the surface of Ti6Al4V pedicle screws in vitro and in vivo. J. Bone Jt. Surg. Am. Vol. 2008, 90, 2485–2498. [Google Scholar] [CrossRef] [Green Version]
- De Bruyn, H.; Christiaens, V.; Doornewaard, R.; Jacobsson, M.; Cosyn, J.; Jacquet, W.; Vervaeke, S. Implant surface roughness and patient factors on long-term peri-implant bone loss. Periodontology 2000 2017, 73, 218–227. [Google Scholar] [CrossRef]
- Cochran, D.L. A comparison of endosseous dental implant surfaces. J. Periodontol. 1999, 70, 1523–1539. [Google Scholar] [CrossRef]
- Larsson, L.; Pilipchuk, S.P.; Giannobile, W.V.; Castilho, R.M. When epigenetics meets bioengineering-A material characteristics and surface topography perspective. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2065–2071. [Google Scholar] [CrossRef]
- Fickl, S.; Kebschull, M.; Calvo-Guirado, J.L.; Hurzeler, M.; Zuhr, O. Experimental Peri-Implantitis around Different Types of Implants—A Clinical and Radiographic Study in Dogs. Clin. Implant. Dent. Relat. Res. 2015, 17 (Suppl. S2), e661–e669. [Google Scholar] [CrossRef]
- Stepanovska, J.; Matejka, R.; Rosina, J.; Bacakova, L.; Kolarova, H. Treatments for enhancing the biocompatibility of titanium implants. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2020, 164, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20 (Suppl. S4), 172–184. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 1—Review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 2004, 17, 536–543. [Google Scholar]
- Zitzmann, N.U.; Berglundh, T. Definition and prevalence of peri-implant diseases. J. Clin. Periodontol. 2008, 35, 286–291. [Google Scholar] [CrossRef]
- Rupp, F.; Scheideler, L.; Rehbein, D.; Axmann, D.; Geis-Gerstorfer, J. Roughness induced dynamic changes of wettability of acid etched titanium implant modifications. Biomaterials 2004, 25, 1429–1438. [Google Scholar] [CrossRef]
- Alamoush, R.A.; Kushnerev, E.; Yates, J.M.; Satterthwaite, J.D.; Silikas, N. Response of two gingival cell lines to CAD/CAM composite blocks. Dent. Mater. 2020, 36, 1214–1225. [Google Scholar] [CrossRef]
- Scientific, T.F. AlamarBlue®Assay; Thermo Fisher Scientific Inc.: Waltham, MA, USA, 2018; Available online: http://tools.thermofisher.com/content/sfs/manuals/PI-DAL1025-1100TI%20alamarBlue%20Rev%201.1.pdf (accessed on 1 January 2020).
- Scientific, T.F. Pierce LDHcytotoxicity Assay Kit; Thermo Fisher Scientific Inc.: Waltham, MA, USA, 2014; Available online: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/MAN0011851PierceLDHCytotoxicityAsyUG.pdf (accessed on 1 January 2020).
- Kieswetter, K.; Schwartz, Z.; Dean, D.D.; Boyan, B.D. The role of implant surface characteristics in the healing of bone. Crit. Rev. Oral Biol. Med. 1996, 7, 329–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, P.G.; Granjeiro, J.M.; Romanos, G.E.; Suzuki, M.; Silva, N.R.; Cardaropoli, G.; Thompson, V.P.; Lemons, J.E. Basic research methods and current trends of dental implant surfaces. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfest, D.M.D.; Coelho, P.G.; Kang, B.S.; Sul, Y.T.; Albrektsson, T. Classification of osseointegrated implant surfaces: Materials, chemistry and topography. Trends Biotechnol. 2010, 28, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Ellingsen, J.E. Surface configurations of dental implants. Periodontology 2000 1998, 17, 36–46. [Google Scholar] [CrossRef]
- Goriainov, V.; Cook, R.; Latham, J.M.; Dunlop, D.G.; Oreffo, R. Bone and metal: An orthopaedic perspective on osseointegration of metals. Acta Biomater. 2014, 10, 4043–4057. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Suggested guidelines for the topographic evaluation of implant surfaces. Int. J. Oral Maxillofac. Implant. 2000, 15, 331–344. [Google Scholar]
- Ronold, H.J.; Lyngstadaas, S.P.; Ellingsen, J.E. Analysing the optimal value for titanium implant roughness in bone attachment using a tensile test. Biomaterials 2003, 24, 4559–4564. [Google Scholar] [CrossRef]
- Yang, A.; Han, Y.; Pan, Y.; Xing, H.; Li, J. Optimum surface roughness prediction for titanium alloy by adopting response surface methodology. Results Phys. 2017, 7, 1046–1050. [Google Scholar] [CrossRef]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef]
- Holthaus, M.G.; Treccani, L.; Rezwan, K. Osteoblast viability on hydroxyapatite with well-adjusted submicron and micron surface roughness as monitored by the proliferation reagent WST-1. J. Biomater. Appl. 2013, 27, 791–800. [Google Scholar] [CrossRef]
- Anselme, K.; Bigerelle, M.; Noel, B.; Iost, A.; Hardouin, P. Effect of grooved titanium substratum on human osteoblastic cell growth. J. Biomed. Mater. Res. 2002, 60, 529–540. [Google Scholar] [CrossRef]
- Brett, P.M.; Harle, J.; Salih, V.; Mihoc, R.; Olsen, I.; Jones, F.H.; Tonetti, M. Roughness response genes in osteoblasts. Bone 2004, 35, 124–133. [Google Scholar] [CrossRef]
- Degasne, I.; Basle, M.F.; Demais, V.; Hure, G.; Lesourd, M.; Grolleau, B.; Mercier, L.; Chappard, D. Effects of roughness, fibronectin and vitronectin on attachment, spreading, and proliferation of human osteoblast-like cells (Saos-2) on titanium surfaces. Calcif. Tissue Int. 1999, 64, 499–507. [Google Scholar] [CrossRef]
- Balloni, S.; Calvi, E.M.; Damiani, F.; Bistoni, G.; Calvitti, M.; Locci, P.; Becchetti, E.; Marinucci, L. Effects of titanium surface roughness on mesenchymal stem cell commitment and differentiation signaling. Int. J. Oral Maxillofac. Implant. 2009, 24, 627–635. [Google Scholar]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Kim, C.W.; Lim, Y.J.; Heo, S.J. Microrough titanium surface affects biologic response in MG63 osteoblast-like cells. J. Biomed. Mater. Res. Part A 2006, 79, 1023–1032. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Gittens, R.A.; Schneider, J.M.; Hyzy, S.L.; Haithcock, D.A.; Ullrich, P.F.; Schwartz, Z.; Boyan, B.D. Osteoblasts exhibit a more differentiated phenotype and increased bone morphogenetic protein production on titanium alloy substrates than on poly-ether-ether-ketone. Spine J. 2012, 12, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, A.C. The regulation of cell size. Cell 2013, 154, 1194–1205. [Google Scholar] [CrossRef] [Green Version]
- Abushahba, F.; Tuukkanen, J.; Aalto-Setala, L.; Miinalainen, I.; Hupa, L.; Narhi, T.O. Effect of bioactive glass air-abrasion on the wettability and osteoblast proliferation on sandblasted and acid-etched titanium surfaces. Eur. J. Oral Sci. 2020, 128, 160–169. [Google Scholar] [CrossRef]
- Ball, M.D.; Downes, S.; Scotchford, C.A.; Antonov, E.N.; Bagratashvili, V.N.; Popov, V.K.; Lo, W.J.; Grant, D.M.; Howdle, S.M. Osteoblast growth on titanium foils coated with hydroxyapatite by pulsed laser ablation. Biomaterials 2001, 22, 337–347. [Google Scholar] [CrossRef]
- Hayashi, K.; Inadome, T.; Tsumura, H.; Nakashima, Y.; Sugioka, Y. Effect of surface roughness of hydroxyapatite-coated titanium on the bone-implant interface shear strength. Biomaterials 1994, 15, 1187–1191. [Google Scholar] [CrossRef]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2001, 22, 87–96. [Google Scholar] [CrossRef]
- Kunzler, T.P.; Drobek, T.; Schuler, M.; Spencer, N.D. Systematic study of osteoblast and fibroblast response to roughness by means of surface-morphology gradients. Biomaterials 2007, 28, 2175–2182. [Google Scholar] [CrossRef]
- Iso, B.; Standard, B. Biological evaluation of medical devices. Part 2009, 1, 10993. [Google Scholar]
- Standardization, I.O.f. Biological Evaluation of Medical Devices: Sample Preparation and Reference Materials; ISO: Geneva, Switzerland, 2007. [Google Scholar]
- Pabst, A.; Walter, C.; Bell, A.; Weyhrauch, M.; Schmidtmann, I.; Scheller, H.; Lehmann, K. Influence of CAD/CAM zirconia for implant-abutment manufacturing on gingival fibroblasts and oral keratinocytes. Clin. Oral Investig. 2016, 20, 1101–1108. [Google Scholar] [CrossRef]
- Pendegrass, C.; Fontaine, C.; Chan, G.; Hosseini, P.; Blunn, G. Intraosseous transcutaneous amputation prostheses vs dental implants: A comparison between keratinocytes and gingival cell adhesion in vitro. Eur. Cells Mater. 2015, 29, 237–249. [Google Scholar] [CrossRef]
- De Melo, W.M.; Maximiano, W.M.A.; Antunes, A.A.; Beloti, M.M.; Rosa, A.L.; de Oliveira, P.T. Cytotoxicity testing of methyl and ethyl 2-cyanoacrylate using direct contact assay on osteoblast cell cultures. J. Oral Maxillofac. Surg. 2013, 71, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Naganawa, T.; Ishihara, Y.; Iwata, T.; Koide, M.; Ohguchi, M.; Ohguchi, Y.; Murase, Y.; Kamei, H.; Sato, N.; Mizuno, M.; et al. In vitro biocompatibility of a new titanium-29niobium-13tantalum-4.6zirconium alloy with osteoblast-like MG63 cells. J. Periodontol. 2004, 75, 1701–1707. [Google Scholar] [CrossRef] [PubMed]

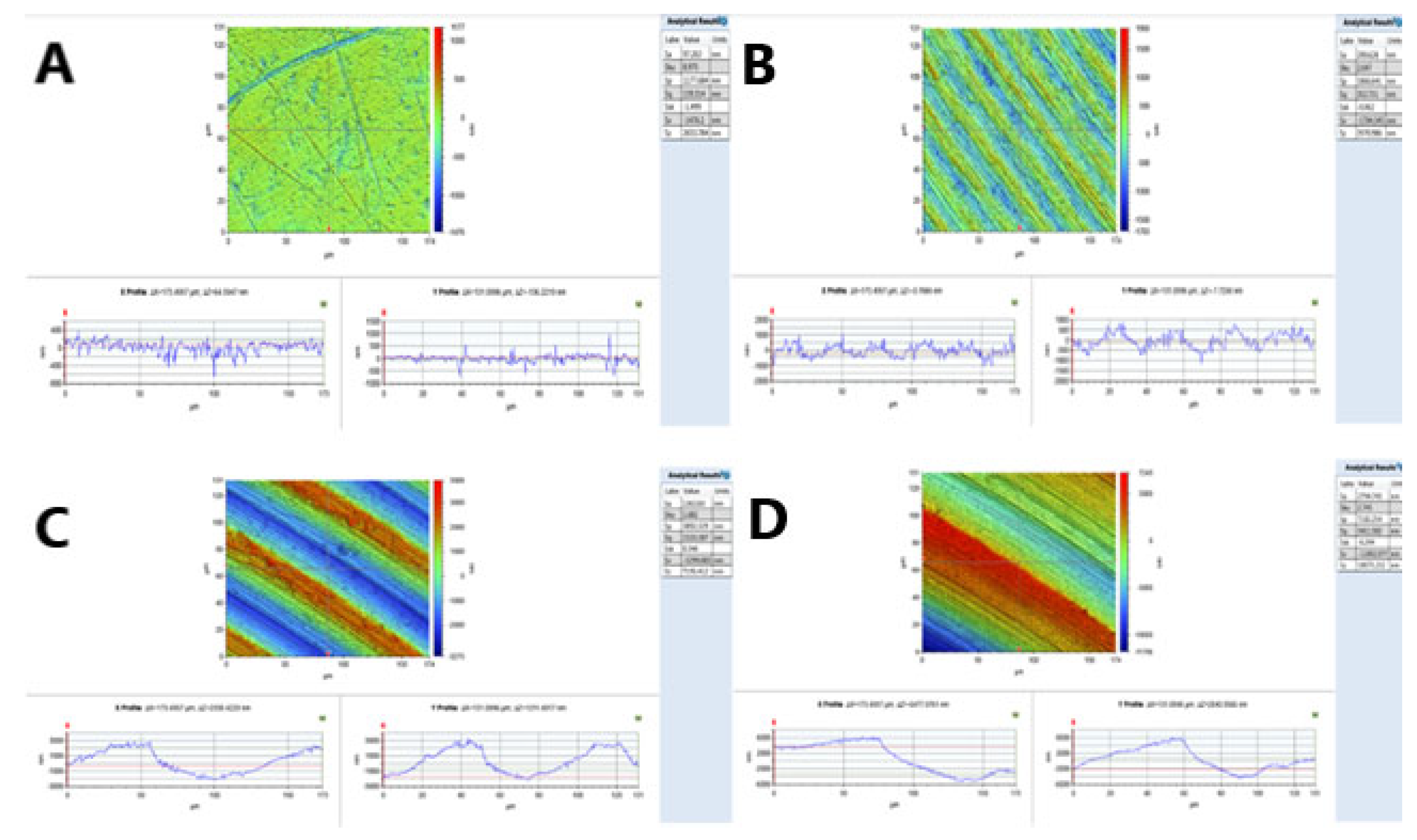
| Roughness Degree | Smooth (S) | Minimally Rough (MM) | Moderately Rough (MR) | Rough (R) |
|---|---|---|---|---|
| Sa value: mean (µm) | 0.11 µm (0.01) | 0.39 µm (0.09) | 1.33 µm (0.02) | 3.34 µm (0.06) |
| Minimum Sa value (µm) | 0.18 µm | 0.27 µm | 1.25 µm | 2.4 µm |
| Maximum Sa value (µm) | 0.08 µm | 0.5 µm | 1.47 µm | 3.79 µm |
| Surface Roughness | Smooth (S) | Minimally Rough (MM) | Moderately Rough (MR) | Rough (R) | ||||
|---|---|---|---|---|---|---|---|---|
| Time (days) | P% | SD% | P% | SD% | P% | SD% | P% | SD% |
| Day 1 | 85.36 | (12.87) | 84.99 | (14.88) | 81.54 | (14.12) | 79.49 | (21.72) |
| Day 3 | 52.94 | (14.99) | 57.57 | (18.17) | 53.61 | (16.30) | 56.03 | (10.49) |
| Day 5 | 38.66 | (17.08) | 33.46 | (18.17) | 36.69 | (15.70) | 41.10 | (18.46) |
| Day 10 | 87.90 | (19.19) | 88.32 | (21.05) | 93.96 | (27.36) | 88.63 | (18.26) |
| Surface Roughness | Smooth (S) | Minimally Rough (MM) | Moderately Rough (MR) | Rough (R) | ||||
|---|---|---|---|---|---|---|---|---|
| Time (Days) | Mean% | SD% | Mean% | SD% | Mean% | SD% | Mean% | SD% |
| Day 1 | 7.33 | (4.77) | 7.53 | (5.07) | 6.24 | (5.48) | 8.46 | (6.68) |
| Day 3 | 14.90 | (11.23) | 11.74 | (9.54) | 10.94 | (9.79) | 11.25 | (9.95) |
| Day 5 | 3.60 | (11.01) | 2.64 | (10.69) | 2.78 | (5.89) | −1.49 | (10.44) |
| Day 10 | 17.45 | (7.19) | 6.84 | (6.15) | 5.21 | (7.25) | 3.13 | (5.45) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, M.A.; Alamoush, R.A.; Kushnerev, E.; Seymour, K.G.; Shawcross, S.; Yates, J.M. In-Vitro Phenotypic Response of Human Osteoblasts to Different Degrees of Titanium Surface Roughness. Dent. J. 2022, 10, 140. https://doi.org/10.3390/dj10080140
Osman MA, Alamoush RA, Kushnerev E, Seymour KG, Shawcross S, Yates JM. In-Vitro Phenotypic Response of Human Osteoblasts to Different Degrees of Titanium Surface Roughness. Dentistry Journal. 2022; 10(8):140. https://doi.org/10.3390/dj10080140
Chicago/Turabian StyleOsman, Muataz A., Rasha A. Alamoush, Evgeny Kushnerev, Kevin G. Seymour, Susan Shawcross, and Julian M. Yates. 2022. "In-Vitro Phenotypic Response of Human Osteoblasts to Different Degrees of Titanium Surface Roughness" Dentistry Journal 10, no. 8: 140. https://doi.org/10.3390/dj10080140
APA StyleOsman, M. A., Alamoush, R. A., Kushnerev, E., Seymour, K. G., Shawcross, S., & Yates, J. M. (2022). In-Vitro Phenotypic Response of Human Osteoblasts to Different Degrees of Titanium Surface Roughness. Dentistry Journal, 10(8), 140. https://doi.org/10.3390/dj10080140