Medication-Related Osteonecrosis of the Jaw in Dental Practice: A Retrospective Analysis of Data from the Milan Cohort
Abstract
1. Introduction
2. Materials and Methods
3. Results
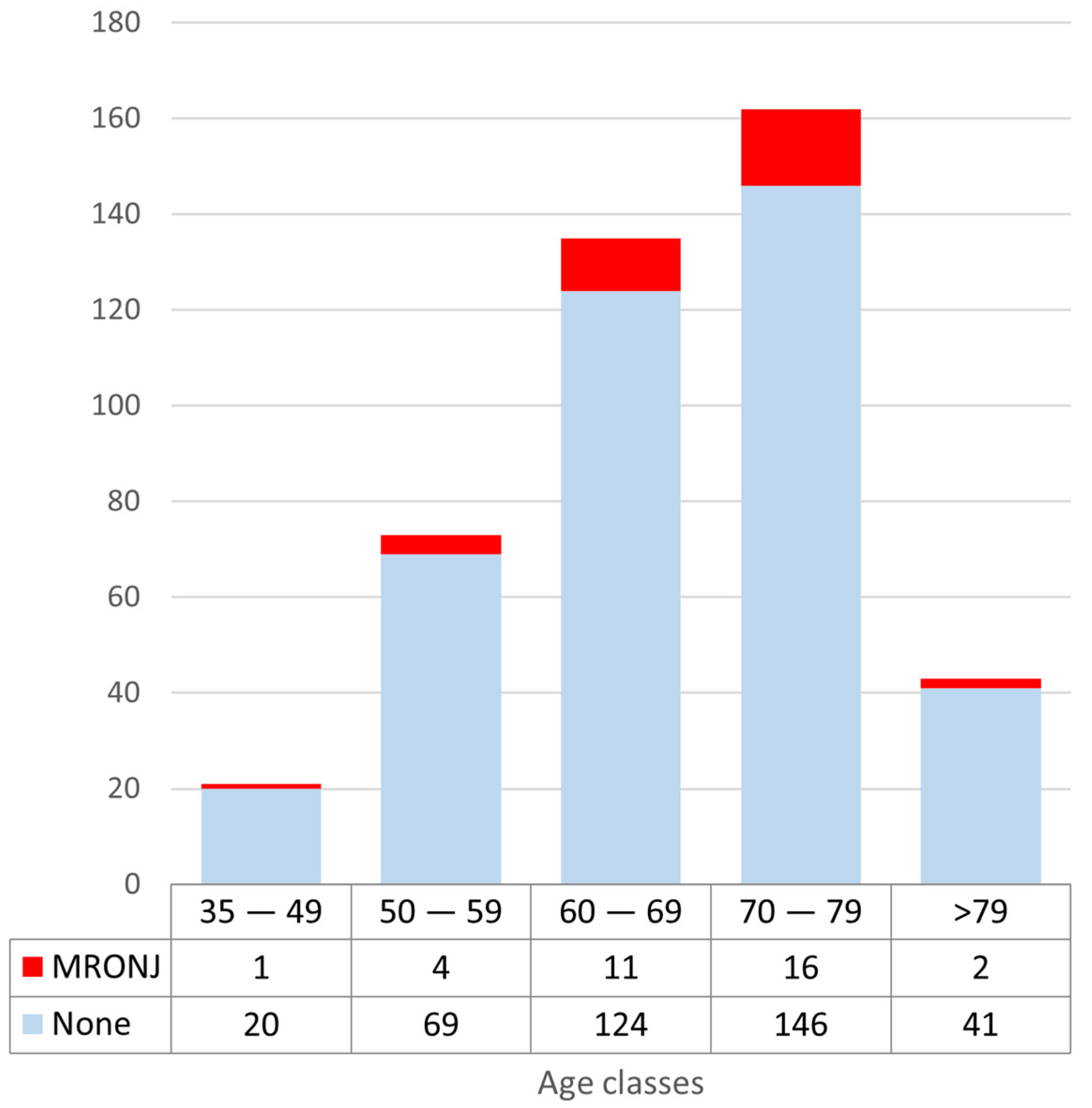
3.1. Incidence of MRONJ by Age
3.2. Incidence of MRONJ by Gender
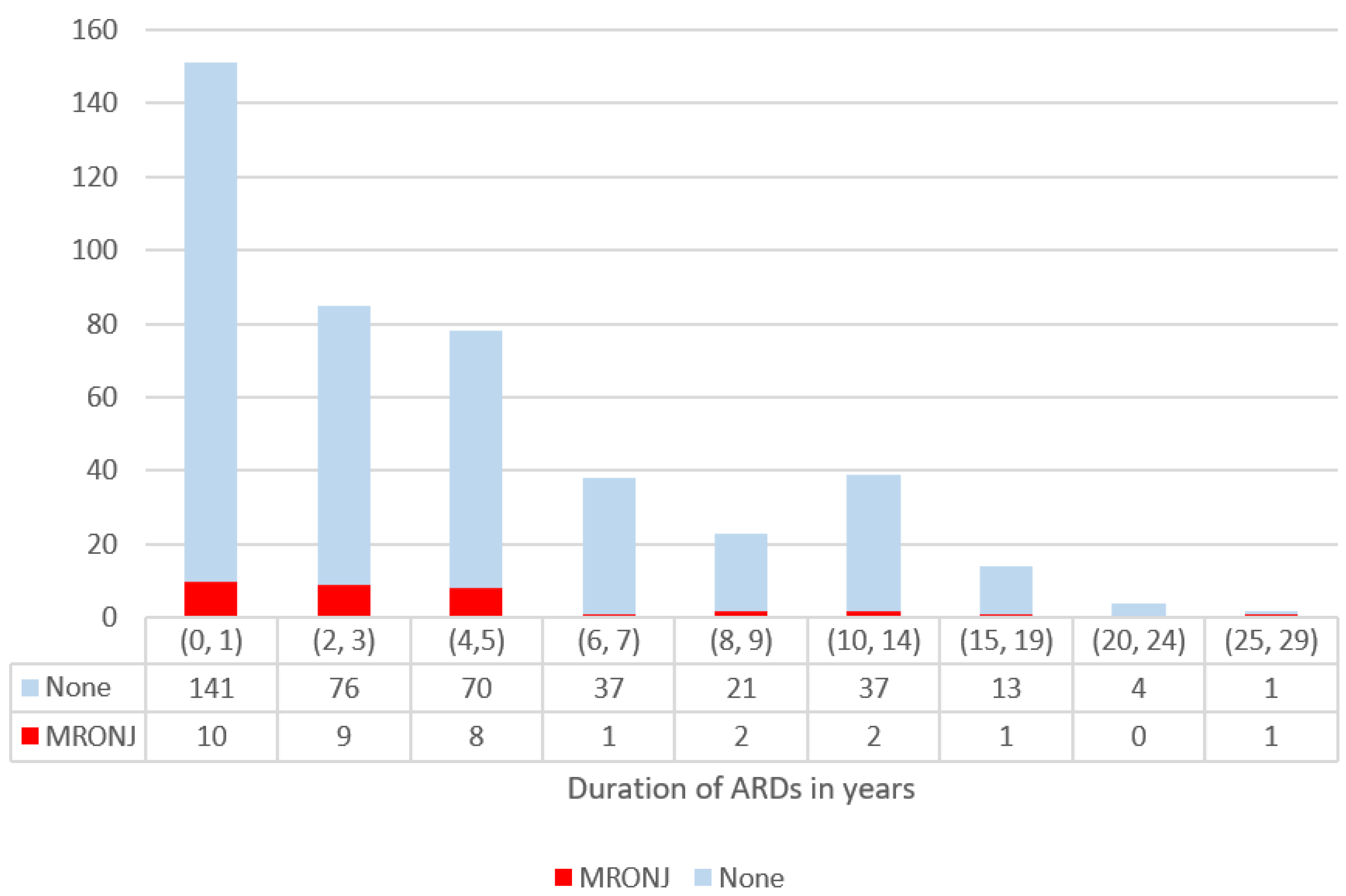
3.3. Incidence of MRONJ by Duration of Treatment with ARD
3.4. Association with Known Pathologies
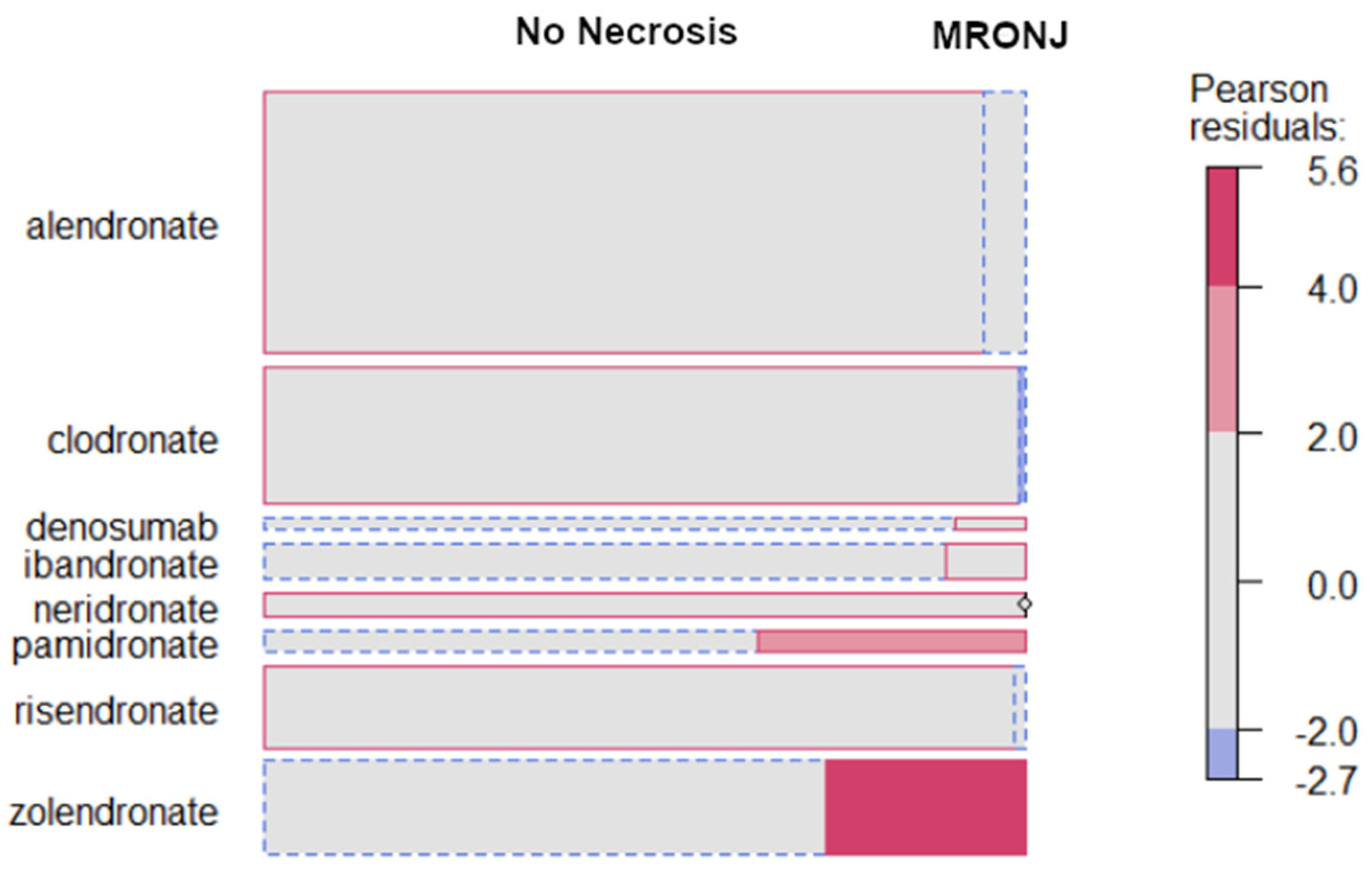
3.5. Incidence of MRONJ by Type of ARD
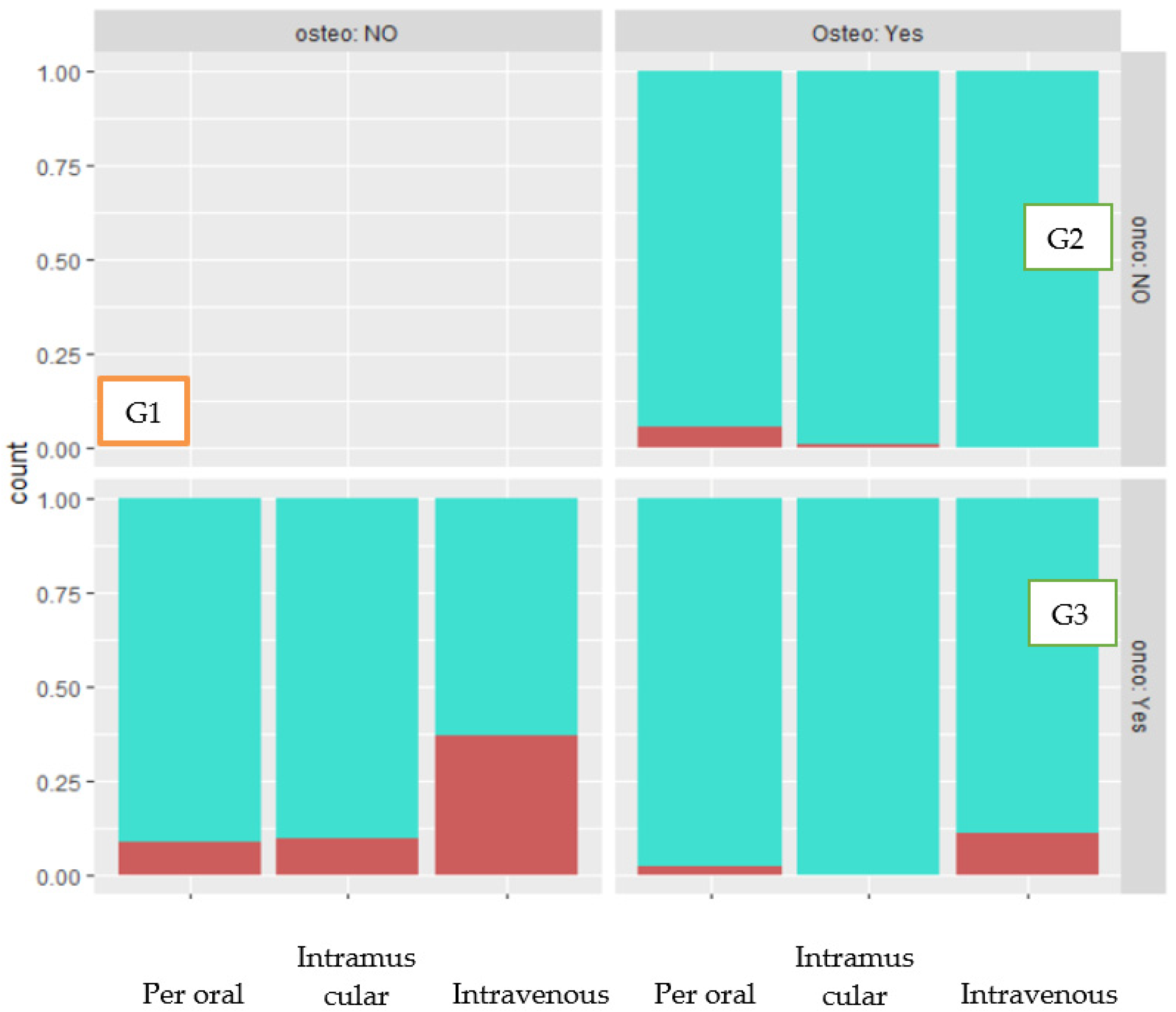
3.6. Incidence of MRONJ by Route of Administration
3.7. Incidence of MRONJ in Patients with Other Concurrent Diseases
3.8. Incidence of MRONJ in Patients Receiving Other Drugs
3.9. Incidence of MRONJ in Relation to Smoking
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Marx, R.E.; Cillo, J.E.; Ulloa, J.J. Oral Bisphosphonate-Induced Osteonecrosis: Risk Factors, Prediction of Risk Using Serum CTX Testing, Prevention, and Treatment. J. Oral Maxillofac. Surg. 2007, 65, 2397–2410. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Mehrotra, B.; Rosenberg, T.J.; Engroff, S.L. Osteonecrosis of the Jaws Associated with the Use of Bisphosphonates: A Review of 63 Cases. J. Oral Maxillofac. Surg. 2004, 62, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, D.; Shetty, S.; Ashworth, D.; Grew, N.; Millar, B. Orofacial pain—A presenting symptom of bisphosphonate associated osteonecrosis of the jaws. Br. Dent. J. 2007, 203, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaw-2022 Update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef]
- Yarom, N.; Shapiro, C.L.; Peterson, D.E.; Van Poznak, C.H.; Bohlke, K.; Ruggiero, S.L.; Migliorati, C.A.; Khan, A.; Morrison, A.; Anderson, H.; et al. Medication-Related Osteonecrosis of the Jaw: MASCC/ISOO/ASCO Clinical Practice Guideline. J Clin. Oncol. 2019, 37, 2270–2290. [Google Scholar] [CrossRef]
- Aljohani, S.; Fliefel, R.; Ihbe, J.; Kühnisch, J.; Ehrenfeld, M.; Otto, S. What is the effect of anti-resorptive drugs (ARDs) on the development of medication-related osteonecrosis of the jaw (MRONJ) in osteoporosis patients: A systematic review. J. Cranio-Maxillofac. Surg. 2017, 45, 1493–1502. [Google Scholar] [CrossRef]
- Khamaisi, M.; Regev, E.; Yarom, N.; Avni, B.; Leitersdorf, E.; Raz, I.; Elad, S. Possible association between diabetes and bisphosphonate-related jaw osteonecrosis. J. Clin. Endocrinol. Metab. 2007, 92, 1172–1175. [Google Scholar] [CrossRef]
- Bamias, A.; Kastritis, E.; Bamia, C.; Moulopoulos, L.A.; Melakopoulos, I.; Bozas, G.; Koutsoukou, V.; Gika, D.; Anagnostopoulos, A.; Papadimitriou, C.; et al. Osteonecrosis of the Jaw in Cancer After Treatment with Bisphosphonates: Incidence and Risk Factors. J. Clin. Oncol. 2005, 23, 8580–8587. [Google Scholar] [CrossRef]
- Gabbert, T.I.; Hoffmeister, B.; Felsenberg, D. Risk factors influencing the duration of treatment with bisphosphonates until occurrence of an osteonecrosis of the jaw in 963 cancer patients. J. Cancer Res. Clin. Oncol. 2015, 141, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.Y.; Chien, J.Y.; Yang, W.S.; Juang, J.M.J.; Lee, J.J.; Tsai, K.S. The risk of osteonecrosis of the jaws in taiwanese osteoporotic patients treated with oral alendronate or raloxifene. J. Clin. Endocrinol. Metab. 2014, 99, 2729–2735. [Google Scholar] [CrossRef] [PubMed]
- Diniz-Freitas, M.; López-Cedrún, J.L.; Fernández-Sanromán, J.; García-García, A.; Fernández-Feijoo, J.; Diz-Dios, P. Oral bisphosphonate-related osteonecrosis of the jaws: Clinical characteristics of a series of 20 cases in Spain. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e751. [Google Scholar] [CrossRef] [PubMed]
- Hess, L.M.; Jeter, J.M.; Benham-Hutchins, M.; Alberts, D.S. Factors Associated with Osteonecrosis of the Jaw among Bisphosphonate Users. Am. J. Med. 2008, 121, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.N.; Palmer, N.O.A.; Lowe, D.; Randall, C. United Kingdom nationwide study of avascular necrosis of the jaws including bisphosphonate-related necrosis. Br. J. Oral Maxillofac. Surg. 2015, 53, 176–182. [Google Scholar] [CrossRef]
- Di Fede, O.; Bedogni, A.; Giancola, F.; Saia, G.; Bettini, G.; Toia, F.; D’Alessandro, N.; Firenze, A.; Matranga, D.; Fedele, S.; et al. BRONJ in patients with rheumatoid arthritis: A multicenter case series. Oral Dis. 2016, 22, 543–548. [Google Scholar] [CrossRef]
- Di Fede, O.; Panzarella, V.; Mauceri, R.; Fusco, V.; Bedogni, A.; Muzio, L.L.; Board, S.O.; Campisi, G. The Dental Management of Patients at Risk of Medication-Related Osteonecrosis of the Jaw: New Paradigm of Primary Prevention. BioMed Res. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Poxleitner, P.; Engelhardt, M.; Schmelzeisen, R.; Voss, P. The Prevention of Medication-related Osteonecrosis of the Jaw. Deutsch. Ärztebl. Int. 2017, 114, 63–69. [Google Scholar] [CrossRef]
- Spanou, A.; Nelson, K.; Ermer, M.A.; Steybe, D.; Poxleitner, P.; Voss, P.J. Primary Wound Closure and Perioperative Antibiotic Therapy for Prevention of Bisphosphonate-Related Osteonecrosis of the Jaw after Tooth Extraction. Quintessence Int. 2020, 51, 220–228. [Google Scholar] [CrossRef]
- Fliefel, R.; Tröltzsch, M.; Kühnisch, J.; Ehrenfeld, M.; Otto, S. Treatment strategies and outcomes of bisphosphonate-related osteonecrosis of the jaw (BRONJ) with characterization of patients: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 568–585. [Google Scholar] [CrossRef]
- Borumandi, F.; Aghaloo, T.; Cascarini, L.; Gaggl, A.; Fasanmade, K. Anti-resorptive Drugs and their Impact on Maxillofacial Bone among Cancer Patients. Anti-Cancer Agents Med. Chem. 2015, 15, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Alhussain, A.; Peel, S.; Dempster, L.; Clokie, C.; Azarpazhooh, A. Knowledge, Practices, and Opinions of Ontario Dentists When Treating Patients Receiving Bisphosphonates. J. Oral Maxillofac. Surg. 2015, 73, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Jeong, S.-R.; Kim, S.-J.; Kim, Y. Perceptions of medical doctors on bisphosphonate-related osteonecrosis of the jaw. BMC Oral Health 2016, 16, 92. [Google Scholar] [CrossRef] [PubMed]
- López-Jornet, P.; Camacho-Alonso, F.; Molina-Miñano, F.; Gomez-Garcia, F. Bisphosphonate-associated osteonecrosis of the jaw. Knowledge and attitudes of dentists and dental students: A preliminary study. J. Eval. Clin. Prac. 2010, 16, 878–882. [Google Scholar] [CrossRef]
- Albu-Stan, I.-A.; Petrovan, C.; Cerghizan, D.; Eremie, L.Y.; Crăciun, A.E.; Copotoiu, C. Knowledge and Attitude of Dentists Regarding Patients Undergoing Bisphosphonate Treatment: A Comparative Questionnaire. J. Interdiscip. Med. 2018, 3, 169–172. [Google Scholar] [CrossRef]
- Al-Maweri, S.A.; Alshammari, M.N.; Alharbi, A.R.; Bahein, A.A.; Alhajj, M.N.; Al-Shamiri, H.M.; Alahmary, A.W.; Doumani, M. Knowledge and Opinions of Saudi Dentists Regarding Dental Treatment of Patients Undergoing Bisphosphonates. Eur. J. Dent. 2020, 14, 144–151. [Google Scholar] [CrossRef]
- De Lima, P.B.; Brasil, V.L.M.; De Castro, J.F.L.; Ramos-Perez, F.; Alves, F.A.; Pontual, M.L.D.A.; Perez, D. Knowledge and attitudes of Brazilian dental students and dentists regarding bisphosphonate-related osteonecrosis of the jaw. Support. Care Cancer 2015, 23, 3421–3426. [Google Scholar] [CrossRef]
- Bruckmoser, E.; Palaoro, M.; Latzko, L.; Schnabl, D.; Neururer, S.B.; Laimer, J. Choosing the Right Partner for Medication Related Osteonecrosis of the Jaw: What Central European Dentists Know. Int. J. Environ. Res. Public Health 2021, 18, 4466. [Google Scholar] [CrossRef]
- Vereb, T.; Boda, K.; Czakó, L.; Vaszilkó, M.; Fülöp, G.; Klenk, G.; Janovszky, Á.; Oberna, F.; Piffkó, J.; Seres, L. Cloud-based multicenter data collection and epidemiologic analysis of bisphosphonate-related osteonecrosis of the jaws in a central european population. J. Clin. Med. 2020, 9, 426. [Google Scholar] [CrossRef]
- Vahtsevanos, K.; Kyrgidis, A.; Verrou, E.; Katodritou, E.; Triaridis, S.; Andreadis, C.G.; Boukovinas, I.; Koloutsos, G.E.; Teleioudis, Z.; Kitikidou, K.; et al. Longitudinal Cohort Study of Risk Factors in Cancer Patients of Bisphosphonate-Related Osteonecrosis of the Jaw. J. Clin. Oncol. 2009, 27, 5356–5362. [Google Scholar] [CrossRef]
- Owosho, A.A.; Liang, S.T.Y.; Sax, A.Z.; Wu, K.; Yom, S.; Huryn, J.; Estilo, C.L. Medication-related osteonecrosis of the jaw: An update on the memorial sloan kettering cancer center experience and the role of premedication dental evaluation in prevention. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Pazianas, M.; Miller, P.; Blumentals, W.A.; Bernal, M.; Kothawala, P. A Review of the Literature on Osteonecrosis of the Jaw in Patients with Osteoporosis Treated with Oral Bisphosphonates: Prevalence, Risk Factors, and Clinical Characteristics. Clin. Ther. 2007, 29, 1548–1558. [Google Scholar] [CrossRef] [PubMed]
- Palaska, P.K.; Cartsos, V.; Zavras, A.I. Bisphosphonates and Time to Osteonecrosis Development. Oncologist 2009, 14, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Lazarovici, T.S.; Yahalom, R.; Taicher, S.; Elad, S.; Hardan, I.; Yarom, N. Bisphosphonate-Related Osteonecrosis of the Jaws: A Single-Center Study of 101 Patients. J. Oral Maxillofac. Surg. 2009, 67, 850–855. [Google Scholar] [CrossRef]
- Mavrokokki, T.; Cheng, A.; Stein, B.; Goss, A. Nature and Frequency of Bisphosphonate-Associated Osteonecrosis of the Jaws in Australia. J. Oral Maxillofac. Surg. 2007, 65, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Fleisher, K.E.; Jolly, A.; Venkata, U.D.C.; Norman, R.G.; Saxena, D.; Glickman, R.S. Osteonecrosis of the Jaw Onset Times Are Based on the Route of Bisphosphonate Therapy. J. Oral Maxillofac. Surg. 2013, 71, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Fung, P.P.L.; Bedogni, G.; Bedogni, A.; Petrie, A.; Porter, S.; Campisi, G.; Bagan, J.; Fusco, V.; Saia, G.; Acham, S.; et al. Time to onset of bisphosphonate-related osteonecrosis of the jaws: A multicentre retrospective cohort study. Oral Dis. 2017, 23, 477–483. [Google Scholar] [CrossRef]
- Hansen, P.J.; Knitschke, M.; Draenert, F.G.; Irle, S.; Neff, A. Incidence of bisphosphonate-related osteonecrosis of the jaws (BRONJ) in patients taking bisphosphonates for osteoporosis treatment-a grossly underestimated risk? Clin. Oral Investig. 2013, 17, 1829–1837. [Google Scholar] [CrossRef]
- Lo, J.C.; O’Ryan, F.S.; Gordon, N.P.; Yang, J.; Hui, R.L.; Martin, D.; Hutchinson, M.; Lathon, P.V.; Sanchez, G.; Silver, P.; et al. Prevalence of Osteonecrosis of the Jaw in Patients with Oral Bisphosphonate Exposure. J. Oral Maxillofac. Surg. 2010, 68, 243–253. [Google Scholar] [CrossRef]
- Migliario, M.; Mergoni, G.; Vescovi, P.; De Martino, I.; Alessio, M.; Benzi, L.; Renò, F.; Fusco, V. Osteonecrosis of the jaw (ONJ) in osteoporosis patients: Report of delayed diagnosis of a multisite case and commentary about risks coming from a restricted ONJ definition. Dent. J. 2017, 5, 13. [Google Scholar] [CrossRef]
- Capsoni, F.; Longhi, M.; Weinstein, R. Bisphosphonate-associated osteonecrosis of the jaw: The rheumatologist’s role. Arthritis Res. Ther. 2006, 8, 219. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fehm, T.; Felsenberg, D.; Krimmel, M.; Solomayer, E.; Wallwiener, D.; Hadjii, P. Bisphosphonate-associated osteonecrosis of the jaw in breast cancer patients: Recommendations for prevention and treatment. Breast 2009, 18, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Soga, Y.; Muro, M.; Kajizono, M.; Kitamura, Y.; Sendo, T.; Sasaki, A. Replacing zoledronic acid with denosumab is a risk factor for developing osteonecrosis of the jaw. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 547–551. [Google Scholar] [CrossRef]
- Migliorati, C.A.; Epstein, J.B.; Abt, E.; Berenson, J.R. Osteonecrosis of the jaw and bisphosphonates in cancer: A narrative review. Nat. Rev. Endocrinol. 2011, 7, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Abu-Id, M.H.; Fedele, S.; Warnke, P.H.; Becker, S.T.; Kolk, A.; Mücke, T.; Mast, G.; Köhnke, R.; Volkmer, E.; et al. Osteoporosis and bisphosphonates-related osteonecrosis of the jaw: Not just a sporadic coincidence—A multi-centre study. J. Cranio-Maxillofac. Surg. 2011, 39, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Sedghizadeh, P.P.; Stanley, K.; Caligiuri, M.; Hofkes, S.; Lowry, B.; Shuler, C.F. Oral bisphosphonate use and the prevalence of osteonecrosis of the jaw. J. Am. Dent. Assoc. 2009, 140, 61–66. [Google Scholar] [CrossRef]
- Benlidayi, I.C.; Guzel, R. Oral Bisphosphonate Related Osteonecrosis of the Jaw: A Challenging Adverse Effect. ISRN Rheumatol. 2013, 2013, 215034. [Google Scholar] [CrossRef]
- Sonis, S.T.; Watkins, B.A.; Lyng, G.D.; Lerman, M.A.; Anderson, K.C. Bony changes in the jaws of rats treated with zoledronic acid and dexamethasone before dental extractions mimic bisphosphonate-related osteonecrosis in cancer patients. Oral Oncol. 2009, 45, 164–172. [Google Scholar] [CrossRef]
- Jadu, F.; Lee, L.; Pharoah, M.; Reece, D.; Wang, L. A retrospective study assessing the incidence, risk factors and comorbidities of pamidronate-related necrosis of the jaws in multiple myeloma patients. Ann. Oncol. 2007, 18, 2015–2019. [Google Scholar] [CrossRef]
- Nisi, M.; La Ferla, F.; Karapetsa, D.; Gennai, S.; Miccoli, M.; Baggiani, A.; Graziani, F.; Gabriele, M. Risk factors influencing BRONJ staging in patients receiving intravenous bisphosphonates: A multivariate analysis. Int. J. Oral Maxillofac. Surg. 2015, 44, 586–591. [Google Scholar] [CrossRef]
- Kotán, E.V.; Bartha-Lieb, T.; Parisek, Z.; Meskó, A.; Vaszilkó, M.; Hankó, B. Database analysis of the risk factors of bisphosphonate-related osteonecrosis of the jaw in Hungarian patients. BMJ Open 2019, 9, e025600. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.-S.; Tay, K.-K.; Chieng, Y.-L. Osteonecrosis of mandible: A rare complication of long-term steroid use. J. Oral Maxillofac. Surg. Med. Pathol. 2015, 27, 255–257. [Google Scholar] [CrossRef]
- Fusco, V.; Porta, C.; Saia, G.; Paglino, C.; Bettini, G.; Scoletta, M.; Bonacina, R.; Vescovi, P.; Merigo, E.; Lo Re, G.; et al. Osteonecrosis of the Jaw in Patients With Metastatic Renal Cell Cancer Treated With Bisphosphonates and Targeted Agents: Results of an Italian Multicenter Study and Review of the Literature. Clin. Genitourin. Cancer 2015, 13, 287–294. [Google Scholar] [CrossRef] [PubMed]
- McGowan, K.; Ivanovski, S.; Acton, C. Osteonecrosis of the jaws: A 14-year retrospective survey of hospital admissions. Aust. Dent. J. 2018, 63, 202–207. [Google Scholar] [CrossRef]
- Pires, F.; Miranda, A.; Cardoso, E.; Cardoso, A.; Fregnani, E.R.; Pereira, C.; Correa, M.; Almeida, J.; De A Alves, F.; Lopes, M.; et al. Oral avascular bone necrosis associated with chemotherapy and biphosphonate therapy. Oral Dis. 2005, 11, 365–369. [Google Scholar] [CrossRef]
- Kuroshima, S.; Sasaki, M.; Nakajima, K.; Tamaki, S.; Hayano, H.; Sawase, T. Prevalence of bisphosphonate-related osteonecrosis of the jaw-like lesions is increased in a chemotherapeutic dose-dependent manner in mice. Bone 2018, 112, 177–186. [Google Scholar] [CrossRef]
- Lai, T.-Y.; Wang, T.-H.; Liu, C.-J.; Chao, T.-F.; Chen, T.-J.; Hu, Y.-W. Risk factors for osteonecrosis of the jaw in oral cancer patients after surgery and eventual adjuvant treatment: The potential role of chemotherapy. Radiother. Oncol. 2017, 123, 406–411. [Google Scholar] [CrossRef]
- Barasch, A.; Cunha-Cruz, J.; Curro, F.A.; Hujoel, P.; Sung, A.H.; Vena, D.; Voinea-Griffin, A.E.; the CONDOR Collaborative Group. Risk Factors for Osteonecrosis of the Jaws. J. Dent. Res. 2011, 90, 439–444. [Google Scholar] [CrossRef]
- Eiken, P.A.; Prieto-Alhambra, D.; Eastell, R.; Abrahamsen, B. Surgically treated osteonecrosis and osteomyelitis of the jaw and oral cavity in patients highly adherent to alendronate treatment: A nationwide user-only cohort study including over 60,000 alendronate users. Osteoporos. Int. 2017, 28, 2921–2928. [Google Scholar] [CrossRef]
- Izzotti, A.; Menini, M.; Pulliero, A.; Dini, G.; Cartiglia, C.; Pera, P.; Baldi, D. Biphosphonates-associated osteonecrosis of the jaw: The role of gene-environment interaction. J. Prev. Med. Hyg. 2013, 54, 138–145. [Google Scholar]
- Quispe, D.; Shi, R.; Burton, G. Osteonecrosis of the Jaw in Patients with Metastatic Breast Cancer: Ethnic and Socio-Economic Aspects. Breast J. 2011, 17, 510–513. [Google Scholar] [CrossRef] [PubMed]

| TOT Nr | 434 |
| Mean age | 68.6 |
| Median age | 69 |
| Age range | 36–91 |
| Interquartile range age | 62–76 |
| Sex | |
| Male | 47 |
| Female | 387 |
| Indication to Treatment | Oncological Diagnosis | |
| Osteoporosis | 298 | |
| Osteoporosis and oncological disease | 57 | 136 |
| Only oncological disease | 79 | |
| Breast cancer | 68 | |
| Multiple myeloma | 22 | |
| Prostate cancer | 12 | |
| Lung cancer | 8 | |
| Uterine cancer | 6 | |
| Others | 20 |
| Environmental Factors | Non-MRONJ Count | MRONJ Count | Statistically Significant Results |
|---|---|---|---|
| Smoking | 69 | 5 | 0.04 |
| Non-smoking | 331 | 29 | |
| Type of medications | |||
| Alendronate | 168 | 9 | 0.0306 |
| Clodronate | 95 | 1 | Non-significant |
| Denosumab | 5 | 1 | Non-significant |
| Ibandronate | 17 | 1 | Non-significant |
| Neridronate | 13 | 0 | n.a.** |
| Pamidronate | 10 | 4 | Non-significant |
| Zoledronate | 46 | 1 | Non-significant |
| Risedronate | 46 | 17 | 0.0333 |
| Drugs | |||
| Antidepressants | 42 | 2 | Non-significant |
| Chemotherapy | 55 | 10 | 0.0019385 * |
| Corticosteroids | 88 | 15 | 0.0004494 * |
| Levothyroxine | 50 | 0 | Non-significant |
| Immunosuppressants | 26 | 4 | Non-significant |
| Proton pump inhibitors (PPIs) | 65 | 8 | Non-significant |
| Metformin | 10 | 2 | Non-significant |
| Strontium ranelate | 6 | 0 | Non-significant |
| Thalidomide | 5 | 3 | 0.0002010 * |
| Hormonal replacement therapy (HRT) | 38 | 5 | Non-significant |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirelli, C.; Marino, S.; Bovio, A.; Pederielli, S.; Dall’Agnola, C.; Gianni, A.B.; Biagi, R. Medication-Related Osteonecrosis of the Jaw in Dental Practice: A Retrospective Analysis of Data from the Milan Cohort. Dent. J. 2022, 10, 89. https://doi.org/10.3390/dj10050089
Mirelli C, Marino S, Bovio A, Pederielli S, Dall’Agnola C, Gianni AB, Biagi R. Medication-Related Osteonecrosis of the Jaw in Dental Practice: A Retrospective Analysis of Data from the Milan Cohort. Dentistry Journal. 2022; 10(5):89. https://doi.org/10.3390/dj10050089
Chicago/Turabian StyleMirelli, Cristina, Sonia Marino, Andrea Bovio, Sara Pederielli, Cristina Dall’Agnola, Aldo Bruno Gianni, and Roberto Biagi. 2022. "Medication-Related Osteonecrosis of the Jaw in Dental Practice: A Retrospective Analysis of Data from the Milan Cohort" Dentistry Journal 10, no. 5: 89. https://doi.org/10.3390/dj10050089
APA StyleMirelli, C., Marino, S., Bovio, A., Pederielli, S., Dall’Agnola, C., Gianni, A. B., & Biagi, R. (2022). Medication-Related Osteonecrosis of the Jaw in Dental Practice: A Retrospective Analysis of Data from the Milan Cohort. Dentistry Journal, 10(5), 89. https://doi.org/10.3390/dj10050089